MDMA
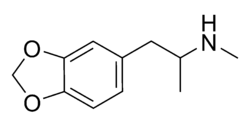  MDMA | |
|
1-(benzo[d][1,3]dioxol-5-yl)-N-methylpropan-2-amine | |
| CAS number |
42542-10-9 |
|---|---|
| Chemical formula | C11H15NO2 |
| Molecular weight | 193.25 g/mol |
| SMILES | CC(NC)CC1=CC=C(OCO2)C2=C1 |
| Elimination half life | The "S" form has a shorter half life (about 4 hours), whereas the "R" form has a much greater half life. (about 14hours) |
| Legal status | Schedule I (USA) Class A (UK) Schedule III (Canada) |
| Delivery | 75-120 mg tablets 100 mg sublingual |
| Indicated for: | |
| Recreational uses: | |
| Other uses: | |
Contraindications:
| |
| Side effects: | |
| Endocrine: | |
Eye:
| |
| Psychological: | |
Skin:
| |
Miscellaneous:
| |
MDMA (3,4-methylenedioxymethamphetamine), most commonly known by the street name ecstasy or XTC, is a synthetic entactogen of the phenethylamine family, whose primary effect is believed to be the stimulation of secretion as well as inhibition of re-uptake of large amounts of serotonin as well as dopamine and norepinephrine in the brain, inducing a general sense of openness, empathy, energy, euphoria, and well-being. Tactile sensations are enhanced for some users, making general physical contact with others more pleasurable; but, contrary to popular mythology it generally does not have aphrodisiac effects. Its reported ability to facilitate self-examination with reduced fear may prove useful in some therapeutic settings, leading in 2001 to permission from the United States FDA for testing in patients with post-traumatic stress disorder in conjunction with psychotherapy.
Acute dehydration is a risk among users who are highly physically active and forget to drink water, as the drug may mask one's normal sense of exhaustion and thirst. Also the opposite, "water intoxication" resulting in acute hyponatremia has been reported as a consequence of use. Sometimes other potentially toxic chemicals such as PMA or methamphetamine alone or in combination with MDMA are added to ecstasy tablets. Long-term effects in humans are largely unknown and the subject of much controversy — particularly with regard to the risks of severe long-term depression as a result of a reduction in the natural production of serotonin.
Refer to this page for a list of street names of MDMA.
History
A patent for MDMA was originally filed on Christmas Eve 1912 by the German pharmaceutical company Merck, and granted exactly two years later. At the time, MDMA was not known to be a drug in its own right; rather, it was patented as an intermediate chemical used in the synthesis of a styptic (a drug intended to control bleeding from wounds.) Over half a century would pass before the first recorded ingestion of MDMA by humans.
The US Army did, however, carry out lethal dose studies on MDMA and several other compounds in the mid-1950s. It was given the name EA-1475, with the EA standing for Edgewood Arsenal. The results of these studies were not declassified until 1969.
MDMA was legal in the United States until 1985. Before then, it was used both as an adjunct to psychotherapy and as a recreational drug. MDMA began to be used therapeutically in the mid 1970s after the chemist Dr. Alexander Shulgin introduced it to psychotherapist Leo Zeff. As Zeff and others spread word about MDMA, it developed a reputation for enhancing communication, reducing psychological defenses, and increasing capacity for introspection. However, no formal measures of these putative effects were made and blinded or placebo-controlled trials were not conducted. A small number of therapists --including George Greer, Joseph Downing, and Philip Wolfson-- used it in their practices until it was made illegal.
MDMA appeared sporadically as a street drug in the early 1970s, but it came into prominence in the early 1980s in certain trendy yuppie bars in the Dallas area, then in gay dance clubs. From there use spread to rave clubs, and then to mainstream society. During the 1990s, along with the growing popularity of the rave subculture, MDMA use became increasingly widespread among young adults in universities and later in high schools. It rapidly became one of the four most widely used illegal drugs in the US, along with cocaine, heroin and marijuana.
In the late 1980s and early 1990s, ecstasy was widely used in the United Kingdom and other parts of Europe, becoming an integral element of rave culture. It was also associated with another psychedelic/dancefloor-influenced music scene, Madchester.
