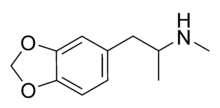
MDMA
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Sublingual salla |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic, CYP extensively involved |
| Elimination half-life | The half-life of MDMA is dose dependent, increasing with higher doses, but is around 6–10 hours at doses of 40–125 mg |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C11H15NO2 |
| Molar mass | 193.25 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
MDMA (3,4-methylenedioxy-N-methylamphetamine), most commonly known today by the street name Ecstasy (often abbreviated E, X, or XTC), is a semisynthetic member of the amphetamine class [2] of psychoactive drugs, a subclass of the phenethylamines.[3] MDMA also falls under many other broad categories of substances, including stimulants, psychedelics, and the empathogenic-entactogens.
MDMA's experiential effects are more consistent than those produced by most psychedelics, and its euphoria appears to be distinct from most stimulants. It is also considered unusual for its tendency to produce a sense of intimacy with others and diminished feelings of fear and anxiety. These effects have led some to suggest it might have therapeutic benefits to some individuals. Before it was made a controlled substance, MDMA was used to aid psychotherapy, often couples therapy, the results of which are poorly documented. Studies have also recently been initiated to examine the therapeutic potential of MDMA for post-traumatic stress disorder and anxiety associated with cancer.
MDMA is criminalized in all countries in the world under a UN agreement,[4] and its possession, manufacture, or sale may result in criminal prosecution. MDMA is one of the most widely used illicit drugs in the world[citation needed] and is taken in a variety of contexts far removed from its roots in psychotherapeutic settings. It is commonly associated with the rave culture and its related genres of music.
There are concerns within science, health care, and drug policy circles about the risks of MDMA. The most controversial of these is the possibility of neurotoxic damage of the central nervous system, the presence, reversibility, extent and significance of which are yet to be determined.[5][6] Based on these and other health concerns, some have suggested that the risks of even single doses of MDMA outweigh its potential benefits.[7][8] Others argue that these concerns are based on inconclusive evidence and that MDMA deserves further study.[9][10] Consistent with this latter point of view, regulatory authorities in several locations around the world have approved studies administering MDMA to humans to examine either its therapeutic potential or, more commonly, its basic effects.[11]
History
At the end of the 19th century, the Merck company of Germany was interested in developing substances that stopped abnormal bleeding. One of the most important compounds was hydrastinine. The plant from which it was isolated became rarer, and they started looking for alternatives. The scientific reports from the laboratory from 1911 and 1912 show that they wanted to use 3-methyl-hydrastinine as an alternative. They believed that this methylated analog of hydrastinine might be similarly effective. Drs. Walther Beck, Otto Wolfes and Anton Köllisch started on the project. In the newly developed synthetic pathway to 3-methyl-hydrastinine, MDMA was mentioned as one of several key precursors under the name of Methylsafrylamin. In 1912 Dr. Anton Köllisch was requested to develop a patentable synthesis for 3-methyl-hydrastinine. The patent started on December 24, 1912. It is a procedural patent for compounds which are key precursors for therapeutics. MDMA was not the purpose of the patent. It was Dr. Max Oberlin (also at Merck) who in 1927 was the first person interested in the pharmacological properties of MDMA. Research on the substance was stopped for economic reasons, and the substance was buried in oblivion for some decades. In the 1950s the American and German armies were interested in psychotropic agents; MDMA was among the tested substances. Most probably for this reason, MDMA was re-synthesized at Merck. In his laboratory journal of 1952 Dr. Albert van Schoor describes how MDMA kills 6 flies in 30 minutes. In 1959 Dr. Fruhstorfer works on MDMA and similar psychotropics, his substance H671 was identified to be MDMA. The research on these substances led to the marketing of Reaktivin in 1960. Its chemical structure is not related to MDMA. The first scientific paper on MDMA appeared in 1960 and described a synthesis for MDMA. It is written in Polish by Biniecki and Krajewski and almost unknown. In 1978 Alexander Shulgin and David Nichols published the first scientific article on the drug’s psychotropic effect in humans.[12]
The U.S. Army did, however, carry out lethal dose studies of MDMA and several other compounds on animals in the mid-1950s. It was given the name EA-1475, with the EA standing for either (accounts vary) "Experimental Agent" or "Edgewood Arsenal."[13] The results of these studies were not declassified until 1969.
MDMA first appeared sporadically as a street drug in the early 1970s after its counterculture analogue, MDA, became criminalized in the United States in 1970.[14] MDMA use, however, remained very limited until the end of the decade. MDMA began to be used therapeutically in the late-1970s after noted chemist Alexander Shulgin tried it himself, in 1977,[15] and subsequently introduced it to psychotherapist Leo Zeff. As Zeff and others spread word about MDMA, it developed a reputation for enhancing communication during clinical sessions, reducing patients' psychological defenses, and increasing capacity for therapeutic introspection. However, no formal measures of these putative effects were made and blinded or placebo-controlled trials were not conducted. A small number of therapists, including George Greer, Joseph Downing, and Philip Wolfson, used it in their practices until it was made illegal.
Although some therapists continued to conduct therapy illegally, MDMA was not legally given to humans until Charles Grob initiated an ascending-dose safety study in healthy volunteers. Subsequent legally-approved MDMA studies in humans have taken place in Detroit (Wayne State University), Chicago (U of Chicago), San Francisco (UCSF and California Pacific Medical Center), Baltimore (NIDA-NIH Intramural Program), and South Carolina, as well as in Switzerland (University Hospital of Psychiatry, Zurich), the Netherlands (Maastricht University), and Spain (Universitat Autònoma de Barcelona).[16]
Due to the wording of the United Kingdom's existing Misuse of Drugs Act of 1971, MDMA was automatically classified as a Class A drug in 1977.
