Dexamethasone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Decadron, Ozurdex, Dexycu, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682792 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous, intramuscular, subcutaneous, intraosseous, intravitreal, eye drop |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80–90% |
| Protein binding | 77% |
| Metabolism | Liver |
| Elimination half-life | biological half-life: 36 to 54 hours; plasma half-life: 4 to 5 hours[8][9] |
| Excretion | Urine (65%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.004 |
| Chemical and physical data | |
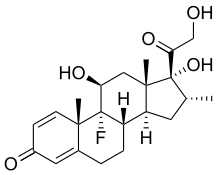
| Formula | C22H29FO5 |
| Molar mass | 392.467 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 262 °C (504 °F) |
| |
| |
| | |
Dexamethasone is a fluorinated glucocorticoid medication[10] used to treat rheumatic problems, a number of skin diseases, severe allergies, asthma, chronic obstructive pulmonary disease (COPD), croup, brain swelling, eye pain following eye surgery, superior vena cava syndrome (a complication of some forms of cancer),[11] and along with antibiotics in tuberculosis.[10] In adrenocortical insufficiency, it may be used in combination with a mineralocorticoid medication such as fludrocortisone.[10] In preterm labor, it may be used to improve outcomes in the baby.[10] It may be given by mouth, as an injection into a muscle, as an injection into a vein, as a topical cream or ointment for the skin or as a topical ophthalmic solution to the eye.[10] The effects of dexamethasone are frequently seen within a day and last for about three days.[10]
The long-term use of dexamethasone may result in thrush, bone loss, cataracts, easy bruising, or muscle weakness.[10] It is in pregnancy category C in the United States, meaning that it should only be used when the benefits are predicted to be greater than the risks.[1] In Australia, the oral use is category A, meaning it has been frequently used in pregnancy and not been found to cause problems to the baby.[12] It should not be taken when breastfeeding.[10] Dexamethasone has anti-inflammatory and immunosuppressant effects.[10]
Dexamethasone was first synthesized in 1957 by Philip Showalter Hench and was approved for medical use in 1958.[13][14][15] It is on the World Health Organization's List of Essential Medicines.[16] In 2022, it was the 234th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[17][18] It is available as a generic medication.[19] In 2022, the combination of dexamethasone with neomycin and polymyxin B was the 274th most commonly prescribed medication in the United States, with more than 800,000 prescriptions.[20][21]
Medical uses
[edit]
Anti-inflammatory
[edit]
Dexamethasone is used to treat many inflammatory and autoimmune disorders, such as rheumatoid arthritis and bronchospasm.[22] Idiopathic thrombocytopenic purpura, a decrease in numbers of platelets due to an immune problem, responds to 40 mg daily for four days; it may be administered in 14-day cycles. It is unclear whether dexamethasone in this condition is significantly better than other glucocorticoids.[23]
It is also given in small amounts before and/or after some forms of dental surgery, such as the extraction of the wisdom teeth, an operation that often causes puffy, swollen cheeks.[24]
Dexamethasone is commonly given as a treatment for croup in children.[25] A single dose can reduce the swelling of the airway to improve breathing and reduce discomfort.[25]
Dexamethasone is sometimes injected into the heel when treating plantar fasciitis or heel pain, sometimes in conjunction with triamcinolone acetonide. There is no evidence that this treatment helps in the long term, however, dexamethasone may provide short-term pain relief.[26]
It may be useful to counteract allergic anaphylactic shock, however this is not usually recommended by clinical guidelines.[27]
It is present in certain eye drops – particularly after eye surgery – and as a nasal spray, and certain ear drops (can be combined with an antibiotic and an antifungal). Dexamethasone intravitreal steroid implants have been approved by the US Food and Drug Administration (FDA) to treat ocular conditions such as diabetic macular edema, central retinal vein occlusion, and uveitis. However, the evidence is poor quality relating to the treatment of uveitis, with the potential side effects (cataract progression and raised intraocular pressure) being significant, and the benefits not certainly greater than standard treatment.[28] Dexamethasone has also been used with antibiotics to treat acute endophthalmitis.[29]
Dexamethasone is used in transvenous screw-in cardiac pacing leads to minimize the inflammatory response of the myocardium. The steroid is released into the myocardium as soon as the screw is extended and can play a significant role in minimizing the acute pacing threshold due to the reduction of inflammatory response. The typical quantity present in a lead tip is less than 1.0 mg.[medical citation needed]
Dexamethasone may be administered before antibiotics in cases of bacterial meningitis. Gram-negative bacteria — to which the causative agent of bacterial meningitis, neisseria meningitidis, belongs — have highly immunogenic lipopolysaccharides as a component of their cell membrane and trigger a strong inflammatory response. Pre-administration of dexamethasone before the administration of antibiotics acts to reduce that response, thus reducing hearing loss and neurological damage.[30]

Cancer
[edit]People with cancer undergoing chemotherapy are often given dexamethasone to counteract certain side effects of their antitumor treatments. Dexamethasone can increase the antiemetic effect of 5-HT3 receptor antagonists, such as ondansetron.[31] The exact mechanism of this interaction is not well-defined, but it has been theorized that this effect may be due to, among many other causes, inhibition of prostaglandin synthesis, anti-inflammatory effects, immunosuppressive effects, decreased release of endogenous opioids, or a combination of the aforementioned.[32]
