Wiskott–Aldrich syndrome protein
The Wiskott–Aldrich Syndrome protein (WASp) is a 502-amino acid protein expressed in cells of the hematopoietic system that in humans is encoded by the WAS gene. In the inactive state, WASp exists in an autoinhibited conformation with sequences near its C-terminus binding to a region near its N-terminus. Its activation is dependent upon CDC42 and PIP2 acting to disrupt this interaction, causing the WASp protein to 'open'. This exposes a domain near the WASp C-terminus that binds to and activates the Arp2/3 complex. Activated Arp2/3 nucleates new F-actin.
WASp is the founding member of a gene family which also includes the broadly expressed N-WASP (neuronal Wiskott–Aldrich Syndrome protein), SCAR/WAVE1, WASH, WHAMM, and JMY.[5][6] WAML (WASP and MIM like), WAWH (WASP without WH1 domain), and WHIMP (WAVE Homology in Membrane Protrusions) have more recently been discovered.[7][8]
Structure and function
The Wiskott–Aldrich syndrome (WAS) family of proteins share similar domain structure, and are involved in transduction of signals from receptors on the cell surface to the actin cytoskeleton. The presence of a number of different motifs suggests they are regulated by a number of different stimuli, and interact with multiple proteins. These proteins, directly or indirectly, associate with the small GTPase CDC42, known to regulate formation of actin filaments, and the cytoskeletal organising complex, Arp2/3.
The WASp family proteins includes WASp, N-WASp, SCAR/WAVE, WHAMM and WASH. The five of them share a C- terminal VCA (verprolin, central, acidic) domain where they interact with actin nucleating complex (ARP2/3) and they differ in their terminal domains. WASp and N-WASP are analogs, they contain an N-terminal EVH1 domain, a C-terminal VCA domain and central B and GBD (GTP binding domain) domains. WASp, is expressed exclusively in hematopoietic cells and neuronal WASp (N-WASp), is ubiquitously expressed. N-WASp contains an output region and a control region that are essential for its regulation. The output region is called the VVCA domain. It is located towards the C-terminal end of the protein and contains four motifs: two verprolin homology motifs (VV) binds actin monomers and delivers them to Arp2/3; the central domain (C) was once thought to bind cofilin but is now believed to enhance the interactions between the V domains and actin monomers, as well as the interaction between the A domain and Arp2/3; and the acidic motif (A) binds Arp2/3.[9] In isolation, the VCA region is constitutively active. However, in full-length N-WASp the control region suppresses VCA domain activity. The control region is located at N-terminal end of N-WASp.[10] The control region contains a CDC42-binding domain (GBP) and a PIP2-binding domain (B), both of which are critical for proper regulation of N-WASp.[10] Cooperative binding of CDC42 and PIP2 relieve the autoinhibition of N-WASp, causing Arp2/3 to carry out actin polymerization.[10] WASp interacting protein (WIP) interacts with WASp N-terminal domain (WH1) preventing it from degradation and stabilising its auto-inhibitory conformation.
In the absence of CDC42 and PIP2, N-WASp is in an inactive, locked conformation.[10] Cooperative binding of both CDC42 and PIP2 relieve the autoinhibition. The cooperative binding of CDC42 and PIP2 is thermodynamically favored; binding of one enhances binding of the other.[10] CDC42 and PIP2 localize the N-WASp-Arp2/3 complex to the plasma membrane. This localization ensures the actin polymers will be able to push through the plasma membrane and form filopodium required for cell motility.[11]
WASp is required for various functions in myeloid and lymphoid immune cells. Many of these, such as phagocytosis and podosome formation, related to its role in regulating the polymerization of actin filaments. Other functions of WASP depend on its activity as a scaffold protein for assembly of effective signalling complexes downstream of antigen receptor or integrin engagement.[12] Particularly in NK cells it participates in the synapse formation and polarization of perforin to the immune synapse for NK cell cytotoxicity. When WASp is absent or mutated T cells and B cells formation of immune synapse and TCR/BCR downstream signaling is also affected.
