Botulinum toxin
 | |
| Clinical data | |
|---|---|
| Trade names | Botox, Myobloc, Jeuveau, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619021 |
| License data | |
| Pregnancy category |
|
| Routes of administration | IM (approved), SC, intradermal, into glands |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| UNII |
|
| KEGG | |
| ECHA InfoCard | 100.088.372 |
| Chemical and physical data | |
| Formula | C6760H10447N1743O2010S32 |
| Molar mass | 149323.05 g·mol−1 |
| | |
| Bontoxilysin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 3.4.24.69 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Botulinum toxin, often shortened to BoNT, is a neurotoxic protein produced by the bacterium Clostridium botulinum and related species.[7] It prevents the release of the neurotransmitter acetylcholine from axon endings at the neuromuscular junction, thus causing flaccid paralysis.[8] The toxin causes the disease botulism. The toxin is also used commercially for medical and cosmetic purposes.
The seven main types of botulinum toxin are named types A to G (A, B, C1, C2, D, E, F and G).[9][10] New types are occasionally found.[11][12] Types A and B are capable of causing disease in humans, and are also used commercially and medically.[13][14][15] Types C–G are less common; types E and F can cause disease in humans, while the other types cause disease in other animals.[16] Botulinum toxin types A and B are used in medicine to treat various muscle spasms.
Botulinum toxins are among the most potent toxins known.[17] Intoxication can occur naturally as a result of either wound or intestinal infection or by ingesting formed toxin in food. The estimated human lethal dose of type A toxin is 1.3–2.1 ng/kg intravenously or intramuscularly, 10–13 ng/kg when inhaled, or 1000 ng/kg when taken by mouth.[18] Commercial forms are marketed under the brand names Botox (onabotulinumtoxinA), Dysport/Azzalure (abobotulinumtoxinA),[19] Xeomin/Bocouture (incobotulinumtoxinA),[20] and Jeuveau (prabotulinumtoxinA).[21][22]
Medical uses
Botulinum toxin is used to treat a number of therapeutic indications, many of which are not part of the approved drug label.[23]
Muscle spasticity
Botulinum toxin is used to treat a number of disorders characterized by overactive muscle movement, including cerebral palsy,[13][14] post-stroke spasticity,[24] post-spinal cord injury spasticity,[25] spasms of the head and neck,[26] eyelid,[27] vagina,[28] limbs, jaw, and vocal cords.[29] Similarly, botulinum toxin is used to relax the clenching of muscles, including those of the esophagus,[30] jaw,[31] lower urinary tract and bladder,[32] or clenching of the anus which can exacerbate anal fissure.[33] Botulinum toxin appears to be effective for refractory overactive bladder.[34]
Other muscle disorders
Strabismus, otherwise known as improper eye alignment, is caused by imbalances in the actions of muscles that rotate the eyes. This condition can sometimes be relieved by weakening a muscle that pulls too strongly, or pulls against one that has been weakened by disease or trauma. Muscles weakened by toxin injection recover from paralysis after several months, so injection might seem to need to be repeated, but muscles adapt to the lengths at which they are chronically held,[35] so that if a paralyzed muscle is stretched by its antagonist, it grows longer, while the antagonist shortens, yielding a permanent effect.[36] If binocular vision is good, the brain mechanism of motor fusion, which aligns the eyes on a target visible to both, can stabilize the corrected alignment.[37]
In January 2014, botulinum toxin was approved by UK's Medicines and Healthcare products Regulatory Agency for treatment of restricted ankle motion due to lower-limb spasticity associated with stroke in adults.[38][39]
On 29 July 2016, the U.S. Food and Drug Administration (FDA) approved abobotulinumtoxinA for injection for the treatment of lower-limb spasticity in pediatric patients two years of age and older.[40][41] AbobotulinumtoxinA is the first and only FDA-approved botulinum toxin for the treatment of pediatric lower limb spasticity.[42] In the U.S., the FDA approves the text of the labels of prescription medicines and for which medical conditions the drug manufacturer may sell the drug. However, prescribers may freely prescribe them for any condition they wish, also known as off-label use.[43] Botulinum toxins have been used off-label for several pediatric conditions, including infantile esotropia.[44]
Excessive sweating
AbobotulinumtoxinA (BTX-A) has been approved for the treatment of excessive underarm sweating of unknown cause, which cannot be managed by topical agents.[29][45]
Migraine
In 2010, the FDA approved intramuscular botulinum toxin injections for prophylactic treatment of chronic migraine headache.[46]
Cosmetic Indications