In the early 1980s in the United States, MDMA rose to prominence as "Adam" in trendy nightclubs in the Dallas area, then in gay dance clubs.[17] From there use spread to rave clubs in major cities around the country, and then to mainstream society. The drug was first proposed for scheduling by the DEA in July 1984,[18] and was classified as a Schedule I controlled substance in the United States from May 31, 1985.[19]
In the late 1980s and early 1990s, MDMA as "ecstasy" was widely used in the United Kingdom and other parts of Europe, becoming an integral element of rave culture and other psychedelic/dancefloor-influenced music scenes, such as Madchester and Acid House. Spreading along with rave culture, illicit MDMA use became increasingly widespread among young adults in universities and later in high schools. MDMA became one of the four most widely used illicit drugs in the United States, along with cocaine, heroin and cannabis.[citation needed] Today in the US, according to some estimates, only cannabis will attract more first-time users.[20]
Therapeutic Uses
There have long been suggestions that MDMA might be useful in psychotherapy, facilitating self-examination with reduced fear.[21][22][23] Indeed, a small number of therapists, including Leo Zeff, George Greer, Joseph Downing, and Philip Wolfson, used MDMA in their practices until it was made illegal. George Greer synthesized MDMA in the lab of Alexander Shulgin and administered it to about 80 of his clients over the course of the remaining years preceding MDMA's Schedule I placement in 1985. In a published summary of the effects,[24] the authors reported patients felt improved in various, mild psychiatric disorders and other personal benefits, especially improved intimate communication with their significant others. In a subsequent publication on the treatment method, the authors reported that one patient with severe pain from terminal cancer experienced lasting pain relief and improved quality of life.[25] However, few of the results in this early MDMA psychotherapy were measured using methods considered reliable or convincing in scientific practice. For example, the questionnaires used might not have been sensitive to negative changes and it is not known to what extent similar patients might improve from chance or from psychotherapy.
The therapeutic potential of MDMA is currently being tested in several ongoing studies, some sponsored by the Multidisciplinary Association for Psychedelic Studies. Studies in the US and other countries are evaluating the efficacy of MDMA-assisted psychotherapy for treating those diagnosed with posttraumatic stress disorder or anxiety related to cancer. In a newspaper interview, the researchers from the South Carolina PTSD study report tendencies for some participants to have reduced disease severity after MDMA psychotherapy.[26] However, these reports focus on individual participants. Statistical results from the entire study will need to be published and, ultimately, results will need to be confirmed in studies by other scientists to demonstrate the efficacy of MDMA as a psychotherapeutic agent.
Psychotherapeutic use of MDMA continues to have its critics. A.C. Parrott reminds that MDMA is powerful and affects neurotransmitter pathways and can intensify psychobiological functions. He argues that although there has been shown to be positive effects when using this drug, the negative effects cannot be ignored. Parrott cautions researchers stating that environmental factors are difficult to control and can effect a patient’s experience on the drug. Parrott also highlights that once the effects of MDMA begin to wear off, “there is a period of neurotransmitter recovery when low moods predominate, and these may exacerbate psychiatric distress. In addition, it remains unclear what drug exposure produces neurotoxicity in humans and proposed doses in clinical trials are close to ones that produce long-term serotonergic changes in animals.[27][28] In addition to these health and safety concerns, historian Erika Dyck, studying LSD, has noted that the nonmedical use of a drug may sometimes contribute to its failure to be considered as a potential medicine in psychotherapy.[29] From either side, it is clear that MDMA should be further researched before any decision is made regarding its use in psychotherapy.[30]
Mechanism of action
The mechanism of MDMA's unusual effects has yet to be fully understood, although it is generally thought that the primary relevant pharmacological characteristic of the drug is its affinity for SERTs. SERTs are the part of the serotonergic neuron which remove serotonin from the synapse to be recycled or stored for later use. Not only does MDMA inhibit the reuptake of serotonin into this pump, but it reverses the action of the transporter so that it begins pumping serotonin into the synapse from inside the cell.[8] In addition, MDMA induces the release of norepinephrine and dopamine.[31]
MDMA's unusual empathic/entactogenic effects have been hypothesized to be at least partly the result of the release of oxytocin,[32] a hormone usually released following such events as orgasm and childbirth, which is thought to facilitate bonding and the establishment of trust. MDMA is thought to cause this release by indirectly stimulating 5-HT1A receptors. However, the evidence that oxytocin is involved in the effects of MDMA is derived from studies conducted on rats where the emotional effects can only be indirectly measured, in this case by the time animals spend in close proximity to one another. Controlled human studies have not yet been carried out, and it is not known conclusively if MDMA has oxytocinergic action in humans. The question of why other serotonergic drugs do not produce a similarly profound emotional state like MDMA also remains unanswered.