Clinical significance
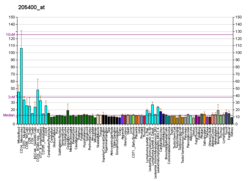
Wiskott–Aldrich syndrome is a rare, inherited, X-linked, recessive disease characterized by immune dysregulation and microthrombocytopenia, and is caused by mutations in the WASp gene. The WASp gene product is a cytoplasmic protein, expressed exclusively in hematopoietic cells, which show signalling and cytoskeletal abnormalities in WAS patients. A transcript variant arising as a result of alternative promoter usage, and containing a different 5' UTR sequence, has been described, but its full-length nature is not known.[13]
WASp is a product of the WASp, and mutations in the WASp can lead to Wiskott–Aldrich syndrome (an X-linked disease that mainly affects males with symptoms that include thrombocytopenia, eczema, recurrent infections, and small-sized platelets) in these patients the protein is usually significantly reduced or absent. Other, less inactivating mutations affecting the WASp cause X-linked thrombocytopenia, or XLT, where there is usually detectable protein levels by flow cytometry. The majority of the mutations causing classic WAS are located in the WH1 domain of the protein[14] and these mutations affect binding with the WASp Interacting Protein.[15] Mutations located in the GBD domain disrupt autoinhibition and lead to an unfolded protein that is constitutively active. Unlike WAS and XLT, WASp in this case is present and active. Activated WASp leads to nuclear localization of actin filaments and this can lead to premature apoptosis, aneuploidy and failure to undergo cytokinesis. This, in turn, causes myelodysplasia and X-linked neutropenia.
A prospective gene therapy for Wiskott–Aldrich syndrome, OTL-103, uses autologous CD34+ lymphocytes that are transfected with a lentiviral vector to produce functional WASp.[16] As of 28 June 2021[update], OTL-103 was undergoing Phase I/II clinical trials at the San Raffaele Hospital in Milan, Italy.[17]
Interactions
Wiskott–Aldrich syndrome protein has been shown to interact with:
See also
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000015285 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000031165 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Pollitt AY, Insall RH (August 2009). "WASP and SCAR/WAVE proteins: the drivers of actin assembly". Journal of Cell Science. 122 (Pt 15): 2575–8. doi:10.1242/jcs.023879. PMC 2954249. PMID 19625501.
- ^ Burianek LE, Soderling SH (April 2013). "Under lock and key: spatiotemporal regulation of WASP family proteins coordinates separate dynamic cellular processes". Seminars in Cell & Developmental Biology. 24 (4): 258–66. doi:10.1016/j.semcdb.2012.12.005. PMC 3656410. PMID 23291261.
- ^ Oda A, Eto K (April 2013). "WASPs and WAVEs: from molecular function to physiology in hematopoietic cells". Seminars in Cell & Developmental Biology. WASP/WAVE proteins: expanding members and functions & The role of ploidy variation on cellular adaptation. 24 (4): 308–13. doi:10.1016/j.semcdb.2013.03.002. PMID 23499790.
- ^ Kabrawala S, Zimmer MD, Campellone KG (14 January 2020). "WHIMP links the actin nucleation machinery to Src-family kinase signaling during protrusion and motility". bioRxiv: 2020.01.14.906784. doi:10.1101/2020.01.14.906784.
- ^ Higgs HN, Pollard TD (2001). "Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins". Annual Review of Biochemistry. 70 (1): 649–76. doi:10.1146/annurev.biochem.70.1.649. PMID 11395419.
- ^ a b c d e Prehoda KE, Scott JA, Mullins RD, Lim WA (October 2000). "Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex". Science. 290 (5492): 801–6. Bibcode:2000Sci...290..801P. doi:10.1126/science.290.5492.801. PMID 11052943.
- ^ Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW (April 1999). "The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly". Cell. 97 (2): 221–31. doi:10.1016/S0092-8674(00)80732-1. PMID 10219243.
- ^ Thrasher AJ, Burns SO (March 2010). "WASP: a key immunological multitasker". Nature Reviews. Immunology. 10 (3): 182–92. doi:10.1038/nri2724. PMID 20182458. S2CID 20764637.
- ^ "Entrez Gene: WAS Wiskott-Aldrich syndrome (eczema-thrombocytopenia)".
- ^ Rajmohan R, Raodah A, Wong MH, Thanabalu T (December 2009). "Characterization of Wiskott-Aldrich syndrome (WAS) mutants using Saccharomyces cerevisiae". FEMS Yeast Research. 9 (8): 1226–35. doi:10.1111/j.1567-1364.2009.00581.x. PMID 19817875.
- ^ Rajmohan R, Meng L, Yu S, Thanabalu T (April 2006). "WASP suppresses the growth defect of Saccharomyces cerevisiae las17Delta strain in the presence of WIP". Biochemical and Biophysical Research Communications. 342 (2): 529–36. doi:10.1016/j.bbrc.2006.01.160. PMID 16488394.