In brain tumors (primary or metastatic), dexamethasone is used to counteract the development of edema, which could eventually compress other brain structures.[33] It is also given in cord compression, where a tumor is compressing the spinal cord.[medical citation needed] Evidence on the safety and efficacy of using dexamethasone to treat malignant brain tumors is not clear.[34]
Dexamethasone is also used as a direct chemotherapeutic agent in certain hematological malignancies, especially in the treatment of multiple myeloma, in which dexamethasone is given alone or in combination with other chemotherapeutic drugs, including most commonly with thalidomide (Thal-dex), lenalidomide, bortezomib (Velcade, Vel-dex),[35] or a combination of doxorubicin (Adriamycin) and vincristine or bortezomib/lenalidomide/dexamethasone.[medical citation needed]
COVID-19
[edit]
Dexamethasone is recommended by the National Health Service in the UK and the National Institutes of Health (NIH) in the US for people with COVID-19 who need either mechanical ventilation or supplemental oxygen (without ventilation).[36][37]
The Infectious Diseases Society of America (IDSA) guideline panel suggests the use of glucocorticoids for people with severe COVID-19, defined as people with SpO2 ≤94% on room air, and those who require supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).[38] The IDSA recommends against the use of glucocorticoids for those with COVID-19 without hypoxemia requiring supplemental oxygen.[38]
The World Health Organization (WHO) recommends systemic corticosteroids rather than no systemic corticosteroids for the treatment of people with COVID-19 (strong recommendation, based on moderate certainty evidence).[39] The WHO suggests not to use corticosteroids in the treatment of people with non-severe COVID-19 (conditional recommendation, based on low certainty evidence).[39]
The Oxford University RECOVERY Trial issued a press release announcing preliminary results that the drug could reduce deaths by about a third in participants on ventilators and by about a fifth in participants on oxygen; it did not benefit people who did not require respiratory support.[40] A meta-analysis of seven clinical trials of critically ill COVID-19 participants, each treated with one of three different corticosteroids found a statistically significant reduction in death.[41] The largest reduction was obtained with dexamethasone (36% compared to placebo).[41][42]
In September 2020, the European Medicines Agency (EMA) endorsed the use of dexamethasone in adults and adolescents, from twelve years of age and weighing at least 40 kilograms (88 lb), who require supplemental oxygen therapy.[43] Dexamethasone can be taken by mouth or given as an injection or infusion (drip) into a vein.[43]
In November 2020, the Public Health Agency of Canada's Clinical Pharmacology Task Group recommended dexamethasone for hospitalized patients requiring mechanical ventilation.[44] Although dexamethasone, and other glucocorticoids, reduce mortality in COVID-19 they have also been associated with an increased risk of secondary infections,[45][46][47] secondary infections being a significant issue in critically ill COVID-19 patients.[48]
The mechanism of action of dexamethasone involves suppression of late-stage interferon type I programs in severe COVID-19 patients.[49]
Surgery
[edit]Dexamethasone is used fairly regularly, often as a single intravenous dose, during surgery to prevent postoperative nausea and vomiting, manage pain, potentially reduce the amount of pain medication required, and help reduce post-surgery hospitalisation time.[50] The adverse effects of taking steroids after surgery on wound healing, blood sugar levels, and in diabetics are not completely understood; however, dexamethasone likely does not increase the risk of postoperative infections.[50][50]
Endocrine
[edit]Dexamethasone is the treatment for the very rare disorder of glucocorticoid resistance.[51][52]
In adrenal insufficiency and Addison's disease, dexamethasone is prescribed when the patient does not respond well to prednisone or methylprednisolone.[medical citation needed]
It can be used in congenital adrenal hyperplasia in older adolescents and adults to suppress adrenocorticotropic hormone (ACTH) production. It is typically given at night.[53]
Pregnancy
[edit]Dexamethasone may be given to women at risk of delivering prematurely to promote maturation of the fetus's lungs. This administration, given from one day to one week before delivery, has been associated with low birth weight, although not with increased rates of neonatal death.[54]
Dexamethasone has also been used during pregnancy as an off-label prenatal treatment for the symptoms of congenital adrenal hyperplasia (CAH) in female babies. CAH causes a variety of physical abnormalities, notably ambiguous genitalia. Early prenatal CAH treatment has been shown to reduce some CAH symptoms, but it does not treat the underlying congenital disorder. This use is controversial: it is inadequately studied, only around one in ten of the fetuses of women treated are at risk of the condition, and serious adverse events have been documented.[55] Experimental use of dexamethasone in pregnancy for fetal CAH treatment was discontinued in Sweden when one in five cases had adverse events.[56]
A small clinical trial found long-term effects on verbal working memory among the small group of children treated prenatally, but the small number of test subjects means the study cannot be considered definitive.[57][58]
High-altitude illnesses
[edit]Dexamethasone is used in the treatment of high-altitude cerebral edema (HACE), as well as high-altitude pulmonary edema (HAPE).[59] It is commonly carried on mountain-climbing expeditions to help climbers deal with complications of altitude sickness.[60][61]
Nausea and vomiting
[edit]Intravenous dexamethasone is effective for the prevention of nausea and vomiting in people who had surgery and whose post-operative pain was treated with long-acting spinal or epidural spinal opioids.[62]