Effects
Acute effects
The primary effects attributable to MDMA consumption are predictable and fairly consistent amongst users.[33][34][35] The most common effects include:
- Euphoria
- Decreased hostility and insecurity
- Increased feelings of intimacy with others
- Feelings of empathy towards others
- Ability to discuss anxiety-provoking topics with markedly-increased ease
- A strong sense of inner peace and self-acceptance
- Feelings of insightfulness and mental clarity
- Intensification of sensory experience, particularly auditory and tactile
- Decreased appetite
- Urinary retention (also see hyponatremia)
- Mydriasis (abnormal pupil dilation)
- Increased physical energy
- Increased heart rate and blood pressure
- Increased mean body temperature
Other effects may include:
- Short-term memory lapses
- Trisma (lockjaw)
- Bruxia (involuntary teeth grinding)
- Nystagmus (rapid, uncontrollable eye movements)
- Several hours of restlessness following primary subjective effects, sometimes accompanied by residual euphoria
- A period of general malaise following primary subjective effects, normally resolving within a few days
- Mildly-blurred vision following primary subjective effects, gradually resolving over a period of up to several days, also known as "plurring"
Serious complications increasing in likelihood with dose, environmental severity, degree of physical activity, and/or certain drug interactions include:
- Hyperthermia (due to an intemperate environment and/or lack of hydration and/or rest from physical activity, usually dancing)
- Dehydration (due to an intemperate environment and/or lack of hydration and/or rest from physical activity, usually dancing)
- Hyponatremia (due to drug induced antidiuretic hormone release and/or excess compensatory intake of fluids, a rare complication)
- Serotonin syndrome (believed to be due to excess release of serotonin, sometimes triggered by coadministration of other serotonergic drugs)
Effects of chronic use
The long-term health effects of ecstasy use are generally not well-known, and the research that has been devoted to addressing the relevant issues thus far has been largely inconclusive. The primary concern is generally that there may be negative long-term consequences that result from the drug's alleged neurotoxic effects on serotonergic neurons.[36] Some further studies have also shown that this damage causes increased rates of depression and anxiety, even after quitting the drug.[37][38] In addition to this, some studies have indicated that MDMA may cause long-term memory and cognition impairment.[39] Many factors, including total lifetime MDMA consumption, the duration of abstinence between uses, the environment of use, poly-drug use/abuse, quality of mental health, various lifestyle choices, and predispositions to develop clinical depression and other disorders may contribute to various possible health consequences. MDMA use has been occasionally associated with liver damage,[40] excessive wear of teeth,[41] and (very rarely) Hallucinogen persisting perception disorder.[42]
Recreational use
MDMA use has increased markedly since the late 1980s, and spread beyond its original subcultures to mainstream use, with prices generally falling, although there is still wide geographical variance, both regionally and between countries. According to the United States Drug Enforcement Administration’s drug information and the results of Monitoring the Future for 2004 and 2005 around 3% of 8th graders, around 4% of 10th graders, and around 6% of 12th graders reported MDMA use.[43] In 2007, MDMA was the only drug reported to have an increase in use among 8th, 10th, and 12th graders. Simultaneously, perceived risk and disapproval ratings have declined among these students.[44]
In 1995 it was reported that the street price per pill in the United Kingdom was "about £15 each,"[45] although two years later this had shifted to a range of £8 to £15 each.[46] A 2001 Home Office study reported that the cost per pill to end-point consumer, "could be as little as £7.50, or as much as £10 to £15 when purchased in clubs."[47] In 2007, the Greater London Authority highlighted regional variations, reporting on the average street price per pill in five selected cities. These prices were much lower than in previous years, ranging from as high as £5 in Bristol to as low as £1.50 in Torquay.[48] Powdered MDMA cost roughly £30-£40 for a gram.
Chemistry

Safrole, a colorless or slightly yellow oil, extracted from the root-bark or the fruit of sassafras plants is the primary precursor for all manufacture of MDMA. There are numerous synthetic methods available in the literature to convert safrole into MDMA via different intermediates. One common route is via the MDP2P (3,4-methylenedioxyphenyl-2-propanone, also known as piperonyl acetone) intermediate. This intermediate can be produced in at least two different ways. One method is to isomerize safrole in the presence of a strong base to isosafrole and then oxidize isosafrole to MDP2P. Another, reportedly better method, is to make use of the Wacker process to oxidize safrole directly to the MDP2P (3,4-methylenedioxy phenyl-2-propanone) intermediate. This can be done with a palladium catalyst. Once the MDP2P intermediate has been produced it is then consumed via a reductive amination to form MDMA as the product.
According to DEA Microgram newsletters very little safrole is actually required to make MDMA.[49] "Ocotea cymbarum is an essential oil... that typically contains between 80 and 94 percent safrole," "a 500-milliliter bottle of Ocotea cymbarum sells for $20 to more than $100," "An MDMA producer with access to the proper chemicals can use a 500-milliliter quantity of Ocotea cymbarum to produce an estimated 1,300 to 2,800 tablets containing 120 milligrams of MDMA."
Legal issues
MDMA is illegal in most of the world under the UN Convention on Psychotropic Substances and other international agreements, although some limited exceptions exist for research. Generally, the use, sale or manufacture of MDMA are all criminal offenses.
A study done on MDMA related crime in 2005 [50] has shown that out of arrested males 16-21, MDMA users are more likely to have completed high-school (70% to 60% that have not). Young men who didn't use 'ecstasy' in the past year were 36% more likely to have been arrested for a crime than those who had used the drug. The only positive correlation (increased likelihood of arrest) between MDMA use and crime was in the category of drug related offenses. Negative correlations include violent types of crimes; among those who had been arrested, those without a history of 'ecstasy' use were 42% more likely to have committed assault, 58% more likely to have committed robbery, and 67% more likely to have committed burglary. Despite these findings, MDMA users were likely to spend 60% more time in prison than non-users.