Recreational use
The primary effects of MDMA include feelings of openness, euphoria, empathy, love, and heightened self-awareness. Its initial adoption by the dance club sub-culture is possibly due to the enhancement of the overall social and musical experience. Taking MDMA or Ecstasy is commonly referred to as rolling, pilling or dropping in the United Kingdom, or "thizzing" in Northern California. Some term the rushing feeling of the drug as blowing up or coming up.
MDMA use has increased markedly since the late 1980s, and spread beyond its original sub-cultures to mainstream use. Prices have also fallen since the 1980s. In countries where distribution is more extensive, such as in the Netherlands and other places in Europe, prices can sometimes be as low as €1 per tablet. In countries where distribution is more difficult, such as the US and Australia, prices are accordingly higher at up to US$20—30 per tablet. Prices are also usually higher when the drug is purchased in a club or at a rave.
Supply and administration
MDMA is usually ingested in pill form, it does however occasionally come in powder form - (often known in the UK as "Madam"). Pills come in a variety of "brands", usually identified by the icons stamped on the pills. However the brands do not consistently designate the actual active compound within the pill, as it is possible for 'copycat' manufacturers to make their own pills which replicate the features of a well-known brand.
Pills sold illegally on the street do not always contain MDMA as the only active ingredient. In British Columbia, Canada, recent government tests showed that some of the pills tested contained methamphetamine in doses as high as 20 milligrams [citation needed]. Analogues of MDMA such as MDEA, MDA and MBDB are often found, and more rarely other psychoactive additives such as amphetamines (speed), DXM, ephedrine, Pseudoephedrine, PMA, 4-MTA, caffeine, ketamine (Special K), 2C-B, 2C-T-7 or other compounds may be present [citation needed]. In addition to MDMA ecstasy pills may contain cocaine, heroin, or mescaline [citation needed]; Mescaline is an especially unlikely contaminant, as a large amount is required for an effective dose [citation needed]. There have been a few cases where an extremely potent synthetic opiate, Fentanyl, has been identified in pills [citation needed], which could potentially be very dangerous if people took several of them thinking that they only contained MDMA [citation needed].

Aspirin, paracetamol (acetaminophen), or even canine heartworm tablets have had the letter E scratched into them and have been sold as ecstasy [citation needed], for enormous profit. While overdose from MDMA itself is rare, many more toxic substances are often sold as ecstasy [citation needed], and overdose or other adverse reaction to adulterants is not uncommon [citation needed].
Although full and proper characterization of ecstasy pills requires advanced lab techniques such as gas chromatography-mass spectrometry, it is also possible to use a less accurate presumptive alkaloid test known as the Marquis reagent. Many organisations sell testing kits containing this reagent. DanceSafe is one such company, and it includes an extensive database of photographs of different pills, along with the results of a laboratory analysis of their contents. EcstasyData.org [1] is a non-profit site that tests the purity of street pills and compiles results.
Effects
Pharmacokinetics
MDMA reaches maximal concentrations in the blood between 1.5 and 3 hours after ingestion. It is then slowly metabolized and excreted, with levels decreasing to half their peak over approximately 8 hrs. Metabolites of MDMA which have been identified in humans include 3,4-methylenedioxyamphetamine (MDA), 4-hydroxy-3-methoxy-methamphetamine (HMMA), 4-hydroxy-3-methoxyamphetamine (HMA), 3,4-dihydroxyamphetamine (DHA, also called alpha-methyldopamine), 3,4-methylenedioxyphenylacetone, and N-hydroxy-3,4-methylenedioxyamphetamine. The contributions of these metabolites to the psychoactive and toxic effects of MDMA are an area of active research.
MDMA is metabolised via three pathways. One such pathway proceeds via N-demethylation; byproducts of which include several active metabolites, including MDA. The metabolism may be primarily by the cytochrome P450 enzymes CYP2D6 (in humans, but CYP2D1 in mice), and CYP3A4. Complex, nonlinear pharmacokinetics arise via autoinhibition of CYP2D6 and CYP2D8, resulting in zeroth order kinetics at higher doses. It is thought that this can result in sustained and higher drug concentrations if the user takes consecutive doses of the drug. A significant quantity is excreted unchanged in the urine, especially when the drug is taken at higher doses.