The combination of dexamethasone and a 5-HT3 receptor antagonist such as ondansetron is more effective than a 5-HT3 receptor antagonist alone in preventing postoperative nausea and vomiting.[63]
Sore throat
[edit]A single dose of dexamethasone or another steroid speeds the improvement of a sore throat.[64]
Contraindications
[edit]Contraindications of dexamethasone include,[65][66] but are not limited to:
- Uncontrolled infections
- Known hypersensitivity to dexamethasone
- Cerebral malaria
- Systemic fungal infection
- Concurrent treatment with live virus vaccines (including smallpox vaccine)
Adverse effects
[edit]The exact incidence of the adverse effects of dexamethasone is not available, hence estimates have been made as to the incidence of the adverse effects below based on the adverse effects of related corticosteroids and on available documentation on dexamethasone.[66][67][68][69][70]
Common
[edit]- Acne
- Amnesia
- Birth defect
- Cataract (in long-term treatment, occurs in about 10% of patients)
- Confusion
- Depression
- Dyspepsia
- Euphoria
- Headaches
- Hiccups (in long-term treatment, occurs in about 11% of patients)
- Hyperglycemia
- Hypertension
- Impaired skin healing and wound repair
- Increased appetite
- Increased risk of viral, bacterial, fungal, and parasitic infections
- Insomnia
- Irritability
- Malaise
- steroid induced Muscle atrophy and myopathy
- Nausea
- Ocular hypertension
- Osteoporosis
- Vertigo
- Vomiting
- Weight gain
Unknown frequency
[edit]- Abdominal distension
- Adrenal suppression
- Allergic reactions (including anaphylaxis)
- Arterial thrombosis
- Aspergillosis
- Bruising
- Candidiasis
- Cardiomyopathy
- Cleft palate
- Corneal or scleral thinning
- Cushing's syndrome
- Edema
- Esophageal ulcer
- Facial plethora
- Glaucoma
- Growth stunting (in children)
- Herpes zoster
- Hypernatremia
- Hypertriglyceridemia
- Hypocalcemia
- Hypokalemia
- Intracranial hypertension (with long-term treatment)
- Leukocytosis
- Mania
- Mucormycosis
- Pancreatitis (inflammation of the pancreas)
- Papilledema
- Peptic ulcer
- Protein catabolism (causing nitrogen depletion)
- Psychological dependence
- Psychosis
- Seizures
- Skin atrophy
- Striae
- Telangiectasia
- Thromboembolism
- Venous thrombosis
- Vertebral collapse
Withdrawal
[edit]Sudden withdrawal after long-term treatment with corticosteroids can lead to[66]
- Adrenal insufficiency
- Arthralgia
- Conjunctivitis
- Death
- Fever
- Hypotension
- Myalgia
- Nodule (medicine) (painful, itchy skin condition)
- Rhinitis
- Weight loss
Interactions
[edit]Known drug interactions include:[66]
- Inducers of hepatic microsomal enzymes such as barbiturates, phenytoin, and rifampicin can reduce the half-life of dexamethasone.[medical citation needed]
- Cotreatment with oral contraceptives can increase its volume of distribution.[medical citation needed]
Pharmacology
[edit]Pharmacodynamics
[edit]As a glucocorticoid, dexamethasone is an agonist of the glucocorticoid receptor (GR).[71] It is highly selective for the GR over the mineralocorticoid receptor (MR),[72] and in relation to this, has minimal mineralocorticoid activity.[73][74][75] This is in contrast to endogenous corticosteroids like cortisol, which bind to and activate both the GR and the MR.[72] Dexamethasone is 25 times more potent than hydrocortisone (cortisol) as a glucocorticoid.[71] Its affinity (Ki) for the GR was about 1.2 nM in one study.[71]
The activation of the GR by dexamethasone results in dose-dependent suppression of the hypothalamic–pituitary–adrenal axis (HPA axis) and of production of endogenous corticosteroids by the adrenal glands, thereby reducing circulating endogenous concentrations of corticosteroids like cortisol and corticosterone.[72]
Dexamethasone poorly penetrates the blood–brain barrier into the central nervous system due to binding to P-glycoprotein.[72][76] However, higher doses of dexamethasone override the export capacity of P-glycoprotein and enter the brain to produce central activation of GRs.[72] In conjunction with the suppression of endogenous corticosteroids by dexamethasone, this results in skewed ratios of activation of peripheral versus central GRs as well as skewed ratios of activation of GRs versus MRs when compared to non-synthetic corticosteroids.[72][76] These differences can have significant clinical relevance.[72][76]
Chemistry
[edit]Dexamethasone is a synthetic pregnane corticosteroid and derivative of cortisol (hydrocortisone) and is also known as 1-dehydro-9α-fluoro-16α-methylhydrocortisone or as 9α-fluoro-11β,17α,21-trihydroxy-16α-methylpregna-1,4-diene-3,20-dione.[77][78] The molecular and crystal structure of dexamethasone has been determined by X-ray crystallography.[79] It is a stereoisomer of betamethasone, the two compounds differing only in the spatial configuration of the methyl group at position 16 (see steroid nomenclature).[80]
Synthesis
[edit]To synthesize dexamethasone, 16β-methylprednisolone acetate is dehydrated to the 9,11-dehydro derivative.[81][82] This is then reacted with a source of hypobromite, such as basic N-bromosuccinimide, to form the 9α-bromo-11β-hydrin derivative, which is then ring-closed to an epoxide. A ring-opening reaction with hydrogen fluoride in tetrahydrofuran gives dexamethasone.[citation needed]

Spectroscopy
[edit]In chemistry, spectroscopy is used to analyze products of reactions. To understand if dexamethasone is synthesized from a reaction, spectroscopy must be taken and compared to the literature spectrum. There are multiple spectroscopy analyses that can be taken including 1H NMR, 13C NMR, IR, Mass spectrometry, and UV/vis spectroscopy.
The NMR spectrum shown above can be used to compare to product synthesized through reactions to figure out if Dexamethasone was synthesized. 1H NMR, among other things, shows that there are 29 hydrogens and 13C NMR shows that there are 22 carbons.
Using IR spectroscopy, the peaks show the functional groups found in the molecule. You can see peaks at 3472, 1662, and 1618 representing alcohol, aldehyde, and alkene functional groups. UV-vis spectroscopy is another way to analyze a product to figure out what it is.