In the United States, MDMA was legal and unregulated until May 31, 1985, at which time it was added to DEA Schedule I, for drugs deemed to have no medical uses and a high potential for abuse. During DEA hearings to criminalize MDMA, most experts recommended DEA Schedule III prescription status for the drug, due to its beneficial usage in psychotherapy. The judge overseeing the hearings, Francis Young, also made this recommendation. Nonetheless, the DEA classified it as Schedule I.[51] In 2001, responding to a mandate from Congress, the U.S. Sentencing Commission, resulted in an increase in the penalties for MDMA by nearly 3,000% [52] despite scientific protest calling for a decrease in the penalties for MDMA possession and distribution. [53] This increase makes 4 ecstasy pills (1g) equal to 33 doses of heroin (1g) or 2.2 pounds (1kg) of marijuana for sentencing purposes at the federal level. [54]
That same year, 1985, the World Health Organization's Expert Committee on Drug Dependence recommended that MDMA be placed in Schedule I of the Convention on Psychotropic Substances. Unlike the Controlled Substances Act, the Convention has a provision in Article 7(a) that allows use of Schedule I drugs for "scientific and very limited medical purposes." The committee's report stated:[55]
- The Expert Committee held extensive discussions concerning therapeutic usefulness of 3,4 Methylenedioxymethamphetamine. While the Expert Committee found the reports intriguing, it felt that the studies lacked the appropriate methodological design necessary to ascertain the reliability of the observations. There was, however, sufficient interest expressed to recommend that investigations be encouraged to follow up these preliminary findings. To that end, the Expert Committee urged countries to use the provisions of article 7 of the Convention on Psychotropic Substances to facilitate research on this interesting substance.
In the United Kingdom, MDMA is a Class A drug under the Misuse of Drugs Act 1971, making it illegal to sell, buy, or possess without a license. Penalties include a maximum of seven years and/or unlimited fine for possession; life and/or unlimited fine for production or trafficking.
Health concerns
While the short-term adverse effects and contraindications of MDMA are fairly well known, there is significant debate within the scientific and medical communities possible regarding long-term physical and psychological effects of MDMA. Short-term physical health risks of MDMA consumption include hyperthermia,[56][57] and hyponatremia.[58] . Continuous activity without sufficient rest or rehydration may cause body temperature to rise to dangerous levels, and loss of fluid via excessive perspiration puts the body at further risk as the stimulatory and euphoric qualities of the drug may render the user oblivious to their energy expenditure for quite some time. Diuretics such as alcohol and caffeine may exacerbate these risks further.
MDMA causes a reduction in the concentration of serotonin transporters (SERTs) in the brain. The rate at which the brain recovers from serotonergic changes is unclear. A number of studies,[59] have demonstrated lasting serotonergic changes occurring due to MDMA exposure. Other studies[60][61] have suggested that that the brain may recover from serotonergic damage; however, damage caused by heavy, prolonged use of MDMA may be long lasting.
Some studies show that MDMA may be neurotoxic in humans.[62][63] Other studies, however, suggest that this brain damage may be at least partially reversible following prolonged abstinence from MDMA.[64][65] However, other studies suggest that SERT-depletion arises from long-term MDMA use due to receptor down-regulation, rather than true neurotoxicity. [66] When any neurotransmitter is present in excess for prolonged periods of time, the brain responds in an attempt to reestablish its own natural neuro-electrical balance. Weekly use of MDMA over a prolonged period may actually cause serotonin receptors to retreat into the dendrite of serotonin nerve cells.[67] Depression and deficits in memory have been shown to occur more frequently in long-term MDMA users.[68][69] However, some recent studies have suggested that MDMA use may not be associated with chronic depression.[70][71]
One now infamous study on MDMA toxicity, which claimed that a single recreational dose of MDMA could cause Parkinson's Disease in later life due to severe dopaminergic stress, was actually retracted by Ricaurte himself after he discovered his lab had administered not MDMA but methamphetamine, which is known to cause dopaminergic changes similar to the serotonergic changes caused by MDMA.[72] Ricaurte blamed this mistake on the chemical supply company that sold the material to his lab. Most studies have found that levels of the dopamine transporter (or other markers of dopamine function) in MDMA users are normal.
Another concern associated with MDMA use is toxicity from chemicals other than MDMA in ecstasy tablets. Due to its near-universal illegality, the purity of a substance sold as Ecstasy is unknown to the typical user. The MDMA content of tablets varies widely between regions and different brands of pills and fluctuates somewhat each year. Pills may contain other active substances meant to stimulate in a way similar to MDMA, such as amphetamine, methamphetamine, ephedrine, or caffeine, all of which may be comparatively cheap to produce and can help to boost profit overall. In some cases, tablets sold as Ecstasy do not even contain any MDMA. Instead they may contain an assortment of presumably undesirable drugs such as paracetamol, ibuprofen, etc.[73]
There have been a number of deaths attributed to PMA, a potent and highly neurotoxic hallucinogenic amphetamine, being sold as Ecstasy. PMA is unique in its ability to quickly elevate body temperature and heart rate at relatively low doses, especially in comparison to MDMA.[74] Hence, a user who believes he is consuming two 120mg pills of MDMA could actually be consuming a dose of PMA that is potentially lethal, depending on the purity of the pill. Not only does PMA cause the release of serotonin, but also acts as an MAO-A inhibitor. When combined with an ecstasy-like substance, serotonin syndrome can result.
Drug interactions
Individuals who have stopped taking any type of SSRI after prolonged medication may not be able to experience the desired effects of MDMA for as long as several months following discontinuation of the medication. This is due to the fact that SSRIs decrease the brain's sensitivity to the presence of serotonin as the brain seeks to reestablish a normal neuro-electrical balance.
Most people who die while under the influence of MDMA have also consumed significant quantities of at least one other drug. The risk of MDMA-induced death overall is minimal.[75]
The use of MDMA can be dangerous when combined with other drugs (particularly monoamine oxidase inhibitors (MAOIs) and antiretroviral drugs, in particular ritonavir). Combining MDMA with MAOIs can precipitate hypertensive crisis, as well as serotonin syndrome which can be fatal.[76] MAO-B inhibitors such as deprenyl do not seem to carry these risks when taken at selective doses, and have been used to completely block neurotoxicity in rats.[77]
Pharmacokinetics
MDMA reaches maximal concentrations in the blood between 1.5 and 3 hours after ingestion. It is then slowly metabolized and excreted, with levels decreasing to half their peak concentration over approximately 8 hrs. Thus, there are still high MDMA levels in the body when the experiential effects have mostly ended, indicating that the brain has developed short-term tolerance to the presence of MDMA. Taking additional MDMA at this point therefore produces higher concentrations of MDMA in the blood and brain than might be expected based on the perceived effects.