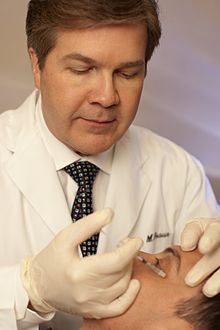
In cosmetic applications, botulinum toxin is considered relatively safe and effective[47] for reduction of facial wrinkles, especially in the uppermost third of the face.[48] Commercial forms are marketed under the brand names Botox Cosmetic/Vistabel from Allergan, Dysport/Azzalure from Galderma and Ipsen, Xeomin/Bocouture from Merz, Jeuveau/Nuceiva from Evolus, manufactured by Daewoong in South Korea.[22] DaxibotulinumtoxinA (Daxxify) was approved for medical use in the United States. In Europe, Letybo is also available, marketed by Croma and manufactured by Hugel in South Korea, along with the first liquid formulated botulinum toxin in Europe, Alluzience, from Galderma and Ipsen.[citation needed] The effects of current botulinum toxin injections for glabellar lines ('11's lines' between the eyes) typically last two to four months and in some cases, product-dependent, with some patients experiencing a longer duration of effect of up to 6 months or longer.[48] Injection of botulinum toxin into the muscles under facial wrinkles causes relaxation of those muscles, resulting in the smoothing of the overlying skin.[48] Smoothing of wrinkles is usually visible three to five days after injection, with maximum effect typically a week following injection.[48] Muscles can be treated repeatedly to maintain the smoothed appearance.[48]
Other
Botulinum toxin is also used to treat disorders of hyperactive nerves including excessive sweating,[45] neuropathic pain,[49] and some allergy symptoms.[29] In addition to these uses, botulinum toxin is being evaluated for use in treating chronic pain.[50] Studies show that botulinum toxin may be injected into arthritic shoulder joints to reduce chronic pain and improve range of motion.[51] The use of botulinum toxin A in children with cerebral palsy is safe in the upper and lower limb muscles.[13][14]
Side effects
While botulinum toxin is generally considered safe in a clinical setting, serious side effects from its use can occur. Most commonly, botulinum toxin can be injected into the wrong muscle group or with time spread from the injection site, causing temporary paralysis of unintended muscles.[52]
Side effects from cosmetic use generally result from unintended paralysis of facial muscles. These include partial facial paralysis, muscle weakness, and trouble swallowing. Side effects are not limited to direct paralysis, however, and can also include headaches, flu-like symptoms, and allergic reactions.[53] Just as cosmetic treatments only last a number of months, paralysis side effects can have the same durations.[54] At least in some cases, these effects are reported to dissipate in the weeks after treatment.[55] Bruising at the site of injection is not a side effect of the toxin, but rather of the mode of administration, and is reported as preventable if the clinician applies pressure to the injection site; when it occurs, it is reported in specific cases to last 7–11 days.[56] When injecting the masseter muscle of the jaw, loss of muscle function can result in a loss or reduction of power to chew solid foods.[53] With continued high doses, the muscles can atrophy or lose strength; research has shown that those muscles rebuild after a break from Botox.[57]
Side effects from therapeutic use can be much more varied depending on the location of injection and the dose of toxin injected. In general, side effects from therapeutic use can be more serious than those that arise during cosmetic use. These can arise from paralysis of critical muscle groups and can include arrhythmia, heart attack, and in some cases, seizures, respiratory arrest, and death.[53] Additionally, side effects common in cosmetic use are also common in therapeutic use, including trouble swallowing, muscle weakness, allergic reactions, and flu-like syndromes.[53]
In response to the occurrence of these side effects, in 2008, the FDA notified the public of the potential dangers of the botulinum toxin as a therapeutic. Namely, the toxin can spread to areas distant from the site of injection and paralyze unintended muscle groups, especially when used for treating muscle spasticity in children treated for cerebral palsy.[58] In 2009, the FDA announced that boxed warnings would be added to available botulinum toxin products, warning of their ability to spread from the injection site.[59] However, the clinical use of botulinum toxin A in cerebral palsy children has been proven to be safe with minimal side effects.[13][14] Additionally, the FDA announced name changes to several botulinum toxin products, to emphasize that the products are not interchangeable and require different doses for proper use. Botox and Botox Cosmetic were given the INN of onabotulinumtoxinA, Myobloc as rimabotulinumtoxinB, and Dysport retained its INN of abobotulinumtoxinA.[59] In conjunction with this, the FDA issued a communication to health care professionals reiterating the new drug names and the approved uses for each.[60] A similar warning was issued by Health Canada in 2009, warning that botulinum toxin products can spread to other parts of the body.[61]