Finally, mass spectroscopy showed peaks at: 393.1, 355.2 147.1 m/z. The peak at 393.1 m/z is the peak for dexamethasone as its molecular weight is 392.46 m/z. [86]
History
[edit]Dexamethasone was first synthesized by Philip Showalter Hench in 1957.[87][14] It was introduced for medical use in 1958.[74]
On 16 June 2020, the RECOVERY Trial announced preliminary results stating that dexamethasone improves survival rates of hospitalized patients with COVID-19 receiving oxygen or on a ventilator. Benefits were only observed in patients requiring respiratory support; those who did not require breathing support saw a worse survival rate than the control group, although the difference may have been due to chance.[88] A preprint containing the full dataset was published on 22 June 2020, and demand for dexamethasone surged after the publication of the preprint.[89] The preliminary report was published in The New England Journal of Medicine on 18 July 2020.[90] The final report was published in February 2021.[91]
The World Health Organization (WHO) states that dexamethasone should be reserved for seriously ill and critical patients receiving COVID-19 treatment in a hospital setting,[92] and the WHO Director-General stated that "WHO emphasizes that dexamethasone should only be used for patients with severe or critical disease, under close clinical supervision. There is no evidence this drug works for patients with mild disease or as a preventative measure, and it could cause harm."[93] In July 2020, the WHO stated they were in the process of updating treatment guidelines to include dexamethasone or other steroids.[94] In September 2020, the WHO released updated guidance on using corticosteroids for COVID-19.[39][95]
In July 2020, the European Medicines Agency (EMA) started reviewing results from the RECOVERY study arm that involved the use of dexamethasone in the treatment of patients with COVID-19 admitted to the hospital to provide an opinion on the results and in particular the potential use of dexamethasone for the treatment of adults with COVID-19.[96][97] In September 2020, the EMA received an application for marketing authorization of dexamethasone for COVID-19.[98]
Society and culture
[edit]Price
[edit]Dexamethasone is inexpensive.[99] In the United States a month of medication is typically priced less than US$25.[10] In India, a course of treatment for preterm labor is about US$0.50.[99] The drug is available in most areas of the world.[99]
Nonmedical use
[edit]Dexamethasone is given in legal Bangladesh brothels to prostitutes not yet of legal age, causing weight gain aimed at making them appear older and healthier to customers and police.[100]
Dexamethasone and most glucocorticoids are banned by sporting bodies including the World Anti-Doping Agency.[101]
Veterinary use
[edit]Combined with marbofloxacin CAS number 115550-35-1and clotrimazole, dexamethasone is available under the name Aurizon, CAS number 50-02-2, and used to treat difficult ear infections, especially in dogs. It can also be combined with trichlormethiazide to treat horses with swelling of distal limbs and general bruising.[102]
References
[edit]- ^ a b "Dexamethasone Use During Pregnancy". Drugs.com. Archived from the original on 17 May 2016. Retrieved 9 June 2016.
Dexamethasone is only recommended for use during pregnancy when there are no alternatives and benefit outweighs risk.
- ^ "Dexamethasone Juno (Juno Pharmaceuticals Pty Ltd)". Department of Health and Aged Care. Archived from the original on 18 March 2023.
- ^ "Ozurdex- dexamethasone implant". DailyMed. 30 October 2020. Archived from the original on 27 March 2022. Retrieved 27 March 2022.
- ^ "Dexamethasone sodium phosphate solution/ drops". DailyMed. 31 July 2020. Archived from the original on 27 January 2022. Retrieved 27 March 2022.
- ^ "Dexycu- dexamethasone injection, suspension". DailyMed. 20 December 2021. Archived from the original on 27 March 2022. Retrieved 27 March 2022.
- ^ "Ozurdex EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 27 October 2020. Retrieved 24 October 2020.
- ^ "Neofordex EPAR". European Medicines Agency (EMA). 25 July 2014. Archived from the original on 7 October 2024. Retrieved 24 October 2020.
- ^ Dogra P, Vijayashankar NP (14 September 2020). "Dexamethasone Suppression Test". StatPearls (14 September 2020). PMID 31194457.
- ^ Jobe AH, Milad MA, Peppard T, Jusko WJ (March 2020). "Pharmacokinetics and Pharmacodynamics of Intramuscular and Oral Betamethasone and Dexamethasone in Reproductive Age Women in India". Clinical and Translational Science. 13 (2): 391–99. doi:10.1111/cts.12724. PMC 7070803. PMID 31808984.
- ^ a b c d e f g h i j "Dexamethasone". The American Society of Health-System Pharmacists. Archived from the original on 31 August 2017. Retrieved 29 July 2015.
- ^ Wilkinson IB (13 July 2017). Oxford Handbook of Clinical Medicine. OUP Oxford. p. 176. ISBN 978-0-19-968990-3.
- ^ "Prescribing medicines in pregnancy database". Australian Government. 3 March 2014. Archived from the original on 8 April 2014. Retrieved 22 April 2014.
Drugs which have been taken by a large number of pregnant women and women of childbearing age without any proven increase in the frequency of malformations or other direct or indirect harmful effects on the fetus having been observed.
- ^ "Drugs@FDA: Dexamethasone". U.S. Food and Drug Administration (FDA). Archived from the original on 30 November 2017. Retrieved 27 March 2022.
- ^ a b Rankovic Z, Hargreaves R, Bingham M (2012). Drug discovery and medicinal chemistry for psychiatric disorders. Cambridge: Royal Society of Chemistry. p. 286. ISBN 9781849733656. Archived from the original on 5 March 2016.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 485. ISBN 9783527607495. Archived from the original on 10 January 2023. Retrieved 17 June 2020.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Dexamethasone Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ "Competitive Generic Therapy Approvals". U.S. Food and Drug Administration (FDA). 29 June 2023. Archived from the original on 29 June 2023. Retrieved 29 June 2023.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Dexamethasone; Neomycin; Polymyxin B Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ Till J. "Paramedic Clinical Training Aid". Archived from the original on 31 March 2012. Retrieved 30 August 2011.
- ^ Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, et al. (January 2010). "International consensus report on the investigation and management of primary immune thrombocytopenia". Blood. 115 (2): 168–86. doi:10.1182/blood-2009-06-225565. PMID 19846889.
