AM-251 (drug)
This is an old revision of this page, as edited by Enix150 (talk | contribs) at 04:30, 22 December 2017 (Enix150 moved page AM251 to AM-251 (drug): AM cannabinoids have a dash in the name). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "AM-251" drug – news · newspapers · books · scholar · JSTOR (December 2010) (Learn how and when to remove this message) |
 | |
 | |
| Identifiers | |
|---|---|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider |
|
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.162.062 |
| Chemical and physical data | |
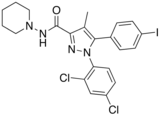
| Formula | C22H21Cl2IN4O |
| Molar mass | 555.238 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
AM-251 is an inverse agonist at the CB1 cannabinoid receptor. AM-251 is structurally very close to SR141716A (rimonabant); both are biarylpyrazole cannabinoid receptor antagonists. In AM-251 the p-chloro group attached to the phenyl substituent at C-5 of the pyrazole ring is replaced with a p-iodo group. The resulting compound exhibits slightly better binding affinity for the CB1 receptor (with a Ki value of 7.5nM) than SR141716A, which has a Ki value of 11.5nM, AM-251 is, however, about two-fold more selective for the CB1 receptor when compared to SR141716A.[1] Like SR141716A, it is additionally a μ-opioid receptor antagonist.[2]
See also
References
- ^ Lan, R; Liu, Q; Fan, P; Lin, S; Fernando, SR; McCallion, D; Pertwee, R; Makriyannis, A. "Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists". J Med Chem. 42: 769–76. doi:10.1021/jm980363y. PMID 10052983.
- ^ Seely, KA; Brents, LK; Franks, LN; Rajasekaran, M; Zimmerman, SM; Fantegrossi, WE; Prather, PL (2012). "AM-251 and rimonabant act as direct antagonists at mu-opioid receptors: implications for opioid/cannabinoid interaction studies". Neuropharmacology. 63: 905–15. doi:10.1016/j.neuropharm.2012.06.046. PMC 3408547. PMID 22771770.
| Receptor (ligands) |
| ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (modulators) |
| ||||||||||||||||||||||||||||||
| Enzyme (modulators) |
| ||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
| μ-opioid (MOR) |
| ||||
|---|---|---|---|---|---|
| δ-opioid (DOR) |
| ||||
| κ-opioid (KOR) |
| ||||
| Nociceptin (NOP) |
| ||||
| Others |
| ||||
This cannabinoid related article is a stub. You can help Wikipedia by expanding it. |
- Articles needing additional references from December 2010
- All articles needing additional references
- ECHA InfoCard ID from Wikidata
- Chem-molar-mass both hardcoded and calculated
- Infobox-drug molecular-weight unexpected-character
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugs missing an ATC code
- Drugs with no legal status
- All stub articles