Role in disease
Botulinum toxin produced by Clostridium botulinum is the cause of botulism.[27] Humans most commonly ingest the toxin from eating improperly canned foods in which C. botulinum has grown. However, the toxin can also be introduced through an infected wound. In infants, the bacteria can sometimes grow in the intestines and produce botulinum toxin within the intestine and can cause a condition known as floppy baby syndrome.[62] In all cases, the toxin can then spread, blocking nerves and muscle function. In severe cases, the toxin can block nerves controlling the respiratory system or heart, resulting in death.[7] Botulism can be difficult to diagnose, as it may appear similar to diseases such as Guillain–Barré syndrome, myasthenia gravis, and stroke. Other tests, such as brain scan and spinal fluid examination, may help to rule out other causes. If the symptoms of botulism are diagnosed early, various treatments can be administered. In an effort to remove contaminated food that remains in the gut, enemas or induced vomiting may be used.[63] For wound infections, infected material may be removed surgically.[63] Botulinum antitoxin is available and may be used to prevent the worsening of symptoms, though it will not reverse existing nerve damage. In severe cases, mechanical respiration may be used to support patients with respiratory failure.[63] The nerve damage heals over time, generally over weeks to months.[16] With proper treatment, the case fatality rate for botulinum poisoning can be greatly reduced.[63]
Two preparations of botulinum antitoxins are available for treatment of botulism. Trivalent (serotypes A, B, E) botulinum antitoxin is derived from equine sources using whole antibodies. The second antitoxin is heptavalent botulinum antitoxin (serotypes A, B, C, D, E, F, G), which is derived from equine antibodies that have been altered to make them less immunogenic. This antitoxin is effective against all main strains of botulism.[64][12]
Mechanism of action

Botulinum toxin exerts its effect by cleaving key proteins required for nerve activation. First, the toxin binds specifically to presynaptic surface of neurons that use the neurotransmitter acetylcholine.[66] Once bound to the nerve terminal, the neuron takes up the toxin into a vesicle by receptor-mediated endocytosis.[67] As the vesicle moves farther into the cell, it acidifies, activating a portion of the toxin that triggers it to push across the vesicle membrane and into the cell cytoplasm.[7] BoNTs recognize distinct classes of receptors simultaneously (gangliosides, synaptotagmin and SV2).[68] Once inside the cytoplasm, the toxin cleaves SNARE proteins (proteins that mediate vesicle fusion, with their target membrane bound compartments) meaning that the acetylcholine vesicles cannot bind to the intracellular cell membrane,[67] preventing the cell from releasing vesicles of neurotransmitter. This stops nerve signaling, leading to paralysis.[7]
The toxin itself is released from the bacterium as a single chain, then becomes activated when cleaved by its own proteases.[29] The active form consists of a two-chain protein composed of a 100-kDa heavy chain polypeptide joined via disulfide bond to a 50-kDa light chain polypeptide.[69] The heavy chain contains domains with several functions; it has the domain responsible for binding specifically to presynaptic nerve terminals, as well as the domain responsible for mediating translocation of the light chain into the cell cytoplasm as the vacuole acidifies.[7][69] The light chain is a M27-family zinc metalloprotease and is the active part of the toxin. It is translocated into the host cell cytoplasm where it cleaves the host protein SNAP-25, a member of the SNARE protein family, which is responsible for fusion. The cleaved SNAP-25 cannot mediate fusion of vesicles with the host cell membrane, thus preventing the release of the neurotransmitter acetylcholine from axon endings.[7] This blockage is slowly reversed as the toxin loses activity and the SNARE proteins are slowly regenerated by the affected cell.[7]
The seven toxin serotypes (A–G) are traditionally separated by their antigenicity. They have different tertiary structures and sequence differences.[69][70] While the different toxin types all target members of the SNARE family, different toxin types target different SNARE family members.[65] The A, B, and E serotypes cause human botulism, with the activities of types A and B enduring longest in vivo (from several weeks to months).[69] Existing toxin types can recombine to create "hybrid" (mosaic, chimeric) types. Examples include BoNT/CD, BoNT/DC, and BoNT/FA, with the first letter indicating the light chain type and the latter indicating the heavy chain type.[71] BoNT/FA received considerable attention under the name "BoNT/H", as it was mistakenly thought it could not be neutralized by any existing antitoxin.[12]