- ^ Schmelzeisen R, Frölich JC (1993). "Prevention of postoperative swelling and pain by dexamethasone after operative removal of impacted third molar teeth". European Journal of Clinical Pharmacology. 44 (3): 275–77. doi:10.1007/BF00271371. PMID 8491244. S2CID 12528750.
- ^ a b "Croup – Diagnosis & Treatment". Mayo Clinic. Archived from the original on 10 October 2017. Retrieved 13 October 2017.
Dexamethasone is usually recommended because of its long-lasting effects (up to 72 hours).
- ^ Arnold MJ, Gruber J (February 2018). "Injected Corticosteroids for Plantar Heel Pain". American Family Physician. 97 (3): 169–170. PMID 29431981.
- ^ Dodd A, Hughes A, Sargant N, Whyte AF, Soar J, Turner PJ (April 2021). "Evidence update for the treatment of anaphylaxis". Resuscitation. 163: 86–96. doi:10.1016/j.resuscitation.2021.04.010. hdl:1983/82ab8f32-67a9-4277-8750-2246aabf5d96. PMC 8139870. PMID 33895231.
- ^ Reddy A, Liu SH, Brady CJ, Sieving PC, Palestine AG (August 2023). "Corticosteroid implants for chronic non-infectious uveitis". The Cochrane Database of Systematic Reviews. 2023 (8): CD010469. doi:10.1002/14651858.CD010469.pub4. PMC 10464657. PMID 37642198.
- ^ Emami S, Kitayama K, Coleman AL (June 2022). "Adjunctive steroid therapy versus antibiotics alone for acute endophthalmitis after intraocular procedure". The Cochrane Database of Systematic Reviews. 2022 (6): CD012131. doi:10.1002/14651858.CD012131.pub3. PMC 9169535. PMID 35665485.
- ^ Brouwer MC, McIntyre P, Prasad K, van de Beek D (September 2015). "Corticosteroids for acute bacterial meningitis". Cochrane Database of Systematic Reviews. 2015 (9): CD004405. doi:10.1002/14651858.CD004405.pub5. PMC 6491272. PMID 26362566.
- ^ Roila F, Ballatori E, Ruggeri B, DeAngelis V (May 2000). "Dexamethasone alone or in combination with ondansetron for the prevention of delayed nausea and vomiting induced by chemotherapy". The New England Journal of Medicine. 342 (21): 1554–59. doi:10.1056/NEJM200005253422102. PMID 10824073.
- ^ Holte K, Kehlet H (November 2002). "Perioperative single-dose glucocorticoid administration: pathophysiologic effects and clinical implications". Journal of the American College of Surgeons. 195 (5): 694–712. doi:10.1016/s1072-7515(02)01491-6. PMID 12437261.
- ^ Kostaras X, Cusano F, Kline GA, Roa W, Easaw J (June 2014). "Use of dexamethasone in patients with high-grade glioma: a clinical practice guideline". Current Oncology. 21 (3): e493–e503. doi:10.3747/co.21.1769. PMC 4059813. PMID 24940109.
- ^ Jessurun CA, Hulsbergen AF, Cho LD, Aglio LS, Nandoe Tewarie RD, Broekman ML (September 2019). "Evidence-based dexamethasone dosing in malignant brain tumors: what do we really know?". Journal of Neuro-Oncology. 144 (2): 249–264. doi:10.1007/s11060-019-03238-4. PMC 6700052. PMID 31346902.
- ^ Harousseau JL, Attal M, Leleu X, Troncy J, Pegourie B, Stoppa AM, et al. (November 2006). "Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II study". Haematologica. 91 (11): 1498–505. PMID 17043025.

- ^ "Dexamethasone and COVID-19". SPS – Specialist Pharmacy Service. Archived from the original on 22 July 2020. Retrieved 22 July 2020.
- ^ "Corticosteroids". COVID-19 Treatment Guidelines. National Institutes of Health. Archived from the original on 19 July 2020. Retrieved 12 July 2020.
- ^ a b "COVID-19 Guideline, Part 1: Treatment and Management". Infectious Diseases Society of America. Archived from the original on 6 October 2020. Retrieved 22 July 2020.
Recommendation 4. Among hospitalized people with severe* COVID-19, the IDSA guideline panel suggests glucocorticoids rather than no glucocorticoids. (Conditional recommendation, Moderate certainty of evidence)
Remark: Dexamethasone 6 mg IV or PO for 10 days (or until discharge if earlier) or equivalent glucocorticoid dose may be substituted if dexamethasone is unavailable. Equivalent total daily doses of alternative glucocorticoids to dexamethasone 6 mg daily are methylprednisolone 32 mg and prednisone 40 mg.
Recommendation 5. Among hospitalized people with COVID-19 without hypoxemia requiring supplemental oxygen, the IDSA guideline panel suggests against the use of glucocorticoids. (Conditional recommendation, Low certainty of evidence) - ^ a b c World Health Organization (2020). Corticosteroids for COVID-19: living guidance, 2 September 2020 (Report). World Health Organization. hdl:10665/334125. WHO/2019-nCoV/Corticosteroids/2020.1. Archived from the original on 11 October 2020. Retrieved 2 September 2020.
- ^ "Dexamethasone reduces death in hospitalized people with severe respiratory complications of COVID-19". University of Oxford. 16 June 2020. Archived from the original on 16 June 2020. Retrieved 16 June 2020.
- ^ a b Sterne JA, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, et al. (The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group) (September 2020). "Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis". JAMA. 324 (13): 1330–1341. doi:10.1001/jama.2020.17023. PMC 7489434. PMID 32876694. S2CID 221467783.
- ^ Prescott HC, Rice TW (September 2020). "Corticosteroids in COVID-19 ARDS: Evidence and Hope During the Pandemic". JAMA. 324 (13): 1292–1295. doi:10.1001/jama.2020.16747. PMID 32876693. S2CID 221468015. Archived from the original on 3 December 2020. Retrieved 2 September 2020.