Metabolites of MDMA that have been identified in humans include 3,4-methylenedioxyamphetamine (MDA), 4-hydroxy-3-methoxy-methamphetamine (HMMA), 4-hydroxy-3-methoxyamphetamine (HMA), 3,4-dihydroxyamphetamine (DHA, also called alpha-methyldopamine), 3,4-methylenedioxyphenylacetone (MDP2P), and N-hydroxy-3,4-methylenedioxyamphetamine (MDOH). The contributions of these metabolites to the psychoactive and toxic effects of MDMA are an area of active research. 65% of MDMA is excreted unchanged in the urine (additionally 7% is metabolised into MDA) during 24 hours after usage.[78]
MDMA is known to be metabolized by two main metabolic pathways: (1) O-demethylenation followed by catechol-O-methyltransferase (COMT)-catalyzed methylation and/or glucuronide/sulfate conjugation; and (2) N-dealkylation, deamination, and oxidation to the corresponding benzoic acid derivatives conjugated with glycine. The metabolism may be primarily by cytochrome P450 enzymes (CYP2D6 (in humans, but CYP2D1 in mice), and CYP3A4) and COMT. Complex, nonlinear pharmacokinetics arise via autoinhibition of CYP2D6 and CYP2D8, resulting in zeroth order kinetics at higher doses. It is thought that this can result in sustained and higher drug concentrations if the user takes consecutive doses of the drug.
Because the enzyme CYP2D6 is deficient or totally absent in some people[79], it was once hypothesized that these people might have elevated risk when taking MDMA. However, there is still no evidence for this theory and available evidence argues against it.[80] It is now thought that the contribution of CYP2D6 to MDMA metabolism in humans is less than 30% of the metabolism. Indeed, an individual lacking CYP2D6 was given MDMA in a controlled clinical setting and a larger study gave MDMA to healthy volunteers after inhibiting CYP2D6 with paroxetine. Lack of the enzyme caused a modest increase in drug exposure and decreases in some metabolites, but physical effects did not appear appreciably elevated. While there is little or no evidence that low CYP2D6 activity increases risks from MDMA, it is likely that MDMA-induced CYP2D inhibition will increase risk of those prescription drugs that are metabolized by this enzyme. MDMA-induced CYP2D inhibition appears to last for up to a week after MDMA exposure.
There are a number of reported potentially dangerous possible interactions between MDMA and other drugs. Several cases have been reported of death in individuals who ingested MDMA while taking ritonavir, which inhibits multple CYP450 enzymes. Toxicity or death has also been reported in people who took MDMA in combination with monoamine oxidase inhibitors, such as phenelzine or moclobemide.
MDMA and metabolites are primarily excreted as conjugates, such as sulfates and glucuronides.[81]
MDMA is a chiral compound and has been almost exclusively administered as a racemate. However, an early uncontrolled report suggests that the S-enantiomer is significantly more potent in humans than the R-enantiomer (Anderson et al. 1978). Studies in humans[82][83] indicate that the disposition of MDMA is stereoselective, with the S-enantiomer having a shorter elimination half-life and greater excretion than the R-enantiomer. For example, Fallon et al.[82] reported that the area under the plasma concentration versus time curve (AUC) was two to four times higher for the R-enantiomer than the S-enantiomer after a 40 mg oral dose in human volunteers. Similarly, the plasma half-life of (R)-MDMA was significantly longer than that of the S-enantiomer (5.8 ± 2.2 hours vs 3.6 ± 0.9 hours). However, because MDMA has dose dependent kinetics, it is likely that these half lives would be higher at more typical doses (100 mg is sometimes considered a typical dose). Given as the racemate, MDMA has a half-life of around 8 hours.
Poly substance use
MDMA is occasionally known for being taken in conjunction with psychedelic drugs, such as LSD or Psilocybin mushrooms. As this practice has become more prevalent, most of the more common combinations have been given nicknames, such as "candy flipping", for MDMA combined with LSD,[84] and "hippie flipping" when combined with Psilocybin Mushrooms. Such combinations have the ability to produce an extremely powerful experience and may carry an increased risk of neurotoxicity, complications and/or injury when compared to any individual substance. Many users use mentholated products while taking MDMA, believing it heightens the drug's effects. Examples include menthol cigarettes, Vicks[85] and lozenges. This sometimes has deleterious results on the upper respiratory tract.[86]
See also
- Effects of MDMA on the human body
- Retracted article on toxicity of MDMA on dopamine cells
- Psychedelic therapy
- Multidisciplinary Association for Psychedelic Studies
- Alexander Shulgin
- MDA
- MDEA
- MDMC (Methylone)
- PMA
- Amphetamine
- Leah Betts & Anna Wood (Deaths attributed to hyponatermia)
- RAVE Act
- Ecstasy Rising, (2004 ABC television documentary hosted by Peter Jennings)
References
- ^ Stimulants, narcotics, hallucinogens - Drugs, Pregnancy, and Lactation, Gerald G. Briggs, OB/GYN News, June 1, 2003.
- ^ http://www.merck.com/mmpe/sec15/ch198/ch198k.html?qt=amphetamine&alt=sh
- ^ http://www.nlm.nih.gov/cgi/mesh/2008/MB_cgi?mode=&term=Phenethylamines&field=entry
- ^ "Where is Ecstacy Legal?"