MDMA is a chiral compound and has been almost exclusively administered as a racemate. However, an early uncontrolled report suggests that the S-enantiomer is significantly more potent in humans than the R-enantiomer (Anderson et al. 1978). Studies in humans [1][2] indicate that the disposition of MDMA is stereoselective, with the S-enantiomer having a shorter elimination half-life and greater excretion that the R-enantiomer. For example, Fallon et al. (1999[3]) reported that the area under the curve (AUC) of plasma concentrations was two to four times higher for the R-enantiomer than the S-enantiomer after 40 mg oral in human volunteers. Similarly, the plasma half-life of (R)-MDMA was significantly longer than that of the S enantiomer ((5.8 ± 2.2 hours) vs 3.6 ± 0.9 hours). However, because MDMA has dose dependent kinetics, it is likely that these half lives would be higher at more typical doses (100 mg is sometimes considered a typical dose). Given as the racemate, MDMA has a half-life around 8 hours.
Short-term neurochemical effects
Serotonin is a neurotransmitter believed to play a role in the regulation of mood and pleasure. MDMA causes serotonin vesicles in the neurons to release quantities of serotonin into the synapses. Although popular press accounts focus on the role of serotonin release, the mechanism by which MDMA causes its unusual psychoactivity is largely unknown. In vitro and nonhuman animal studies have established that MDMA also induces dopamine, norepinephrine, and acetylcholine release and can act directly on a number of receptors, including α2-adrenergic and 5HT2A receptors. MDMA promotes the release of several hormones including prolactin and the antidiuretic hormone vasopressin, which may be important in its occasional production of water intoxication or hyponatremia.
Subjective effects
Effects desired by users include:
- intense euphoria
- a feeling of connection with other people, especially if they are also using the drug
- a marked increase in the salience and expression of happiness, love or other positive emotions
- the feeling that something "tremendously important" or "fundamental and positive" is occurring
- intense feelings of love, closeness and mutual understanding with complete strangers
- a child-like sense of wonder at the world; a feeling of reclaimed innocence
- a sense of mental clarity
- a relieving sense that problems in life are insignificant
MDMA, particularly with larger doses, is sometimes reported to cause visual distortions. In a review of studies in which 1.5 to 1.7 mg/kg oral MDMA was administered in their laboratory to 74 people, Vollenweider et al. reported that scenic hallucinations were reported only once, while simple patterns, distorted objects, and flashes of light were commonly reported [4].
Other short-term effects
Acute physiological effects include:
- Pupil dilation with attendant photosensitivity and color perception
- eyes roll to the back of the head
- Tightening of facial muscles, including rolling of tongue and jaw (known by users as "Gurning")
- Shutter vision (nystagmus)
- General restlessness
- Loss of appetite/taste sensation changes
- Lack of focus / concentration - which can alternate with periods of fascinated fixation on a person or object
- A need to take exaggerated, deep breaths, particularly when "rushing" (i.e. when the effects of MDMA are coming on strongly)
- Tingling
- Sweaty palms
- Increased heart rate
Acute toxic (dangerous) effects
Apart from the dangers from impurities, the primary acute risks of taking MDMA resemble those of other stimulant amphetamines. The majority of fatalities and cases requiring emergency care involve hyperthermic syndromes. MDMA appears to decrease heat loss in the body by causing constriction of blood vessels near the skin. In addition, it may sometimes increase heat production by muscles and the brain. These effects may be amplified in people who become dehydrated and are unable to cool by sweating. MDMA can mask the body's normal thirst and exhaustion responses, particularly if a user is dancing or is otherwise physically active for long periods of time without hydration. Because of these effects, MDMA can temporarily reduce the body's ability to regulate its core temperature, and in high-temperature surroundings (e.g. clubs) combined with physical exertion this may lead to hyperpyrexia if precautions are not taken to remain cool. Sustained hyperpyrexia may lead to rhabdomyolysis (muscle breakdown), which in turn can cause renal failure and death.