Botulinum toxins are closely related to tetanus toxin; the two are collectively known as Clostridium neurotoxins and the light chain is classified by MEROPS as family M27. Nonclassical types include BoNT/X (P0DPK1), which is toxic in mice and possibly in humans;[11] a BoNT/J (A0A242DI27) found in cow Enterococcus;[72] and a BoNT/Wo (A0A069CUU9) found in the rice-colonizing Weissella oryzae.[71]
History
Initial descriptions and discovery of Clostridium botulinum
One of the earliest recorded outbreaks of foodborne botulism occurred in 1793 in the village of Wildbad in what is now Baden-Württemberg, Germany. Thirteen people became sick and six died after eating pork stomach filled with blood sausage, a local delicacy. Additional cases of fatal food poisoning in Württemberg led the authorities to issue a public warning against consuming smoked blood sausages in 1802 and to collect case reports of "sausage poisoning".[73] Between 1817 and 1822, the German physician Justinus Kerner published the first complete description of the symptoms of botulism, based on extensive clinical observations and animal experiments. He concluded that the toxin develops in bad sausages under anaerobic conditions, is a biological substance, acts on the nervous system, and is lethal even in small amounts.[73] Kerner hypothesized that this "sausage toxin" could be used to treat a variety of diseases caused by an overactive nervous system, making him the first to suggest that it could be used therapeutically.[74] In 1870, the German physician Müller coined the term "botulism" to describe the disease caused by sausage poisoning, from the Latin word botulus, meaning "sausage".[74]
In 1895 Émile van Ermengem, a Belgian microbiologist, discovered what is now called Clostridium botulinum and confirmed that a toxin produced by the bacteria causes botulism.[75] On 14 December 1895, there was a large outbreak of botulism in the Belgian village of Ellezelles that occurred at a funeral where people ate pickled and smoked ham; three of them died. By examining the contaminated ham and performing autopsies on the people who died after eating it, van Ermengem isolated an anaerobic microorganism that he called Bacillus botulinus.[73] He also performed experiments on animals with ham extracts, isolated bacterial cultures, and toxins extracts from the bacteria. From these he concluded that the bacteria themselves do not cause foodborne botulism, but rather produce a toxin that causes the disease after it is ingested.[76] As a result of Kerner's and van Ermengem's research, it was thought that only contaminated meat or fish could cause botulism. This idea was refuted in 1904 when a botulism outbreak occurred in Darmstadt, Germany, because of canned white beans. In 1910, the German microbiologist J. Leuchs published a paper showing that the outbreaks in Ellezelles and Darmstadt were caused by different strains of Bacillus botulinus and that the toxins were serologically distinct.[73] In 1917, Bacillus botulinus was renamed Clostridium botulinum, as it was decided that term Bacillus should only refer to a group of aerobic microorganisms, while Clostridium would be only used to describe a group of anaerobic microorganisms.[75] In 1919, Georgina Burke used toxin-antitoxin reactions to identify two strains of Clostridium botulinum, which she designated A and B.[75]
Food canning
This section needs additional citations for verification. (August 2018) |
Over the next three decades, 1895–1925, as food canning was approaching a billion-dollar-a-year industry, botulism was becoming a public health hazard. Karl Friedrich Meyer, a Swiss-American veterinary scientist, created a center at the Hooper Foundation in San Francisco, where he developed techniques for growing the organism and extracting the toxin, and conversely, for preventing organism growth and toxin production, and inactivating the toxin by heating. The California canning industry was thereby preserved.[77]
World War II
With the outbreak of World War II, weaponization of botulinum toxin was investigated at Fort Detrick in Maryland. Carl Lamanna and James Duff[78] developed the concentration and crystallization techniques that Edward J. Schantz used to create the first clinical product. When the Army's Chemical Corps was disbanded, Schantz moved to the Food Research Institute in Wisconsin, where he manufactured toxin for experimental use and provided it to the academic community.
