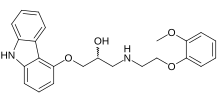
Carvedilol
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Coreg, others |
| Other names | BM-14190 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697042 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 25–35% |
| Protein binding | 98% |
| Metabolism | Liver (CYP2D6, CYP2C9) |
| Elimination half-life | 7–10 hours |
| Excretion | Urine (16%), feces (60%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.117.236 |
| Chemical and physical data | |
| Formula | C24H26N2O4 |
| Molar mass | 406.482 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Carvedilol, sold under the brand name Coreg among others, is a medication used to treat high blood pressure, congestive heart failure (CHF), and left ventricular dysfunction in people who are otherwise stable.[1] For high blood pressure, it is generally a second-line treatment.[1] It is taken by mouth.[1]
Common side effects include dizziness, tiredness, joint pain, low blood pressure, nausea, and shortness of breath.[1] Severe side effects may include bronchospasm.[1] Safety during pregnancy or breastfeeding is unclear.[2] Use is not recommended in those with liver problems.[3] Carvedilol is a nonselective beta blocker and alpha-1 blocker.[1] How it improves outcomes is not entirely clear but may involve dilation of blood vessels.[1]
Carvedilol was patented in 1978 and approved for medical use in the United States in 1995.[1][4] It is on the World Health Organization's List of Essential Medicines.[5] It is available as a generic medication.[1] In 2020, it was the 26th most commonly prescribed medication in the United States, with more than 23 million prescriptions.[6][7]
Medical uses
Carvedilol is indicated in the management of congestive heart failure (CHF), commonly as an adjunct to angiotensin-converting-enzyme inhibitor (ACE inhibitors) and diuretics. It has been clinically shown to reduce mortality and hospitalizations in people with CHF.[8] The mechanism behind its positive effect when used long-term in clinically stable CHF patients is not fully understood, but is thought to contribute to remodeling of the heart, improving upon its structure and function.[9][10]
Carvedilol reduces the risk of death, hospitalizations, and recurring heart attacks for patients with reduced heart function following a heart attack.[11] [12] Carvedilol has also been proven to reduce death and hospitalization in patients with severe heart failure.[13]
In practice, carvedilol has been used in the treatment of uncomplicated hypertension, yet studies suggest it has relatively ineffective blood pressure-lowering effects when compared with other blood pressure-lowering therapies or other beta blockers.[14]
Available forms
Carvedilol is available in the following forms:
Contraindications
Carvedilol should not be used in patients with bronchial asthma or bronchospastic conditions due to increased risk of bronchoconstriction.[17][18] It should not be used in people with second- or third-degree atrioventricular block, sick sinus syndrome, severe bradycardia (unless a permanent pacemaker is in place), or a decompensated heart condition. People with severe hepatic impairment should use carvedilol with caution.[19][20][21]
Side effects
The most common side effects (>10% incidence) of carvedilol include:[15]
- Dizziness
- Fatigue
- Low blood pressure
- Diarrhea
- Weakness
- Slowed heart rate
- Weight gain
- Erectile dysfunction
Carvedilol is not recommended for people with uncontrolled bronchospastic disease (e.g. current asthma symptoms) as it can block receptors that assist in opening the airways.[15]
Carvedilol may mask symptoms of low blood sugar,[15] resulting in hypoglycemia unawareness. This is termed beta blocker induced hypoglycemia unawareness.