It has been argued that "the seriousness of the effects can be dependent on environmental factors other than the drug concentration," as blood concentrations of the drug spanned a large range in cases of death in MDMA users. This notwithstanding, "most of the cases of serious toxicity or fatality have involved blood levels... up to 40 times higher than the usual recreational range." (Kalant H., 2001) [5]
While dehydration is undesirable, there also have been a number of users suffering from water intoxication and associated hyponatremia (dilution of the blood that can cause swelling of the brain). Although many cases of this clearly involved individuals drinking large amounts of water, there are cases where there is no evidence of excessive water consumption. There cases may be caused by MDMA inducing release of the antiduretic hormone vasopressin by the pituitary gland. This causes one to retain water to a greater extent. The death of British teen Leah Betts may be the most widely publicised MDMA-related fatality, and resulted from her consuming too much water due to concerns over dehydration. Signs of hyponatremia include confusion, nausea, headache and loss of consciousness. Hyponatremia in MDMA users is a medical emergency and requires prompt treatment. In general, females are at greater risk of developing symptoms and dying from hyponatremia than males.
MDMA users have also been recorded to demonstrate Bruxism (teeth grinding) and Trisma (jaw clenching) as a short term effect from the drug [6] Many users of MDMA alleviate this by using chewing gum [7], however this can result in temporary mouth ulcers through inadvertent biting of the mouth lining. Temporary jaw ache often results from jaw clenching or excessive chewing. Some users also report decreased libido or impotence; however, studies have had conflicting results [8], [9] There are reported allergic reactions, which are extremely rare. Liver damage, which may have an immunological cause, has been seen in a small number of users. Animal studies suggest risk and extent of liver damage is increased by high body temperature.
Long-term adverse effects
Long-term effects are still unknown and heavily debated among scientists. There are several reports of Hallucinogen Persisting Perception Disorder being induced by MDMA. In some cases, the disorder appears to be permanent. The disorder seems to occur in only a small fraction of a percentage of users, and its mechanism of causation is unknown.
Some experiments indicate that use at very high doses may lead to the synaptic terminals of serotonin neurons being damaged. The precise mechanism of this action is unknown, but recent evidence (Jones 2004; Miller 1997; Monks et al. 2004) suggests that the metabolic breakdown of MDMA includes the formation of reactive oxygen species (ROS), chemicals known to cause oxidative cell damage when taken up into the releasing synapse.
This effect has been demonstrated experimentally in the brains of rats, where the serotonin terminals of animals who are given extremely high doses of MDMA over a prolonged period of time (usually ten to one hundred times greater than a typical human dose) become withered and useless, although this isn't certain[2][3]. This hypothesis is supported by the fact that the administration of selective serotonin reuptake inhibitors ("SSRIs", which bind to the serotonin cell's reuptake transporters and thus block ROS from entering the serotonin cells) along with or immediately following MDMA seems to block neuron damage in rats given MDMA.
The mechanism proposed as a large part of this neurotoxicity and its functional consequences appear to involve the induction of oxidative stress. This stress results from an increase in free radicals and a decrease in antioxidants in the brain. (Shankaran, 2001) Oxidation is part of the normal metabolic processes of the body. As the cell goes about its life, by-products called oxidative radicals are formed, also called free radicals. These molecules have an unpaired electron that makes them highly reactive. They pull strongly on the electrons of neighboring molecules and destabilize the electrical balance of those molecules, sometimes causing those molecules to fall apart. This can become a chain reaction.
In normal functioning, there are antioxidants in the system that act as free radical scavengers. These are molecules with an extra electron that they are willing to give up to the free radicals, making both the free radical and the antioxidant more stable. MDMA rapidly increases the levels of free-radicals in the system and overwhelms the reserves of scavengers. The radicals then damage cell walls, reduce the flexibility of blood vessels, destroy enzymes, and cause other molecular damage in the neurological pathways. (Erowid, 2001) It has been shown that MDMA-neurotoxic effects are increased by a hyperthermic environment and decreased by a hypothermic one. (Yeh, 1997)
Studies have suggested that the neurotoxic molecules are not hydroxyl free radicals, but superoxide free radicals. When rats are injected with salicylate, a molecule that scavenges hydroxyl free radicals, the neurotoxic effects of MDMA are not attenuated, but actually potentiated. Further evidence of this superoxide theory comes from the observation that CuZn-superoxide dismutase transgenic mice (mice with excess human antioxidant enzyme) demonstrate neuroprotective mechanisms that protect the mice from MDMA-induced depletion of 5-HT and 5-HIAA and lethal effects. (Baggott, 2001 and Yeh, 1997)
Studies giving animal species injections have shown that ascorbic acid, alpha lipoic acid, l-cysteine, and some other radical scavengers are effective in reducing oxidative stress caused by MDMA. (Erowid, 2001) A combination of antioxidants, including Vitamin A, C, and E are recommended; taking multivitamins including selenium, riboflavin, zinc, cartenoids, etc. should help reduce oxidative damage. Many of these vitamins, though, are water soluble, and are quickly excreted from the body. The typical MDMA user is psychoactive for 4-6 hours and may not have an appetite from the time of taking until the following sleep cycle or many hours later. These vitamins flush through the system in 3-4 hours. Damage occurs in the absence of these antioxidants.