The mechanism of botulinum toxin action – blocking the release of the neurotransmitter acetylcholine from nerve endings – was elucidated in the mid-20th century,[79] and remains an important research topic. Nearly all toxin treatments are based on this effect in various body tissues.
Strabismus
Ophthalmologists specializing in eye muscle disorders (strabismus) had developed the method of EMG-guided injection (using the electromyogram, the electrical signal from an activated muscle, to guide injection) of local anesthetics as a diagnostic technique for evaluating an individual muscle's contribution to an eye movement.[80] Because strabismus surgery frequently needed repeating, a search was undertaken for non-surgical, injection treatments using various anesthetics, alcohols, enzymes, enzyme blockers, and snake neurotoxins. Finally, inspired by Daniel B. Drachman's work with chicks at Johns Hopkins,[81] Alan B. Scott and colleagues injected botulinum toxin into monkey extraocular muscles.[82] The result was remarkable; a few picograms induced paralysis that was confined to the target muscle, long in duration, and without side effects.
After working out techniques for freeze-drying, buffering with albumin, and assuring sterility, potency, and safety, Scott applied to the FDA for investigational drug use, and began manufacturing botulinum type A neurotoxin in his San Francisco lab. He injected the first strabismus patients in 1977, reported its clinical utility in 1980,[83] and had soon trained hundreds of ophthalmologists in EMG-guided injection of the drug he named Oculinum ("eye aligner").
In 1986, Oculinum Inc, Scott's micromanufacturer and distributor of botulinum toxin, was unable to obtain product liability insurance, and could no longer supply the drug. As supplies became exhausted, patients who had come to rely on periodic injections became desperate. For four months, as liability issues were resolved, American blepharospasm patients traveled to Canadian eye centers for their injections.[84]
Based on data from thousands of patients collected by 240 investigators, Oculinum Inc (which was soon acquired by Allergan) received FDA approval in 1989 to market Oculinum for clinical use in the United States to treat adult strabismus and blepharospasm. Allergan then began using the trademark Botox.[85] This original approval was granted under the 1983 US Orphan Drug Act.[86]
Cosmetics

The effect of BTX-A on reducing and eliminating forehead wrinkles was first described and published by Richard Clark, MD, a plastic surgeon from Sacramento, California. In 1987 Clark was challenged with eliminating the disfigurement caused by only the right side of the forehead muscles functioning after the left side of the forehead was paralyzed during a facelift procedure. This patient had desired to look better from her facelift, but was experiencing bizarre unilateral right forehead eyebrow elevation while the left eyebrow drooped, and she constantly demonstrated deep expressive right forehead wrinkles while the left side was perfectly smooth due to the paralysis. Clark was aware that Botulinum toxin was safely being used to treat babies with strabismus and he requested and was granted FDA approval to experiment with Botulinum toxin to paralyze the moving and wrinkling normal functioning right forehead muscles to make both sides of the forehead appear the same. This study and case report of the cosmetic use of Botulinum toxin to treat a cosmetic complication of a cosmetic surgery was the first report on the specific treatment of wrinkles and was published in the journal Plastic and Reconstructive Surgery in 1989.[87] Editors of the journal of the American Society of Plastic Surgeons have clearly stated "the first described use of the toxin in aesthetic circumstances was by Clark and Berris in 1989."[88]
Jean and Alastair Carruthers observed that blepharospasm patients who received injections around the eyes and upper face also enjoyed diminished facial glabellar lines ("frown lines" between the eyebrows). Alastair Carruthers reported that others at the time also noticed these effects and discussed the cosmetic potential of botulinum toxin.[89] Unlike other investigators, the Carruthers did more than just talk about the possibility of using botulinum toxin cosmetically. They conducted a clinical study on otherwise normal individuals whose only concern was their eyebrow furrow. They performed their study during 1987-1989 and presented their results at the 1990 annual meeting of the American Society for Dermatologic Surgery. Their findings were subsequently published in 1992.[90]