- ^ a b "EMA endorses use of dexamethasone in COVID-19 patients on oxygen or mechanical ventilation". European Medicines Agency (EMA) (Press release). 18 September 2020. Archived from the original on 14 February 2021. Retrieved 21 September 2020. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ Public Health Agency of Canada (23 November 2020). "Ad-hoc COVID-19 Clinical Pharmacology Task Group: Statement on dexamethasone". Government of Canada. Archived from the original on 27 December 2020. Retrieved 1 April 2022.
- ^ Conway Morris A, Kohler K, De Corte T, Ercole A, De Grooth HJ, Elbers PW, et al. (August 2022). "Co-infection and ICU-acquired infection in COIVD-19 ICU patients: a secondary analysis of the UNITE-COVID data set". Critical Care. 26 (1): 236. doi:10.1186/s13054-022-04108-8. PMC 9347163. PMID 35922860.
- ^ Meynaar IA, van Rijn S, Ottens TH, van Burgel ND, van Nieuwkoop C (July 2022). "Increased risk of central line-associated bloodstream infection in COVID-19 patients associated with dexamethasone but not with interleukin antagonists". Intensive Care Medicine. 48 (7): 954–957. doi:10.1007/s00134-022-06750-w. PMC 9171741. PMID 35670819.
- ^ Scaravilli V, Guzzardella A, Madotto F, Beltrama V, Muscatello A, Bellani G, et al. (June 2022). "Impact of dexamethasone on the incidence of ventilator-associated pneumonia in mechanically ventilated COVID-19 patients: a propensity-matched cohort study". Critical Care. 26 (1): 176. doi:10.1186/s13054-022-04049-2. PMC 9191402. PMID 35698155.
- ^ Maes M, Higginson E, Pereira-Dias J, Curran MD, Parmar S, Khokhar F, et al. (January 2021). "Ventilator-associated pneumonia in critically ill patients with COVID-19". Critical Care. 25 (1): 25. doi:10.1186/s13054-021-03460-5. PMC 7797892. PMID 33430915.
- ^ Sinha S, Rosin NL, Arora R, Labit E, Jaffer A, Cao L, et al. (January 2022). "Dexamethasone modulates immature neutrophils and interferon programming in severe COVID-19". Nature Medicine. 28 (1): 201–211. doi:10.1038/s41591-021-01576-3. PMC 8799469. PMID 34782790. S2CID 244132637.
- ^ a b c Polderman JA, Farhang-Razi V, Van Dieren S, Kranke P, DeVries JH, Hollmann MW, et al. (November 2018). "Adverse side effects of dexamethasone in surgical patients". The Cochrane Database of Systematic Reviews. 11 (11): CD011940. doi:10.1002/14651858.CD011940.pub3. PMC 6426282. PMID 30480776.
- ^ Chrousos GP, Detera-Wadleigh SD, Karl M (December 1993). "Syndromes of glucocorticoid resistance" (PDF). Annals of Internal Medicine. 119 (11): 1113–24. doi:10.7326/0003-4819-119-11-199312010-00009. PMID 8239231. S2CID 26040431. Archived from the original (PDF) on 14 August 2017. Retrieved 4 July 2020.
- ^ Charmandari E, Kino T, Ichijo T, Chrousos GP (May 2008). "Generalized glucocorticoid resistance: clinical aspects, molecular mechanisms, and implications of a rare genetic disorder". The Journal of Clinical Endocrinology and Metabolism. 93 (5): 1563–72. doi:10.1210/jc.2008-0040. PMC 2386273. PMID 18319312.
- ^ Dan L. Longo, Anthony Fauci, Dennis Kasper, Stephen Hauser, J. Jerry Jameson and Joseph Loscalzo, Harrison's Principles of Internal Medicine, 18th edition, p. 3055
- ^ Bloom SL, Sheffield JS, McIntire DD, Leveno KJ (April 2001). "Antenatal dexamethasone and decreased birth weight". Obstetrics and Gynecology. 97 (4): 485–90. doi:10.1016/S0029-7844(00)01206-0. PMID 11275014. S2CID 33601749.
- ^ Elton C (18 June 2010). "A Prenatal Treatment Raises Questions of Medical Ethics". Archived from the original on 31 August 2016 – via content.time.com.
- ^ Hirvikoski T, Nordenström A, Wedell A, Ritzén M, Lajic S (June 2012). "Prenatal dexamethasone treatment of children at risk for congenital adrenal hyperplasia: the Swedish experience and standpoint". The Journal of Clinical Endocrinology and Metabolism. 97 (6): 1881–83. doi:10.1210/jc.2012-1222. PMID 22466333.
- ^ Hirvikoski T, Nordenström A, Lindholm T, Lindblad F, Ritzén EM, Wedell A, et al. (February 2007). "Cognitive functions in children at risk for congenital adrenal hyperplasia treated prenatally with dexamethasone". The Journal of Clinical Endocrinology and Metabolism. 92 (2): 542–48. doi:10.1210/jc.2006-1340. PMID 17148562.
- ^ Lajic S, Nordenström A, Hirvikoski T (2011). "Long-term outcome of prenatal dexamethasone treatment of 21-hydroxylase deficiency". Endocrine Development. 20: 96–105. doi:10.1159/000321228. ISBN 978-3-8055-9643-5. PMID 21164263.
- ^ Simancas-Racines D, Arevalo-Rodriguez I, Osorio D, Franco JV, Xu Y, Hidalgo R (June 2018). "Interventions for treating acute high altitude illness". Cochrane Database Syst Rev. 6 (12): CD009567. doi:10.1002/14651858.CD009567.pub2. PMC 6513207. PMID 29959871.