There are some fallacies in applying these animal studies to human use. Firstly, it is difficult to equate rat doses to human doses, rats metabolise MDMA twice as fast humans and often larger doses or multiple doses are administered to simulate human plasma levels. The doses given in experiments are far greater than typical human use of 100-300 mg in order to notice the problems caused so that we may say that if this happens at large doses, then a lesser form should happen at low doses. There could be a threshold of nothing happening or a threshold of the worst problems at low doses.
Secondly, the doses of antioxidants given to these animals are much higher than humans would ever take both in its vehicle (injected vs. oral) and in its dosage. Essentially both the neurotoxic and neuroprotective effects are exaggerated, but it is not possible to say if this scales down the same way.
Some MDMA users administer an SSRI while, or shortly after taking MDMA, in an attempt to prevent possible neurotoxicity. These SSRIs are typically antidepressants such as fluoxetine or sertraline. However, administration of SSRIs before using MDMA is known to block the euphoric high from the drug, due to the regulation of serotonin. This blocking effect can last several weeks, depending on the half-life of the SSRI. The same effects are seen with recent cocaine use, which itself is an SSRI.
It should be noted, however, that MDMA use in conjunction with a different class of antidepressants, namely Monoamine oxidase inhibitors, is strongly contra-indicated due to danger of serotonin syndrome and the possibility of life-threatening hypertension. The safety of this practice has not been systematically evaluated.
Many users also attempt to replenish the deficit of serotonin which follows the use of MDMA by administering 5-HTP. The serotonin precursor 5-HTP, which is commercially available as a dietary supplement, reportedly supplies the user with more of the raw materials to synthesize the neurotransmitter. Pre-loading with 5-HTP has not been shown to increase the subjective effects of MDMA.
Because the neurotoxicity of MDMA is believed by some to be highly dependent on its metabolic disposition (Jones 2004; de la Torre & Farré 2004), it is unclear how to generalize to humans from experiments in rats and monkeys.
Considerable research has been done into possible cognitive-behavioral deficits among ecstasy users but data have been largely inconclusive. At least two meta-analyses of these studies have been completed (Morgan 2000; Sumnall & Cole 2005). Morgan's analysis of 17 studies showed that ecstasy users had a slight tendency to be more impulsive and depressed than controls. Sumnall and Cole's analysis showed a slight increase in the prevalence of depressive symptoms in ecstasy users over controls. Of course, in retrospective studies like these we are always faced with a chicken-or-egg question: did these impulsive and depressed people use ecstasy to self-medicate or did otherwise normal people become depressed and impulsive after using ecstasy? This question has not been answered. Moreover, such research is problematic as ecstasy users are much more likely than control subjects to have taken other drugs in addition to ecstasy. This makes it difficult for researchers to establish a direct causal relationship.
Although some experimental evidence exists indicating that long-term ecstasy users experience memory difficulties, a large study in 2002 (Strote et al.) showed that ecstasy users in 4-year colleges have GPAs which do not differ significantly from those of non-users.
MDMA and Parkinson's
Research at the University of Manchester indicates that MDMA dramatically reduces tremors in patients receiving L-DOPA treatment for Parkinson's Disease.
