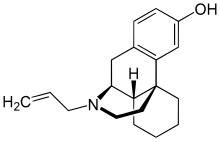
Dextrallorphan
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C19H25NO |
| Molar mass | 283.415 g·mol−1 |
| 3D model (JSmol) | |
| |
Dextrallorphan (DXA) is a chemical of the morphinan class that is used in scientific research. It acts as a σ1 receptor agonist and NMDA receptor antagonist.[1][2][3][4] It has no significant affinity for the σ2, μ-opioid, or δ-opioid receptor, or for the serotonin or norepinephrine transporter.[2][5] As an NMDA receptor antagonist, in vivo, it is approximately twice as potent as dextromethorphan, and five-fold less potent than dextrorphan.[3]
Uses in Scientific Research
[edit]Masking of sigma-1 receptor
[edit]Dextrallorphan is often used in research to block σ1 receptor sites so that σ2 receptor sites (which have not been cloned yet [when?]) can be studied.[6][7][8] It was hypothesized that both of these sigma (σ) receptors were opioid receptors, due to their affinity for psychoactive drugs. However, it is now understood that they are non-opioid receptors that bind to certain psychoactive drugs, like dextrallorphan.[9] One example of dextrallorphan being used to mask σ1 receptor sites was seen in a study on the localization of the σ2 receptor in detergent-resistant lipid raft domains.[6] It has also been used to mask σ1 receptor sites so that σ2 receptor binding characteristics in the rat liver could be determined, by labeling σ2 receptor sites with [3H]l,3-di-o-tolylguanidine (DTG) in the presence of 1 μM dextrallorphan solution.[8]
Animal Studies
[edit]Dextrallorphan was used in Spraque-Dawley rats to study cerebellar Purkinje neurons electrophysical responses to the drug when it was applied iontophoretically as a sigma (σ) receptor ligand. Dextrallorphan increased the firing rate by 14%, suggesting that sigma (σ) ligands (like dextrallorphan) alter the spontaneous firing of Purkinje neurons and cause motor effects.[10]
In another study, dextrallorphan, along with other opioid derivatives, was found to be a potent inhibitor of etorphine-inaccessible (EI) sites in the guinea-pig brain. Dextrallorphan was of the top three most potent opioid inhibitors of those studied, with a concentration of 67 nM required to show 50% inhibition.[1]
History
[edit]In 1955, dextrallorphan has been used to study inhibition of cholinesterases and to look at the relationship between analgetics and acetylcholine metabolism.[11] It was found that dextrallorphan inhibits 25% of bovine erythrocyte cholinesterase at a dose of 10−3 mole/liter, which corresponds to a concentration of up to 0.2 mg/kg in dog intestine. However, at this dose the drug showed no effect on the gut tone. Dextrallorphan was classified as a potent inhibitor of the intestinal and red blood cell cholinesterase based on the concentration of the drug needed to inhibit these enzymes in the cholinesterase preparations from the animals systems utilized. Simultaneously, dextrallorphan showed no analgesia and no change in intestinal tone. With these results dextrallorphan helped proved that there is no correlation between the inhibition of cholinesterase systems and analgetic or intestinal effects.[12]
In 1979, dextrallorphan was found to have a half maximal inhibitory concentration (IC50) for binding to the pituitary and brain receptor of 10,000 ± 1000 nM and 10,000 ± 1500 nM, respectively. While its stereoisomer, levallorphan, had a 10,000 times more potent dose, thus proving that binding to these receptors is stereospecific.[13]
See also
[edit]References
[edit]- ^ a b Su TP (November 1982). "Evidence for sigma opioid receptor: binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain" (PDF). The Journal of Pharmacology and Experimental Therapeutics. 223 (2): 284–90. PMID 6290634.
- ^ a b Codd EE, Shank RP, Schupsky JJ, Raffa RB (September 1995). "Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: structural determinants and role in antinociception" (PDF). The Journal of Pharmacology and Experimental Therapeutics. 274 (3): 1263–70. PMID 7562497.
- ^ a b Shukla VK, Lemaire S (January 1997). "N-methyl-D-aspartate antagonist activity of alpha- and beta-sulfallorphans" (PDF). The Journal of Pharmacology and Experimental Therapeutics. 280 (1): 357–65. PMID 8996216.
- ^ Shannon HE (April 1983). "Pharmacological evaluation of N-allynormetazocine (SKF 10,047) on the basis of its discriminative stimulus properties in the rat". The Journal of Pharmacology and Experimental Therapeutics. 225 (1): 144–52. PMID 6834266.
- ^ He XS, Bowen WD, Lee KS, Williams W, Weinberger DR, de Costa BR (March 1993). "Synthesis and binding characteristics of potential SPECT imaging agents for sigma-1 and sigma-2 binding sites". Journal of Medicinal Chemistry. 36 (5): 566–71. doi:10.1021/jm00057a006. PMID 8496936.
- ^ a b Gebreselassie D, Bowen WD (June 2004). "Sigma-2 receptors are specifically localized to lipid rafts in rat liver membranes". European Journal of Pharmacology. 493 (1–3): 19–28. doi:10.1016/j.ejphar.2004.04.005. PMID 15189760.
- ^ Maeda DY, Williams W, Bowen WD, Coop A (January 2000). "A sigma-1 receptor selective analogue of BD1008. A potential substitute for (+)-opioids in sigma receptor binding assays". Bioorganic & Medicinal Chemistry Letters. 10 (1): 17–8. doi:10.1016/s0960-894x(99)00590-9. PMID 10636233.
- ^ a b Torrence-Campbell C, Bowen WD (May 1996). "Differential solubilization of rat liver sigma 1 and sigma 2 receptors: retention of sigma 2 sites in particulate fractions". European Journal of Pharmacology. 304 (1–3): 201–10. doi:10.1016/0014-2999(96)00109-4. PMID 8813603.
- ^ Hayashi T, Su T (October 2005). "The sigma receptor: evolution of the concept in neuropsychopharmacology". Current Neuropharmacology. 3 (4): 267–80. doi:10.2174/157015905774322516. PMC 2268997. PMID 18369400.
- ^ Martin WJ, De Costa BR, Walker JM (1994). "Effects of sigma ligands on rat cerebellar Purkinje neuron firing: an iontophoretic study". Brain Research Bulletin. 35 (4): 303–9. doi:10.1016/0361-9230(94)90106-6. PMID 7850479. S2CID 54255450.
- ^ Eikenburg DC, Stickney JL (1979). "Anti-cholinesterase activity of 1-alpha-acetylmethadol: relationship to bradycardia". General Pharmacology. 10 (3): 195–200. doi:10.1016/0306-3623(79)90089-2. PMID 467958.
- ^ Young DC, Ploeg RA, Featherstone RM, Gross EG (May 1955). "The interrelationships among the central, peripheral and anticholinesterase effects of some morphinan derivatives" (pdf). The Journal of Pharmacology and Experimental Therapeutics. 114 (1): 33–7. PMID 14392568.
- ^ Simantov R, Snyder SH (March 1977). "Opiate receptor binding in the pituitary gland". Brain Research. 124 (1): 178–84. doi:10.1016/0006-8993(77)90877-0. PMID 191146. S2CID 40173550.