- ^ The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, "ecstasy") [1]
- ^ The neuropsychology of ecstasy (MDMA) use: a quantitative review.
- ^ McCann & Ricaurte (2001) 'Caveat Emptor: Editors Beware' Neuropsychopharmacology 24,3:333-4 http://www.maps.org/w3pb/new/2001/2001_mccann_1129_1.pdf
- ^ Gijsman et al. (1999) 'MDMA study' Neuropsychopharmacology 21,4:597 http://www.maps.org/w3pb/new/1999/1999_gijsman_295_1.pdf
- ^ Kish (2002) 'How strong is the evidence that brain serotonin neurons are damaged in human users of ecstasy?' Pharmacol Biochem Behav 71,4:845–855 http://www.maps.org/w3pb/new/2003/2003_kish_6239_1.pdf
- ^ Aghajanian & Liebermann (2001) 'Caveat Emptor: Reseearchers Beware' Neuropsychopharmacology 24,3:335-6 http://www.maps.org/w3pb/new/2001/2001_aghajanian_1130_1.pdf
- ^ MAPS: Psychedelic Research Worldwide
- ^ Bernschneider-Reif S, Oxler F, Freudenmann RW. The origin of MDMA ("ecstasy")--separating the facts from the myth. Pharmazie. 2006;61:966-72. http://www.erowid.org/references/refs_view.php?A=ShowDoc1&ID=6707
- ^ Saunders, Nicholas. Ecstasy Reconsidered (1997), page 7.
- ^ http://www.erowid.org/chemicals/mdma/mdma_info6.shtml
- ^ Tom Shroder, "The Peace Drug", Washington Post Magazine, November 25, 2007
- ^ Bibliography of psychadelic research studies collected by the Multidisciplinary Association for Psychedelic Studies
- ^ The Austin Chronicle - "Countdown to Ecstasy" by Marc Savlov
- ^ "Pharmaceutical company unravels drug's chequered past" (HTML). 2005. Retrieved 18 August.
{{cite web}}: Check date values in:|accessdate=(help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) - ^ Erowid MDMA Vault : Info #3 on scheduling
- ^ Primetime with Peter Jennings
- ^ Greer G. Tolbert R. "The Therapeutic Use of MDMA in Ecstasy: The clinical, pharmacological and neurotoxicological effects of the drug MDMA" 1990 (ed Peroutka, SJ) Boston, p. 21-36
- ^ Doblin, R. ( 2002) A clinical plan for MDMA (ecstasy) in the treatment of post-traumatic stress disorder (PTSD): Partnering with the FDA. J. Psychoactive Drugs, 35, 185–194.
- ^ Sessa, B., & Nutt, D. (2007) MDMA, politics and medical research: Have we thrown the baby out with the bathwater? Journal of Psychopharmacology, 21, 787-791.
- ^ Greer, G.; Tolbert, R. (1986). "Subjective Reports of the Effects of MDMA in a Clinical Setting" (reprint). Journal of Psychoactive Drugs. 18 (4): 319–327.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Greer, G.; Tolbert, R. (1998). "A Method of Conducting Therapeutic Sessions with MDMA" (reprint). Journal of Psychoactive Drugs. 30 (4): 371–379.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Shroder, T. (November 25, 2007). "The Peace Drug". Washington Post. p. W12.
{{cite news}}: Check date values in:|date=(help) - ^ http://scienceblogs.com/drugmonkey/2008/06/clinical_mdma_brief_20_june_20.php
- ^ http://scienceblogs.com/drugmonkey/2008/01/clinical_use_of_mdma_part_2.php
- ^ Dyck, E. (2005) Flashback: Psychiatric Experimentation With LSD in Historical Perspective. The Canadian Journal of Psychiatry, 50, 381-387.
- ^ Parrott, A. C. (2006, June) The psychotherapeutic potential of MDMA (3,4-methylenedioxymethamphetamine): an evidence-based review. Psychopharmacology, 191, 181-193.
- ^ "This is your brain". TheDEA.
- ^ "Ecstasy really does unleash the love hormone". New Scientist. April 4, 2007.
{{cite web}}: Check date values in:|date=(help) - ^ http://www.erowid.org/chemicals/mdma/mdma_effects.shtml
- ^ http://www.dancesafe.org/documents/druginfo/ecstasy.php
- ^ http://www.thedea.org/technicalFAQ.html
- ^ Parrott AC, Lasky J (1998). "Ecstasy (MDMA) effects upon mood and cognition: before, during and after a Saturday night dance". Psychopharmacology (Berl.). 139 (3): 261–8. PMID 9784083.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Verheyden SL, Henry JA, Curran HV (2003). "Acute, sub-acute and long-term subjective consequences of 'ecstasy' (MDMA) consumption in 430 regular users". Hum Psychopharmacol. 18 (7): 507–17. doi:10.1002/hup.529. PMID 14533132.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^
Verheyden SL, Maidment R, Curran HV (2003). "Quitting ecstasy: an investigation of why people stop taking the drug and their subsequent mental health". J. Psychopharmacol. (Oxford). 17 (4): 371–8. PMID 14870948.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Rodgers J, Buchanan T, Scholey AB, Heffernan TM, Ling J, Parrott AC (2003). "Patterns of drug use and the influence of gender on self-reports of memory ability in ecstasy users: a web-based study". J. Psychopharmacol. (Oxford). 17 (4): 389–96. PMID 14870950.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Jones and Simpson (1999) "Mechanisms and management of hepatotoxicity in ecstasy (MDMA) and amphetamine intoxications" Aliment Pharmacol Ther 13,2:129-33
- ^ Milosevic et al. (1999) "The occurrence of toothwear in users of Ecstasy (3,4-methylenedioxymethamphetamine) Community Dent Oral Epidemiol 27,4:283-7
- ^ [http://www.maps.org/w3pb/new/1991/1991_creighton_526_1.pdf Creighton et al. 1991 ‘Ecstasy’ psychosis and flashbacks. Br. J. Psychiatry 159, 713�/715.]