In a now-retracted study, a research team led by Dr. George A. Ricaurte at Johns Hopkins University implicated MDMA as a cause of Parkinson's-like brain abnormalities in monkeys, suggesting that a single use of MDMA caused permanent and serious brain damage. These claims were hotly disputed by physicians, therapists, and other experts in the field, including a team of scientists at New York University. Criticisms of the study included its use of injection rather than oral administration; that this type and scale of damage (>20% mortality) would translate to hundreds of thousands or millions of deaths which had not materialized in the real world amidst extremely broad global MDMA usage; and, perhaps most important, that other research teams could not duplicate the study's findings.
On September 6, 2003, Dr. George A. Ricaurte and his team announced that they were retracting all results of their commonly cited and controversial study. The researchers said that the labels on the drugs had been somehow switched, and they had inadvertently injected their experimental monkeys and baboons with extremely high doses of methamphetamine instead of MDMA. The chemical supplier, Research Triangle Institute, has publicly claimed that the proper drug was supplied, and Ricaurte has yet to pursue them for their alleged error.
Ricaurte had also come under fire for supplying PET scans to the U.S. Office of National Drug Control Policy that were used in anti-drug literature (Plain Brain/Brain After Ecstasy) that seemed to suggest MDMA created holes in human brains, an implication that critics called misleading. Ricaurte later asked the Agency to change the literature, citing the "poor quality" of the images. These images are still circulating in educational systems across the U.S., however, and the myth that ecstasy users develop "holes in their brains" remains quite popular.
Legal issues
Use, supply and trafficking of ecstasy are currently illegal in most countries. In the United States, MDMA was legal and unregulated until May 31st 1985, at which time it was added to DEA Schedule I, for drugs deemed to have no medical uses and a high potential for abuse. During DEA hearings to criminalize MDMA, most experts recommended DEA Schedule III prescription status for the drug, due to its beneficial usage in psychotherapy. The judge overseeing the hearings, Francis Young, also made this recommendation. Nonetheless, the DEA classified it as Schedule I[4].
That same year, the World Health Organization's Expert Committee on Drug Dependence recommended that MDMA be placed in Schedule I of the Convention on Psychotropic Substances. Unlike the Controlled Substances Act, the Convention has a provision (in Article 7(a)) that allows use of Schedule I drugs for "scientific and very limited medical purposes". The Committee's report stated[10]:
- It should be noted that the Expert Committee held extensive discussions concerning therapeutic usefulness of 3,4 Methylenedioxymethamphetamine. While the Expert Committee found the reports intriguing, it felt that the studies lacked the appropriate methodological design necessary to ascertain the reliability of the observations. There was, however, sufficient interest expressed to recommend that investigations be encouraged to follow up these preliminary findings. To that end, the Expert Committee urged countries to use the provisions of article 7 of the Convention on Psychotropic Substances to facilitate research on this interesting substance.
In the United Kingdom, MDMA is Schedule I/Class A, making it illegal to sell, buy, or possess without a license. Penalties include a maximum of seven years and/or unlimited fine for possession; life and/or unlimited fine for production or trafficking. A mandatory seven year sentence is now the penalty for a third conviction for trafficking.
Medical use and clinical studies
In 2001, the FDA granted permission for experimental administration of MDMA to patients suffering from post-traumatic stress disorder. This research is being sponsored by the Multidisciplinary Association for Psychedelic Studies (MAPS). For further information on this, see MAPS's MDMA Research Information and the recent article from MSNBC/Newsweek. This research in patients builds on studies in which MDMA was given to healthy volunteers. The first of these healthy volunteer studies was conducted by Dr. Charles Grob, with other studies done by Dr. Franz Vollenweider in Switzerland, Drs. John Mendelson and Reese Jones at the University of California San Francisco, and Drs. Magi Faree and Rafael de la Torre in Spain.
Safety and contradictions
The illegality of this drug in many countries makes exact study of its effects difficult. Some of the effects ascribed to ecstasy, which may or may not be conclusive, are the following:
- Because of its illegality, the dose and purity of an ecstasy pill varies dramatically. The dose may be stronger than is advertised, may be adulterated, or might not even contain MDMA.
- Ecstasy affects the regulation of the body's internal systems. Continuous dancing without sufficient breaks or drinks can lead to dangerous overheating and dehydration. Drinking too much water without consuming a corresponding amount of salt can lead to hyponatremia or Water intoxication.