- ^ Orchard Therapeutics (Europe) Limited (29 July 2019). "Orchard Therapeutics Announces FDA Regenerative Medicine Advanced Therapy (RMAT) Designation Granted for OTL-103 for the Treatment of Wiskott-Aldrich Syndrome". GlobeNewswire News Room. Retrieved 28 June 2021.
- ^ Clinical trial number NCT03837483 for "A Single Arm, Open-label Clinical Trial of Hematopoietic Stem Cell Gene Therapy With Cryopreserved Autologous CD34+ Cells Transduced With Lentiviral Vector Encoding WAS cDNA in Subjects With Wiskott-Aldrich Syndrome (WAS)" at ClinicalTrials.gov}
- ^ a b Tian L, Nelson DL, Stewart DM (March 2000). "Cdc42-interacting protein 4 mediates binding of the Wiskott-Aldrich syndrome protein to microtubules". The Journal of Biological Chemistry. 275 (11): 7854–61. doi:10.1074/jbc.275.11.7854. PMID 10713100.
- ^ Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK (March 2000). "Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein". Nature. 404 (6774): 151–8. Bibcode:2000Natur.404..151K. doi:10.1038/35004513. PMID 10724160. S2CID 4416185.
- ^ Kolluri R, Tolias KF, Carpenter CL, Rosen FS, Kirchhausen T (May 1996). "Direct interaction of the Wiskott-Aldrich syndrome protein with the GTPase Cdc42". Proceedings of the National Academy of Sciences of the United States of America. 93 (11): 5615–8. Bibcode:1996PNAS...93.5615K. doi:10.1073/pnas.93.11.5615. PMC 39296. PMID 8643625.
- ^ Symons M, Derry JM, Karlak B, Jiang S, Lemahieu V, Mccormick F, Francke U, Abo A (March 1996). "Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization". Cell. 84 (5): 723–34. doi:10.1016/S0092-8674(00)81050-8. PMID 8625410.
- ^ Oda A, Ochs HD, Lasky LA, Spencer S, Ozaki K, Fujihara M, Handa M, Ikebuchi K, Ikeda H (May 2001). "CrkL is an adapter for Wiskott-Aldrich syndrome protein and Syk". Blood. 97 (9): 2633–9. doi:10.1182/blood.v97.9.2633. PMID 11313252.
- ^ a b She HY, Rockow S, Tang J, Nishimura R, Skolnik EY, Chen M, Margolis B, Li W (September 1997). "Wiskott-Aldrich syndrome protein is associated with the adapter protein Grb2 and the epidermal growth factor receptor in living cells". Molecular Biology of the Cell. 8 (9): 1709–21. doi:10.1091/mbc.8.9.1709. PMC 305731. PMID 9307968.
- ^ a b c d e f Banin S, Truong O, Katz DR, Waterfield MD, Brickell PM, Gout I (August 1996). "Wiskott-Aldrich syndrome protein (WASp) is a binding partner for c-Src family protein-tyrosine kinases". Current Biology. 6 (8): 981–8. doi:10.1016/s0960-9822(02)00642-5. PMID 8805332.
- ^ a b c Finan PM, Soames CJ, Wilson L, Nelson DL, Stewart DM, Truong O, Hsuan JJ, Kellie S (October 1996). "Identification of regions of the Wiskott-Aldrich syndrome protein responsible for association with selected Src homology 3 domains". The Journal of Biological Chemistry. 271 (42): 26291–5. doi:10.1074/jbc.271.42.26291. PMID 8824280.
- ^ a b c Rivero-Lezcano OM, Marcilla A, Sameshima JH, Robbins KC (October 1995). "Wiskott-Aldrich syndrome protein physically associates with Nck through Src homology 3 domains". Molecular and Cellular Biology. 15 (10): 5725–31. doi:10.1128/MCB.15.10.5725. PMC 230823. PMID 7565724.
- ^ Banin S, Gout I, Brickell P (August 1999). "Interaction between Wiskott-Aldrich Syndrome protein (WASP) and the Fyn protein-tyrosine kinase". Molecular Biology Reports. 26 (3): 173–7. doi:10.1023/A:1006954206151. PMID 10532312. S2CID 36018089.