- ^ Cymerman A, Rock PB (1994). Medical Problems in High Mountain Environments. A Handbook for Medical Officers (PDF). Vol. USARIEM-TN94-2. US Army Research Inst. of Environmental Medicine Thermal and Mountain Medicine Division Technical Report. Archived from the original (PDF) on 17 June 2017. Retrieved 17 June 2020.
- ^ Eledrisi MS (April 2007). "First-line therapy for hypertension". Annals of Internal Medicine. 146 (8): 615. doi:10.7326/0003-4819-146-8-200704170-00021. PMID 17438328.
- ^ Grape S, Usmanova I, Kirkham KR, Albrecht E (April 2018). "Intravenous dexamethasone for prophylaxis of postoperative nausea and vomiting after administration of long-acting neuraxial opioids: a systematic review and meta-analysis". Anaesthesia. 73 (4): 480–89. doi:10.1111/anae.14166. PMID 29226971.
- ^ Kovac AL (May 2006). "Meta-analysis of the use of rescue antiemetics following PONV prophylactic failure with 5-HT3 antagonist/dexamethasone versus single-agent therapies". The Annals of Pharmacotherapy. 40 (5): 873–87. doi:10.1345/aph.1G338. PMID 16670361. S2CID 32843029.
- ^ Sadeghirad B, Siemieniuk RA, Brignardello-Petersen R, Papola D, Lytvyn L, Vandvik PO, et al. (September 2017). "Corticosteroids for treatment of sore throat: systematic review and meta-analysis of randomised trials". BMJ. 358: j3887. doi:10.1136/bmj.j3887. PMC 5605780. PMID 28931508.
- ^ "Decadron, Dexamethasone Intensol (dexamethasone) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 12 December 2013. Retrieved 11 December 2013.
- ^ a b c d "Product Information Dexamethsone (dexamethasone)" (PDF). TGA eBusiness Services. Aspen Pharmacare Australia Pty Ltd. 10 August 2010. Archived from the original on 28 January 2017. Retrieved 11 December 2013. (Accept terms and conditions to open PDF, which doesn't work in archived version)
- ^ "Product Information Dexmethsone Injection" (PDF). TGA eBusiness ServicesAspen Pharmacare Australia Pty Ltd. Aspen Pharmacare Australia Pty Ltd. 2 March 2011. Archived from the original on 29 January 2017. Retrieved 11 December 2013. (Accept terms and conditions to open PDF, which doesn't work in archived version)
- ^ "Dexamethasone tablet [ECR Pharmaceuticals]". DailyMed. ECR Pharmaceuticals. December 2010. Archived from the original on 13 December 2013. Retrieved 11 December 2013.
- ^ "Dexamethasone Tablet BP 2.0 mg – Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Merck Sharp & Dohme Limited. 26 January 2018. Archived from the original on 17 June 2020. Retrieved 17 June 2020.
- ^ "Dexamethasone 4.0 mg/ml injection – Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Merck Sharp & Dohme Limited. 4 December 2013. Archived from the original on 8 May 2015. Retrieved 11 December 2013.
- ^ a b c Cole TJ (2006). "Glucocorticoid action and the development of selective glucocorticoid receptor ligands". Biotechnol Annu Rev. Biotechnology Annual Review. 12: 269–300. doi:10.1016/S1387-2656(06)12008-6. ISBN 9780444527240. PMID 17045197.
- ^ a b c d e f g Meijer OC, de Kloet ER (March 2017). "A Refill for the Brain Mineralocorticoid Receptor: The Benefit of Cortisol Add-On to Dexamethasone Therapy". Endocrinology. 158 (3): 448–454. doi:10.1210/en.2016-1495. PMID 27967238. S2CID 7311584.
- ^ De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, et al. (2000). "Endotext". Glucocorticoid Therapy and Adrenal Suppression. MDText.com. PMID 25905379. Archived from the original on 1 July 2020. Retrieved 17 June 2020.
- ^ a b Khan MO, Park KK, Lee HJ (2005). "Antedrugs: an approach to safer drugs". Current Medicinal Chemistry. 12 (19): 2227–39. doi:10.2174/0929867054864840. PMID 16178782.
- ^ Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, et al. (August 2013). "A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy". Allergy, Asthma, and Clinical Immunology. 9 (1): 30. doi:10.1186/1710-1492-9-30. PMC 3765115. PMID 23947590.
- ^ a b c De Kloet ER (October 1997). "Why Dexamethasone Poorly Penetrates in Brain". Stress. 2 (1): 13–20. doi:10.3109/10253899709014734. PMID 9787252.
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 366–. ISBN 978-1-4757-2085-3. Archived from the original on 15 February 2017.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 310–. ISBN 978-3-88763-075-1. Archived from the original on 24 August 2017.
- ^ Raynor JW, Minor W, Chruszcz M (2007). "Dexamethasone at 119 K". Acta Crystallographica Section E. 63 (6): o2791–93. Bibcode:2007AcCrE..63o2791R. doi:10.1107/S1600536807020806.
- ^ Antignac JP, Le Bizec B, Monteau F, Andre F (January 2002). "Differentiation of betamethasone and dexamethasone using liquid chromatography/positive electrospray tandem mass spectrometry and multivariate statistical analysis". Journal of Mass Spectrometry. 37 (1): 69–75. Bibcode:2002JMSp...37...69A. doi:10.1002/jms.260. PMID 11813313.
- ^ Arth GE, Fried J, Johnston DBR, Hoff DR, Sarett HL, Silber RH, et al. (1958). "16-Methylated steroids. II. 16α-Methyl analogs of cortisone, a new group of anti-inflammatory steroids. 9α-Halo derivatives". Journal of the American Chemical Society. 80 (12): 3161–63. Bibcode:1958JAChS..80.3161A. doi:10.1021/ja01545a063.
- ^ Taub D, Hoffsommer RD, Slates HL, lWendler NL (1958). "16β-Methyl cortical steroids". Journal of the American Chemical Society. 80 (16): 4435. Bibcode:1958JAChS..80.4435T. doi:10.1021/ja01549a095.