- ^ DEA, Drug Information, MDMA
- ^ Johnston, L. D., O'Malley, P. M., Bachman, J. G., & Schulenberg, J. E. (2007, December 11). National press release, "Overall, illicit drug use by American teens continues gradual decline in 2007." University of Michigan News Service, Ann Arbor, 57 pp.
- ^ Saunders, Nicholas. Ecstatsy and the Dance Culture (1995), page 168
- ^ Saunders, Nicholas. Ecstasy Reconsidered (1997), page 237
- ^ Home Office Research Study 227 Middle market drug distribution, page 22
- ^ London: The highs and the lows 2—A report from the Greater London Alcohol and Drug Alliance. City Hall, London: Greater London Authority. 2007. ISBN 1-85261-974-0.
{{cite book}}: Unknown parameter|month=ignored (help) - ^ Nov 05 DEA Microgram newsletter
- ^ [2]
- ^ MAPS. "Documents from the DEA Scheduling Hearing of MDMA,".
- ^ [3]
- ^ [4]
- ^ [5]
- ^ E for Ecstasy by Nicholas Saunders, Appendix 1: Reference Section
- ^ Nimmo S M, Kennedy B W, Tullett W M, Blyth A S, Dougall J R (1993) Drug-induced hyperthermia. Anaesthesia 48: 892–895
- ^ Malberg J E, Seiden L S (1998) Small changes in ambient temperature causes large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J. Neuroscience 18: 5086–5094
- ^ Wolff K, Tsapakis E M, Winstock A R, Hartley D, Holt D, Forsling M L, Aitchison K J(2006) Vasopressin and oxytocin secretion in response to the consumption of ecstasy in a clubbing population. Journal of Psychopharmacology 20(3): 400–410
- ^ Fischer, C. and Hatzidimitriou, G. and Wlos, J. and Katz, J. and Ricaurte, G. (1995). "Reorganization of ascending 5-HT axon projections in animals previously exposed to the recreational drug (+/-) 3, 4-methylenedioxymethamphetamine (MDMA," ecstasy")". Journal of Neuroscience. Soc Neuroscience.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Scheffel U, Szabo Z, Mathews WB, Finley PA, Dannals RF, Ravert HT, Szabo K, Yuan J, Ricaurte GA. “In vivo detection of short- and long-term MDMA neurotoxicity--a positron emission tomography study in the living baboon brain”. Synapse. 1998;29(2):183-92. Abstract
- ^ Reneman L, Lavalaye J, Schmand B, de Wolff FA, van den Brink W, den Heeten GJ, Booij J. “Cortical Serotonin Transporter Density and Verbal Memory in Individuals Who Stopped Using 3,4-Methylenedioxymethamphetamine (MDMA or 'Ecstasy'): Preliminary Findings”. Archives of General Psychiatry. 2001;58(10):901-906. Abstract
- ^ Neurotoxicity at Dancesafe.org
- ^ Does MDMA Cause Brain Damage?, Matthew Baggott, and John Mendelson
- ^ Research on Ecstasy Is Clouded by Errors, Donald G. McNeil Jr., New York Times, December 2, 2003.
- ^ Cortical Serotonin Transporter Density and Verbal Memory in Individuals Who Stopped Using 3,4-Methylenedioxymethamphetamine (MDMA or 'Ecstasy'): Preliminary Findings, Reneman L, Lavalaye J, Schmand B, de Wolff FA, van den Brink W, den Heeten GJ, Booij J. Archives of General Psychiatry 2001;58(10):901-906.
- ^ "p-Chlorophenylalanine Changes Serotonin Transporter mRNA Levels and Expression of the Gene Product", Journal of Neurochemistry, 1996. Online copy (pdf)
- ^ Down-regulation of Receptors: The most probable cause of ecstasy-related depression at DanceSafe.org
- ^ Depression at DanceSafe.org
- ^ Wareing, M. and Fisk, J.E. and Murphy, P.N. (2000). "Working memory deficits in current and previous users of MDMA ('ecstasy')" (PDF). Br J Psychol. pp. 181--8.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Study claims recreational ecstasy use and depression unrelated, Wikinews, April 26, 2006]
- ^ Recreational Ecstacy Use and Depression, Journal of Psychopharmacology, Vol. 20, No. 3, 411-416 (2006) DOI: 10.1177/0269881106063265
- ^ Ecstasy Study Botched, Retracted, Kristen Philipkoski, Wired.com, 09.05.03
- ^ Summary Statistics for EcstasyData.org Lab Testing Results
- ^ Club Drug Update, Jeff Morelock, PRP Online, Spring 2003
- ^ Primetime video
- ^ Death following ingestion of MDMA (ecstasy) and moclobemide, Vuori et al. Addiction 2003 Mar;98(3):365-368
- ^ The Prozac Misunderstanding, at TheDEA.org
- ^ Verebey K, Alrazi J, Jaffe JH (1988). "The complications of 'ecstasy' (MDMA)". JAMA. 259 (11): 1649–50. doi:10.1001/jama.259.11.1649. PMID 2893845 : 2893845.