- ^ Cory GO, MacCarthy-Morrogh L, Banin S, Gout I, Brickell PM, Levinsky RJ, Kinnon C, Lovering RC (November 1996). "Evidence that the Wiskott-Aldrich syndrome protein may be involved in lymphoid cell signaling pathways". Journal of Immunology. 157 (9): 3791–5. doi:10.4049/jimmunol.157.9.3791. PMID 8892607.
- ^ Bunnell SC, Henry PA, Kolluri R, Kirchhausen T, Rickles RJ, Berg LJ (October 1996). "Identification of Itk/Tsk Src homology 3 domain ligands". The Journal of Biological Chemistry. 271 (41): 25646–56. doi:10.1074/jbc.271.41.25646. PMID 8810341.
- ^ McGavin MK, Badour K, Hardy LA, Kubiseski TJ, Zhang J, Siminovitch KA (December 2001). "The intersectin 2 adaptor links Wiskott Aldrich Syndrome protein (WASp)-mediated actin polymerization to T cell antigen receptor endocytosis". The Journal of Experimental Medicine. 194 (12): 1777–87. doi:10.1084/jem.194.12.1777. PMC 2193569. PMID 11748279.
- ^ Krause M, Sechi AS, Konradt M, Monner D, Gertler FB, Wehland J (April 2000). "Fyn-binding protein (Fyb)/SLP-76-associated protein (SLAP), Ena/vasodilator-stimulated phosphoprotein (VASP) proteins and the Arp2/3 complex link T cell receptor (TCR) signaling to the actin cytoskeleton". The Journal of Cell Biology. 149 (1): 181–94. doi:10.1083/jcb.149.1.181. PMC 2175102. PMID 10747096.
- ^ Okabe S, Fukuda S, Broxmeyer HE (July 2002). "Activation of Wiskott-Aldrich syndrome protein and its association with other proteins by stromal cell-derived factor-1alpha is associated with cell migration in a T-lymphocyte line". Experimental Hematology. 30 (7): 761–6. doi:10.1016/s0301-472x(02)00823-8. PMID 12135674.
- ^ Wu Y, Spencer SD, Lasky LA (March 1998). "Tyrosine phosphorylation regulates the SH3-mediated binding of the Wiskott-Aldrich syndrome protein to PSTPIP, a cytoskeletal-associated protein". The Journal of Biological Chemistry. 273 (10): 5765–70. doi:10.1074/jbc.273.10.5765. PMID 9488710.
- ^ Ramesh N, Antón IM, Hartwig JH, Geha RS (December 1997). "WIP, a protein associated with wiskott-aldrich syndrome protein, induces actin polymerization and redistribution in lymphoid cells". Proceedings of the National Academy of Sciences of the United States of America. 94 (26): 14671–6. Bibcode:1997PNAS...9414671R. doi:10.1073/pnas.94.26.14671. PMC 25088. PMID 9405671.
- ^ Antón IM, Lu W, Mayer BJ, Ramesh N, Geha RS (August 1998). "The Wiskott-Aldrich syndrome protein-interacting protein (WIP) binds to the adaptor protein Nck". The Journal of Biological Chemistry. 273 (33): 20992–5. doi:10.1074/jbc.273.33.20992. PMID 9694849.
Further reading
- O'Sullivan E, Kinnon C, Brickell P (1999). "Wiskott-Aldrich syndrome protein, WASP". The International Journal of Biochemistry & Cell Biology. 31 (3–4): 383–7. doi:10.1016/S1357-2725(98)00118-6. PMID 10224664.
- Snapper SB, Rosen FS (1999). "The Wiskott-Aldrich syndrome protein (WASP): roles in signaling and cytoskeletal organization". Annual Review of Immunology. 17: 905–29. doi:10.1146/annurev.immunol.17.1.905. PMID 10358777.
- Thrasher AJ, Kinnon C (April 2000). "The Wiskott-Aldrich syndrome". Clinical and Experimental Immunology. 120 (1): 2–9. doi:10.1046/j.1365-2249.2000.01193.x. PMC 1905602. PMID 10759756.
External links
- GeneReviews/NIH/NCBI/UW entry on WAS-Related Disorders including Wiskott-Aldrich syndrome (WAS), X-linked thrombocytopenia (XLT), and X-linked congenital neutropenia (XLN)
- Online Mendelian Inheritance in Man (OMIM): 300392
- Online Mendelian Inheritance in Man (OMIM): 313900
- Wiskott-Aldrich+Syndrome+Protein at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Overview of all the structural information available in the PDB for UniProt: P42768 (Wiskott-Aldrich syndrome protein) at the PDBe-KB.