- ^ a b Speakman, N. M. A., Heard, A. W., & Nitschke, J. R. (2024). A CuI6L4 Cage Dynamically Reconfigures to Form Suit[4]anes and Selectively Bind Fluorinated Steroids. Journal of the American Chemical Society, 146(15), 10234–10239. https://doi.org/10.1021/jacs.4c00257
- ^ Shing, C. (2023). Novel cocrystals of dexamethasone. Hong Kong:
- ^ Takegami, S., Kitamura, K., Funakoshi, T., & Kitade, T. (2008). Partitioning of anti-inflammatory steroid drugs into phosphatidylcholine and phosphatidylcholine-cholesterol small unilamellar vesicles as studied by second-derivative spectrophotometry. Chemical and Pharmaceutical Bulletin, 56(5), 663–667. doi:10.1248/cpb.56.663
- ^ Mulabagal, V., Wilson, C., & Hayworth, J. S. (2017). An ultrahigh-performance chromatography/tandem mass spectrometry quantitative method for trace analysis of potential endocrine disrupting steroid hormones in estuarine sediments. Rapid Communications in Mass Spectrometry, 31(5), 419–429. doi:10.1002/rcm.7807
- ^ "Dexamethasone". 17 June 2020. Archived from the original on 1 October 2020. Retrieved 18 June 2020.
- ^ "Dexamethasone in COVID-19" (PDF). www.gpni.co.uk. 16 June 2020. Archived (PDF) from the original on 17 June 2020. Retrieved 18 June 2020.
- ^ Mahase E (June 2020). "Covid-19: Demand for dexamethasone surges as RECOVERY trial publishes preprint". BMJ. 369: m2512. doi:10.1136/bmj.m2512. PMID 32576548.
- ^ Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. (July 2020). "Dexamethasone in Hospitalized Patients with Covid-19 – Preliminary Report". New England Journal of Medicine. 384 (8): 693–704. doi:10.1056/NEJMoa2021436. PMC 7383595. PMID 32678530.
- ^ Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. (February 2021). "Dexamethasone in Hospitalized Patients with Covid-19". N Engl J Med. 384 (8): 693–704. doi:10.1056/NEJMoa2021436. PMC 7383595. PMID 32678530.
- ^ "Important to use dexamethasone only for serious COVID cases – WHO". 17 June 2020. Archived from the original on 17 June 2020. Retrieved 18 June 2020.
- ^ "WHO Director-General's opening remarks at the media briefing on COVID-19". World Health Organization. 22 June 2020. Archived from the original on 12 September 2020. Retrieved 26 June 2020.
- ^ "Q&A: Dexamethasone and COVID-19". World Health Organization (WHO). Archived from the original on 11 October 2020. Retrieved 12 July 2020.
- ^ "WHO updates clinical care guidance with corticosteroid recommendations". World Health Organization (WHO) (Press release). 2 September 2020. Archived from the original on 6 May 2021. Retrieved 27 March 2022.
- ^ "EMA starts review of dexamethasone for treating adults with COVID-19 requiring respiratory support". European Medicines Agency (EMA) (Press release). 24 July 2020. Archived from the original on 30 January 2021. Retrieved 27 July 2020. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "EMA to review results from study testing common steroid drug against COVID-19". Reuters. 24 July 2020. Archived from the original on 27 July 2020. Retrieved 27 July 2020.
- ^ "EMA receives application for marketing authorisation of Dexamethasone Taw COVID-19". European Medicines Agency (EMA). 2 September 2020. Archived from the original on 3 September 2020. Retrieved 2 September 2020.
- ^ a b c "Dexamethasone for Accelerating Lung Maturation in Preterm Babies" (PDF). Archived (PDF) from the original on 22 December 2015. Retrieved 29 July 2015.
- ^ Dummett M (30 May 2010). "Bangladesh's dark brothel steroid secret". BBC News Online. Archived from the original on 22 February 2012.
- ^ "Prohibited In-Competition: Glucocorticoids". World Anti-Doping Agency. Archived from the original on 16 July 2018. Retrieved 16 July 2018.
- ^ "Trichlormethiazide and Dexamethasone for veterinary use". Wedgewood Pharmacy. Archived from the original on 12 December 2007. Retrieved 23 January 2008.
External links
[edit]- "Dexamethasone Ophthalmic". MedlinePlus.
- "Dexamethasone Injection". MedlinePlus.
- Drugs developed by AbbVie
- Chemical substances for emergency medicine
- COVID-19 drug development
- CYP3A4 inducers
- Fluorinated corticosteroids
- Glucocorticoids
- Halohydrins
- Drugs developed by Novartis
- Organofluorides
- Otologicals
- Peripherally selective drugs
- Pregnane X receptor agonists
- Pregnanes
- World Health Organization essential medicines

![1H NMR for Dexamethasone [83]](http://upload.wikimedia.org/wikipedia/commons/thumb/c/c5/1H_NMR_for_Dexamethasone.png/400px-1H_NMR_for_Dexamethasone.png)
![13C NMR for Dexamethasone [83]](http://upload.wikimedia.org/wikipedia/commons/thumb/f/f4/13C_NMR_for_Dexamethasone.png/400px-13C_NMR_for_Dexamethasone.png)
![Infrared spectroscopy of Dexamethasone [84]](http://upload.wikimedia.org/wikipedia/commons/thumb/7/7c/Infrared_spectroscopy_of_Dexamethasone.png/400px-Infrared_spectroscopy_of_Dexamethasone.png)
![UV-vis spectroscopy of Dexamethasone [85]](http://upload.wikimedia.org/wikipedia/commons/thumb/5/53/UV-vis_spectroscopy_of_Dexamethasone.png/301px-UV-vis_spectroscopy_of_Dexamethasone.png)