{{cite journal}}: Check|pmid=value (help)CS1 maint: multiple names: authors list (link) - ^ eMedicine - Toxicity, MDMA : Article by David Yew
- ^ [6]
- ^ [7]
- ^ a b J. K. Fallon, A. T. Kicman, J. A. Henry, P. J. Milligan, D. A. Cowan, A. J. Hutt (1999). "Stereospecific Analysis and Enantiomeric Disposition of 3,4-Methylenedioxymethamphetamine (Ecstasy) in Humans". Clinical Chemistry. 45 (7): 1058–1069.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ D. Hensley, J. T. Cody (1999). "Simultaneous Determination of Amphetamine, Methamphetamine, Methylenedioxyamphetamine (MDA), Methylenedioxymethamphetamine (MDMA), and Methylenedioxyethylamphetamine (MDEA) Enantiomers by GC–MS" (PDF). Journal of Analytical Toxicology. 23 (6): 518–523.
- ^ UMD Center for Substance Abuse Research. "Ecstasy:CESAR".
- ^ "Director's Report to the National Advisory Council on Drug Abuse".
- ^ Erowid Experience Vaults: Vicks used with Ecstasy - Important Warning About Vicks on Ecstasy - 4287
Further reading
- Baggott, Matthew, and John Mendelson. “MDMA Neurotoxicity”. Ecstasy: The Complete Guide. Ed. Julie Holland. Spring 2001 from www.erowid.com.
- de la Torre, Rafael et al. (2000), Non-linear pharmacokinetics of MDMA (`ecstasy') in humans. Br J Clin Pharmacol, 2000; 49(2):104-9
- de la Torre, Rafael & Farré, Magí (2004). Neurotoxicity of MDMA (ecstasy): the limitations of scaling from animals to humans. Trends in Pharmacological Sciences 25, 505-508.
- Eisner, Bruce. Ecstasy: The MDMA Story, 2nd ed. Berkeley, CA: Ronin Publishing, 1994.
- Erowid, Earth. “Do Antioxidants Protect Against MDMA Hangover, Tolerance, and Neurotoxicity?” Erowid Extracts. December 2001; 2:6-11.
- Jones, Douglas C. et al. (2004). Thioether Metabolites of 3,4-Methylenedioxyamphetamine and 3,4-Methylenedioxymethamphetamine Inhibit Human Serotonin Transporter (hSERT) Function and Simultaneously Stimulate Dopamine Uptake into hSERT-Expressing SK-N-MC Cells. J Pharmacol Exp Ther 311, 298-306.
- Kalant H. (2001) The pharmacology and toxicology of "ecstasy" (MDMA) and related drugs. CMAJ. October 2;165(7):917-28. Review. PMID Full Text
- Miller, R.T. et al. (1997). 2,5-Bis-(glutathione-S-yl)-alpha-methyldopamine, a putative metabolite of (+/-)-3,4-methylenedioxyamphetamine, decreases brain serotonin concentrations. Eur J Pharmaco. 323(2-3), 173-80. Abstract retrieved April 17, 2005, from PubMed.
- Monks, T.J. et al. (2004). The role of metabolism in 3,4-(+)-methylenedioxyamphetamine and 3,4-(+)-methylenedioxymethamphetamine (ecstasy) toxicity. Ther Drug Monit 26(2), 132-136.
- Morgan, Michael John (2000). Ecstasy (MDMA): a review of its possible persistent psychological effects. Psychopharmacology 152, 230-248.
- Shankaran, Mahalakshmi, Bryan K. Yamamoto, and Gary A. Gudelsky. “Ascorbic Acid Prevents 3,4,-Methylenedioxymethamphetamine (MDMA)- Induced Hydroxyl Radical Formation and the Behavioral and Neurochemical Consequences of the Depletion of Brain 5-HT”. Synapse. 2001; 40:55-64.
- Strote, Jared et al. (2002). Increasing MDMA use among college students: results of a national survey. Journal of Adolescent Health 30, 64-72.
- Sumnall, Harry R. & Cole, Jon C. (2005). Self-reported depressive symptomatology in community samples of polysubstance misusers who report Ecstasy use: a meta-analysis. Journal of Psychopharmacology 19(1), 84-92.
- Vollmer, Grit. "Crossing the Barrier." Scientific American Mind. June/July 2006, 34-39.
- Yeh, S. Y. “Effects of Salicylate on 3,4-Methylenedioxymethamphetamine (MDMA)-Induced Neurotoxicity in Rats”. Pharmacology Biochemistry and Behavior. 1997; Vol. 58, No. 3: 701-708.
- Gerra G, Zaimovic A, Ampollini R, Giusti F, Delsignore R, Raggi MA, Laviola G, Macchia T, Brambilla F. "Experimentally induced aggressive behavior in subjects with 3,4-methylenedioxy-methamphetamine ("Ecstasy") use history: psychobiological correlates." J Subst Abuse. 2001;13(4):471-91
- Reid LW, Elifson KW, Sterk CE. "Hug drug or thug drug? Ecstasy use and aggressive behavior". Violence Vict. 2007;22(1):104-19.
- Ksir, Charles, Carl L. Hart, and Oakley Ray. Drugs, Society and Human Behavior, 12th ed. New York, NY: McGraw Hill, 2006.
External links
- MDMA PiHKAL entry
- Erowid Vaults: MDMA
- Jennings, Peter. "Primetime Special: Peter Jennings — Ecstasy Rising." ABC News, April 1, 2004.
- Multidisciplinary Association for Psychedelic Studies MDMA Resarch Page - A regularly updated information source about MDMA research, including efforts to get MDMA approved as a prescription medicine.
- EcstasyData.org A database of photos and lab-test results of over 1500 pills of "Ecstasy."
- American Council for Drug Education factsheet on Ecstasy