Pharmacology
Pharmacodynamics
Carvedilol is both a non-selective β-adrenergic receptor antagonist (β1, β2) and an α-adrenergic receptor antagonist (α1). The S(–) enantiomer accounts for the beta-blocking activity whereas the S(–) and R(+) enantiomers have alpha-blocking activity.[15] The affinity (Ki) of carvedilol for the β-adrenergic receptors is 0.32 nM for the human β1-adrenergic receptor and 0.13 to 0.40 nM for the β2-adrenergic receptor.[22]
Using rat proteins, carvedilol has shown affinity for a variety of targets including the β1-adrenergic receptor (Ki = 0.24–0.43 nM), β2-adrenergic receptor (Ki = 0.19–0.25 nM), α1-adrenergic receptor (Ki = 3.4 nM), α2-adrenergic receptor (Ki = 2,168 nM), 5-HT1A receptor (Ki = 3.4 nM), 5-HT2 receptor (Ki = 207 nM), H1 receptor (Ki = 3,034 nM), D2 receptor (Ki = 213 nM), μ-opioid receptor (Ki = 2,700 nM), veratridine site of voltage-gated sodium channels (IC50 = 1,260 nM), serotonin transporter (Ki = 528 nM), norepinephrine transporter (Ki = 2,406 nM), and dopamine transporter (Ki = 627 nM).[23] It is an antagonist of the human 5-HT2A receptors with moderate affinity (Ki = 547 nM), although it is unclear if this is significant for its pharmacological actions given its much stronger activity at adrenergic receptors.[24]
Carvedilol reversibly binds to β-adrenergic receptors on cardiac myocytes. Inhibition of these receptors prevents a response to the sympathetic nervous system, leading to decreased heart rate and contractility. This action is beneficial in heart failure patients where the sympathetic nervous system is activated as a compensatory mechanism.[25] Carvedilol blockade of α1-adrenergic receptors causes vasodilation of blood vessels. This inhibition leads to decreased peripheral vascular resistance and an antihypertensive effect. There is no reflex tachycardia response due to carvedilol blockade of β1-adrenergic receptors on the heart.[26]
Pharmacokinetics
Carvedilol is about 25% to 35% bioavailable following oral administration due to extensive first-pass metabolism. Absorption is slowed when administered with food, however, it does not show a significant difference in bioavailability. Taking carvedilol with food decreases the risk of orthostatic hypotension.[15]
The majority of carvedilol is bound to plasma proteins (98%), mainly to albumin. Carvedilol is a basic, hydrophobic compound with a steady-state volume of distribution of 115 L. Plasma clearance ranges from 500 to 700 mL/min.[15] Carvedilol is lipophilic and easily crosses the blood–brain barrier in animals, and hence is not thought to be peripherally selective.[27][28]
The compound is metabolized by liver enzymes, CYP2D6 and CYP2C9 via aromatic ring oxidation and glucuronidation, then further conjugated by glucuronidation and sulfation. The three active metabolites exhibit only one-tenth of the vasodilating effect of the parent compound. However, the 4'-hydroxyphenyl metabolite is about 13-fold more potent in β-blockade than the parent.[15]
The mean elimination half-life of carvedilol following oral administration ranges from 7 to 10 hours. The pharmaceutical product is a mix of two enantiomorphs, R(+)-carvedilol and S(–)-carvedilol, with differing metabolic properties. R(+)-Carvedilol undergoes preferential selection for metabolism, which results in a fractional half-life of about 5 to 9 hours, compared with 7 to 11 hours for the S(-)-carvedilol fraction.[15]
References
- ^ a b c d e f g h i "Carvedilol Monograph for Professionals". Drugs.com. AHFS. Retrieved 24 December 2018.
- ^ "Carvedilol Use During Pregnancy". Drugs.com. Retrieved 24 December 2018.
- ^ British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 147. ISBN 9780857113382.
- ^ Fischer, Janos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 463. ISBN 9783527607495.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ "The Top 300 of 2020". ClinCalc. Retrieved 7 October 2022.
- ^ "Carvedilol - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.
- ^ Yancy, Clyde W.; Jessup, Mariell; Bozkurt, Biykem; Butler, Javed; Casey, Donald E.; Drazner, Mark H.; Fonarow, Gregg C.; Geraci, Stephen A.; Horwich, Tamara; Januzzi, James L.; Johnson, Maryl R.; Kasper, Edward K.; Levy, Wayne C.; Masoudi, Frederick A.; McBride, Patrick E.; McMurray, John J. V.; Mitchell, Judith E.; Peterson, Pamela N.; Riegel, Barbara; Sam, Flora; Stevenson, Lynne W.; Tang, W. H. Wilson; Tsai, Emily J.; Wilkoff, Bruce L.; Wilkoff, B. L. (15 October 2013). "2013 AHA Guidelines for the Management of Heart Failure" (PDF). Circulation. 128 (16): e240-327. doi:10.1161/CIR.0b013e31829e8776. PMID 23741058.