- The use of ecstasy can exacerbate depression and may produce temporary depression as an after-effect for some users. Some individuals also might experience wild or unexpected mood swings the first couple of days following the use of MDMA.
- The use of ecstasy can be very dangerous when combined with other drugs (particularly monoamine oxidase inhibitors (MAOIs) and antiretroviral drugs, in particular Ritonavir). Combining MDMA with MAOIs can precipitate a hypertensive crisis and can result in a near-fatal repercussion.
- In many cases, pills marketed as ecstasy do not contain MDMA, but instead are substituted with various substances like ketamine, methamphetamine and caffeine. Some users purchase testing kits to verify that pills are actually MDMA. Organizations such as DanceSafe provide testing kits [11].
- Long-term after-effects are greatly exacerbated by high doses and frequent use.
- A small percentage of users may be highly sensitive to MDMA; this may make first-time use especially hazardous. This includes but is not limited to people with congenital heart defects. Some scientists have suggested that a small percentage of people who lack the proper enzymes to break down the drug. One enzyme involved in MDMA's breakdown is CYP2D6, which is deficient or totally absent in 5-10% of the caucasian population and those of African descent and 1-2% of Asians.[12]. However, there is no clear evidence linking lack of this enzyme to problems in users and the connection remains theoretical.
See also
- Sextasy
- Empathogen-entactogen
- Amphetamine
- Phenethylamines
- Psychedelic therapy
- Psychoactive drug
- RAVE Act
- Retracted article on neurotoxicity of ecstasy
- Leah Betts & Anna Wood (people who have died as a result of drinking too much water while on ecstasy)
External links
Media
- Jennings, Peter. "Primetime Special: Peter Jennings - Ecstasy Rising." ABC News, April 1, 2004.
- Conant, Eve. "Ecstasy: A Possible New Role for a Banned Club Drug." Newsweek, May 2, 2005.
- Generation on X: An undercover look at the growing trend of teens using Ecstasy FOX News, April 26, 2005.
- Weiss, Rick. "Use Studied to Ease Fear in Terminally Ill." The Washington Post, December 27, 2004.
- Philipkoski, Kristen. "Long Trip for Psychedelic Drugs." Wired, September 27, 2004.
- Philipkoski, Kristen. "DEA Approves Ecstasy Tests." Wired, March 2, 2004.
- Darman, Jonathan. "Out of the Club, Onto the Couch Newsweek.com, December 5, 2003. - An interview with NYU's Dr. Julie Holland
- Weiss, Rick. "Results Retracted on Ecstasy Study." The Washington Post, September 6, 2003.
- Recer, Paul "Ecstasy-Parkinson's Connections?."CBS News, September 26, 2002.
- Man found to have taken 40,000 tabs in 9 years - London.; The Guardian, April 4, 2006.
- Wittlin, Maggie. "Hitting a High E: Italian scientists find loud music intensifies and extends the brain’s response to MDMA," Seed Magazine (02/15/2006)
Academic
- The Multidisciplinary Association for Psychedelic Studies (MAPS): MDMA project MAPS is at the forefront of human MDMA research, having obtained FDA permits for two studies administering MDMA to human volunteers in order to explore the drug's potential psychiatric benefits (one study is already underway.)
- The DEA.org's extensive critique/review of the evidence against MDMA ('ecstasy') causing brain damage at common recreational doses.
- This is your brain on Ecstasy - A slideshow that illustrates the neuropharmacokinetics of Ecstasy (how the drug affects the brain.) Some of the information on this page is at present (July 2005) outdated.
- PiHKAL entry
- The MAPS research library, containing downloadable copies of most of the MDMA and LSD research ever done.
General
- Multidisciplinary Association for Psychedelic Studies - A non-profit organization currently conducting FDA-approved studies with MDMA.
- EcstasyData.org A database of photos and lab-test results of over 1500 pills of "Ecstasy".
- Pillreports A similar database to EcstasyData, but with user-contributed photos of pills and subjective "pill reports" and ratings. Over 1600 listings (as of Jan. 2006).
- DanceSafe - a risk reduction site with lots of information on Ecstasy. Includes a large database of photographs of different pill types, along with laboratory analysis of what was actually found in the pill.
