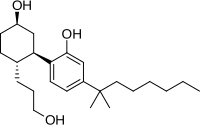
CP 55,940
This is an old revision of this page, as edited by Dcirovic (talk | contribs) at 18:06, 28 May 2016 (clean up using AWB). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C24H40O3 |
| Molar mass | 376.573 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
CP 55,940 is a cannabinoid which mimics the effects of naturally occurring THC (one of the psychoactive compounds found in cannabis). CP 55,940 was created by Pfizer in 1974 but was never marketed. It is currently used to study the endocannabinoid system.
A study found that CP 55,940 can upregulate 5-HT2A receptors in mice.[1]
CP 55,940 is 45 times more potent than Δ9-THC, and fully antagonized by rimonabant (SR141716A).[2]
CP 55,940 is considered a full agonist at both CB1 and CB2 receptors and has Ki values of 0.58nM and 0.68nM respectively, but is an antagonist at GPR55, the putative "CB3" receptor.[3]
CP 55,940 showed protective effects on rat brain mitochondria upon paraquat exposure.[4]
It also showed neuroprotective effects by reducing intracellular calcium release and reducing hippocampal cell death in cultured neurons subjected to high levels of NMDA.[5]
CP 55,940 induced cell death in NG 108-15 Mouse neuroblastoma x Rat glioma hybrid brain cancer (genetically engineered mouse x rat brain cancer) cells.[6][7]
See also
References
- ^ Franklin, J. M.; Carrasco, G. A. (2013). "Cannabinoid receptor agonists upregulate and enhance serotonin 2A (5-HT2A) receptor activity via ERK1/2 signaling". Synapse. 67 (3): 145–159. doi:10.1002/syn.21626. PMID 23151877.
- ^ Rinaldi-Carmona, M.; Pialot, F.; Congy, C.; Redon, E.; Barth, F.; Bachy, A.; Brelière, J. C.; Soubrié, P.; le Fur, G. (1996). "Characterization and distribution of binding sites for [3H]-SR 141716A, a selective brain (CB1) cannabinoid receptor antagonist, in rodent brain". Life Sciences. 58 (15): 1239–1247. doi:10.1016/0024-3205(96)00085-9. PMID 8614277.
- ^ Kapur, A.; Zhao, P.; Sharir, H.; Bai, Y.; Caron, M. G.; Barak, L. S.; Abood, M. E. (2009). "Atypical responsiveness of the orphan receptor GPR55 to cannabinoid ligands". The Journal of Biological Chemistry. 284 (43): 29817–29827. doi:10.1074/jbc.M109.050187. PMC 2785612. PMID 19723626.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Velez-Pardo, C.; Jimenez-Del-Rio, M.; Lores-Arnaiz, S.; Bustamante, J. (2010). "Protective effects of the synthetic cannabinoids CP55,940 and JWH-015 on rat brain mitochondria upon paraquat exposure". Neurochemical Research. 35 (9): 1323–1332. doi:10.1007/s11064-010-0188-1. PMID 20514518.
- ^ Zhuang, S. Y.; Bridges, D.; Grigorenko, E.; McCloud, S.; Boon, A.; Hampson, R. E.; Deadwyler, S. A. (2005). "Cannabinoids produce neuroprotection by reducing intracellular calcium release from ryanodine-sensitive stores". Neuropharmacology. 48 (8): 1086–1096. doi:10.1016/j.neuropharm.2005.01.005. PMID 15910885.
- ^ Tomiyama, K.; Funada, M. (2011). "Cytotoxicity of synthetic cannabinoids found in 'Spice' products: The role of cannabinoid receptors and the caspase cascade in the NG 108-15 cell line". Toxicology Letters. 207 (1): 12–17. doi:10.1016/j.toxlet.2011.08.021. PMID 21907772.
- ^ "General Cell Collection: NG108-15". Public Health England Culture Collections.
| Psychedelics (5-HT2A agonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dissociatives (NMDAR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deliriants (mAChR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Receptor (ligands) |
| ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (modulators) |
| ||||||||||||||||||||||||||||||
| Enzyme (modulators) |
| ||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |
- CS1 maint: unflagged free DOI
- Chem-molar-mass both hardcoded and calculated
- Infobox-drug molecular-weight unexpected-character
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- All stub articles