- ^ Biaggioni, MD, Italo (2009). Basic and Clinical Pharmacology 11th Edition. New York: McGraw-Hill Medical Publishing Division. p. 189. ISBN 9780071604055.
- ^ Reis Filho JR, Cardoso JN, Cardoso CM, Pereira-Barretto AC (June 2015). "Reverse Cardiac Remodeling: A Marker of Better Prognosis in Heart Failure". Arquivos Brasileiros de Cardiologia. 104 (6): 502–6. doi:10.5935/abc.20150025. PMC 4484683. PMID 26131706.
- ^ Dargie, H. J. (2001). "Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial". The Lancet. 357 (9266): 1385–1390. doi:10.1016/s0140-6736(00)04560-8. ISSN 0140-6736. PMID 11356434. S2CID 1840228.
- ^ Huang, Bao-Tao, MD, PhD, Huang, Fang-Yang, MD, Zuo, Zhi-Liang, MD, Liao, Yan-Biao, MD, PhD, Heng, Yue, MD, Wang, Peng-Ju, MD, Gui, Yi-Yue, MD, Xia, Tian-Li, MD, Xin, Zhe-Mei, MD, Liu, Wei, MD, PhD, Zhang, Chen, MD, PhD, Chen, Shi-Jian, MD, PhD, Pu, Xiao-Bo, MD, PhD, Chen, Mao, MD, PhD, & Huang, De-Jia, MD, PhD. (2015). Meta-Analysis of Relation Between Oral β-Blocker Therapy and Outcomes in Patients With Acute Myocardial Infarction Who Underwent Percutaneous Coronary Intervention. The American Journal of Cardiology, 115(11), 1529–1538. https://doi.org/10.1016/j.amjcard.2015.02.057
- ^ Cleland, Bunting, K. V., Flather, M. D., Altman, D. G., Holmes, J., Coats, A. J. S., Manzano, L., McMurray, J. J. V., Ruschitzka, F., van Veldhuisen, D. J., von Lueder, T. G., Böhm, M., Andersson, B., Kjekshus, J., Packer, M., Rigby, A. S., Rosano, G., Wedel, H., Hjalmarson, Åke, … Kotecha, D. (2018). Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: An individual patient-level analysis of double-blind randomized trials. European Heart Journal, 39(1), 26–35. https://doi.org/10.1093/eurheartj/ehx564
- ^ Wong, Gavin WK; Laugerotte, Alexandra; Wright, James M (26 August 2015). "Blood pressure lowering efficacy of dual alpha and beta blockers for primary hypertension". Cochrane Database of Systematic Reviews. 2015 (8): CD007449. doi:10.1002/14651858.cd007449.pub2. ISSN 1465-1858. PMC 6486308. PMID 26306578.
- ^ a b c d e f g h i "Coreg - Food and Drug Administration" (PDF).
- ^ "Drug Approval Package". www.accessdata.fda.gov. Retrieved 5 November 2015.
- ^ Morales, D.R., Lipworth, B.J., Donnan, P.T. et al. Respiratory effect of beta-blockers in people with asthma and cardiovascular disease: population-based nested case control study. BMC Med. 2017 Jan; 15 (18). https://doi.org/10.1186/s12916-017-0781-0
- ^ Kotlyar E, Keogh A, Macdonald P, Arnold R, McCaffrey D, Glanville A. Tolerability of carvedilol in patients with heart failure and concomitant chronic obstructive pulmonary disease or asthma. J Heart Lung Transplant. 2002 March; 21 (12): 1290-1295. https://doi.org/10.1016/S1053-2498(02)00459-X
- ^ Sinha R, Lockman KA, Mallawaarachchi N, Robertson M, Plevris JN, Hayes PC. Carvedilol use is associated with improved survival in patients with liver cirrhosis and ascites. Journal of hepatology. 2017;67(1):40–6.