- Erowid's Ecstasy page - lots of information
- UK National Drugs Line factsheet on Ecstasy
- American Council for Drug Education factsheet on Ecstasy
- Congressional Research Service (CRS) Reports regarding Ecstasy
- [13]
- Utopian Pharmacology. Detailed essay discussing the history and uses of MDMA
- TheDEA.org An ecstasy user's guide with detailed discussions of risks and scientific research.
- MDMA, Personality and Human Nature: The Power to Transform People. Essay by Bruce Eisner, author of Ecstasy: The MDMA Story
References
- ^ The Ecstasy Testing Program
- ^ Baumann MH, Wang X, Rothman RB. (2006). "3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings". Psychopharmacology. PMID 16541247.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Saunders, Nicholas (1995). "Interviews with two foremost researchers into neurotoxicity who hold opposing views".
- ^ MAPS. "Documents from the DEA Scheduling Hearing of MDMA, 1984-1988".
- Baggott, Matthew, and John Mendelson. “MDMA Neurotoxicity”. Ecstasy: The Complete Guide. Ed. Julie Holland. Spring 2001 from www.erowid.com.
- de la Torre, Rafael et al. (2000), Non-linear pharmacokinetics of MDMA (`ecstasy') in humans. Br J Clin Pharmacol, 2000; 49(2):104-9
- de la Torre, Rafael & Farré, Magí (2004). Neurotoxicity of MDMA (ecstasy): the limitations of scaling from animals to humans. Trends in Pharmacological Sciences 25, 505-508.
- Erowid, Earth. “Do Antioxidants Protect Against MDMA Hangover, Tolerance, and Neurotoxicity?” Erowid Extracts. Dec 2001; 2:6-11.
- Jennings, Peter. Ecstasy Rising, ABC television documentary. 2004-01-04.
- Jones, Douglas C. et al. (2004). Thioether Metabolites of 3,4-Methylenedioxyamphetamine and 3,4-Methylenedioxymethamphetamine Inhibit Human Serotonin Transporter (hSERT) Function and Simultaneously Stimulate Dopamine Uptake into hSERT-Expressing SK-N-MC Cells. J Pharmacol Exp Ther 311, 298-306.
- Kalant H. (2001) The pharmacology and toxicology of "ecstasy" (MDMA) and related drugs. CMAJ. Oct 2;165(7):917-28. Review. PMID 11599334 Full Text
- Miller, R.T. et al. (1997). 2,5-Bis-(glutathione-S-yl)-alpha-methyldopamine, a putative metabolite of (+/-)-3,4-methylenedioxyamphetamine, decreases brain serotonin concentrations. Eur J Pharmaco. 323(2-3), 173-80. Abstract retrieved Apr 17, 2005, from PubMed.
- Monks, T.J. et al. (2004). The role of metabolism in 3,4-(+)-methylenedioxyamphetamine and 3,4-(+)-methylenedioxymethamphetamine (ecstasy) toxicity. Ther Drug Monit 26(2), 132-136.
- Morgan, Michael John (2000). Ecstasy (MDMA): a review of its possible persistent psychological effects. Psychopharmacology 152, 230-248.
- Shankaran, Mahalakshmi, Bryan K. Yamamoto, and Gary A. Gudelsky. “Ascorbic Acid Prevents 3,4,-Methylenedioxymethamphetamine (MDMA)- Induced Hydroxyl Radical Formation and the Behavioral and Neurochemical Consequences of the Depletion of Brain 5-HT”. Synapse. 2001; 40:55-64.
- Strote, Jared et al. (2002). Increasing MDMA use among college students: results of a national survey. Journal of Adolescent Health 30, 64-72.
- Sumnall, Harry R. & Cole, Jon C. (2005). Self-reported depressive symptomatology in community samples of polysubstance misusers who report Ecstasy use: a meta-analysis. Journal of Psychopharmacology 19(1), 84-92.
Yeh, S. Y. “Effects of Salicylate on 3,4-Methylenedioxymethamphetamine (MDMA)-Induced Neurotoxicity in Rats”. Pharmacology Biochemistry and Behavior. 1997; Vol. 58, No. 3: 701-708.