- ^ Zacharias AP, Jeyaraj R, Hobolth L, Bendtsen F, Gluud LL, Morgan MY, et al. Carvedilol versus traditional, non‐selective beta‐blockers for adults with cirrhosis and gastroesophageal varices. Cochrane database of systematic reviews. 2018;2018(10):CD011510
- ^ 3.Lo G-H, Chen W-C, Wang H-M, Yu H-C. Randomized, controlled trial of carvedilol versus nadolol plus isosorbide mononitrate for the prevention of variceal rebleeding. Journal of gastroenterology and hepatology. 2012;27(11):1681–7.
- ^ "Carvedilol | Ligand page | IUPHAR/BPS Guide to PHARMACOLOGY".
- ^ Pauwels PJ, Gommeren W, Van Lommen G, Janssen PA, Leysen JE (December 1988). "The receptor binding profile of the new antihypertensive agent nebivolol and its stereoisomers compared with various beta-adrenergic blockers". Mol Pharmacol. 34 (6): 843–51. PMID 2462161.
- ^ Murnane KS, Guner OF, Bowen JP, Rambacher KM, Moniri NH, Murphy TJ, Daphney CM, Oppong-Damoah A, Rice KC (June 2019). "The adrenergic receptor antagonist carvedilol interacts with serotonin 2A receptors both in vitro and in vivo". Pharmacology, Biochemistry, and Behavior. 181: 37–45. doi:10.1016/j.pbb.2019.04.003. PMC 6570414. PMID 30998954.
- ^ Manurung D, Trisnohadi HB (2007). "Beta blockers for congestive heart failure" (PDF). Acta Medica Indonesiana. 39 (1): 44–8. PMID 17297209. Archived from the original (PDF) on 4 March 2016. Retrieved 12 November 2015.
- ^ Ruffolo RR, Gellai M, Hieble JP, Willette RN, Nichols AJ (1990). "The pharmacology of carvedilol". European Journal of Clinical Pharmacology. 38 Suppl 2: S82–8. doi:10.1007/BF01409471. PMID 1974511. S2CID 2901620.
- ^ Wang J, Ono K, Dickstein DL, Arrieta-Cruz I, Zhao W, Qian X, Lamparello A, Subnani R, Ferruzzi M, Pavlides C, Ho L, Hof PR, Teplow DB, Pasinetti GM (December 2011). "Carvedilol as a potential novel agent for the treatment of Alzheimer's disease". Neurobiol Aging. 32 (12): 2321.e1–12. doi:10.1016/j.neurobiolaging.2010.05.004. PMC 2966505. PMID 20579773.
- ^ Bart J, Dijkers EC, Wegman TD, de Vries EG, van der Graaf WT, Groen HJ, Vaalburg W, Willemsen AT, Hendrikse NH (August 2005). "New positron emission tomography tracer [(11)C]carvedilol reveals P-glycoprotein modulation kinetics". Br J Pharmacol. 145 (8): 1045–51. doi:10.1038/sj.bjp.0706283. PMC 1576233. PMID 15951832.
Further reading
- Chakraborty S, Shukla D, Mishra B, Singh S (February 2010). "Clinical updates on carvedilol: a first choice beta-blocker in the treatment of cardiovascular diseases". Expert Opinion on Drug Metabolism & Toxicology. 6 (2): 237–50. doi:10.1517/17425250903540220. PMID 20073998. S2CID 25670550.
- Dean L (2018). "Carvedilol Therapy and CYP2D6 Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 30067327. Bookshelf ID: NBK518573.
External links
- "Carvedilol". Drug Information Portal. U.S. National Library of Medicine.