Chronic pain
William J. Binder reported in 2000, that patients who had cosmetic injections around the face reported relief from chronic headache.[91] This was initially thought to be an indirect effect of reduced muscle tension, but the toxin is now known to inhibit release of peripheral nociceptive neurotransmitters, suppressing the central pain processing systems responsible for migraine headache.[92][93]
Society and culture
Economics
This article needs to be updated. (October 2017) |
As of 2018[update], botulinum toxin injections are the most common cosmetic operation, with 7.4 million procedures in the United States, according to the American Society of Plastic Surgeons.[94] Qualifications for Botox injectors vary by county, state, and country. Botox cosmetic providers include dermatologists, plastic surgeons, aesthetic spa physicians, dentists, nurse practitioners, nurses, and physician assistants.[95]
The global market for botulinum toxin products, driven by their cosmetic applications, was forecast to reach $2.9 billion by 2018. The facial aesthetics market, of which they are a component, was forecast to reach $4.7 billion ($2 billion in the U.S.) in the same timeframe.[96]
- Global Market
- In 2019, 6,271,488 Botulinum Toxin procedures were administered worldwide.[97] The Global Botulinum Toxin market size was US$4.83 billion in 2019 and is projected to reach US$7.71 billion by 2027.[97]
- US Market
- In 2020, 4,401,536 Botulinum Toxin Type A procedures were administered.[98] In 2019 the botulinum Toxin market made US$3.19 billion.[97]
- Botox cost
- Botox cost is generally determined by the number of units administered (avg. $10.00 - $30.00 per unit) or by the area ($200–1000) and depends on expertise of a physician, clinic location, number of units, and treatment complexity.
- Insurance
- Botox for medical purposes is usually covered by insurance if deemed medically necessary by your doctor and covers a plethora of medical problems including overactive bladder (OAB), urinary incontinence due to neurologic conditions, headaches and migraines, TMJ, spasticity in adult patients, cervical dystonia in adult patients, severe axillary hyperhidrosis (or other areas of the body), blepharospasm, upper or lower limb spasticity.[99][100]
- Migraines
- For migraine induced headaches the FDA-recommended dosage is 155 units and costs between $300 to $600 per treatment out of pocket when covered by insurance.[101]
- Hyperhidrosis
- Botox for excessive sweating is FDA approved.[52]
- Cosmetic
- Standard areas for aesthetics botox injections include facial and other areas that can form fine lines and wrinkles due to every day muscle contractions and/or facial expressions such as smiling, frowning, squinting, and raising eyebrows. These areas include the glabellar region between the eyebrows, horizontal lines on the forehead, crow's feet around the eyes, and even circular bands that form around the neck secondary to platysmal hyperactivity.[102]
Bioterrorism
Botulinum toxin has been recognized as a potential agent for use in bioterrorism.[103] It can be absorbed through the eyes, mucous membranes, respiratory tract, and non-intact skin.[104] The effects of botulinum toxin are different from those of nerve agents involved insofar in that botulism symptoms develop relatively slowly (over several days), while nerve agent effects are generally much more rapid. Evidence suggests that nerve exposure (simulated by injection of atropine and pralidoxime) will increase mortality by enhancing botulinum toxin's mechanism of toxicity.[105] With regard to detection, protocols using NBC detection equipment (such as M-8 paper or the ICAM) will not indicate a "positive" when samples containing botulinum toxin are tested.[106] To confirm a diagnosis of botulinum toxin poisoning, therapeutically or to provide evidence in death investigations, botulinum toxin may be quantitated by immunoassay of human biological fluids; serum levels of 12–24 mouse LD50 units per milliliter have been detected in poisoned patients.[107]
Japanese doomsday cult Aum Shinrikyo produced botulinum toxin and spread it as an aerosol in downtown Tokyo during the 1990s, but the attacks caused no fatalities.[108]
During the early 1980s, German and French newspapers reported that the police had raided a Baader-Meinhof gang safe house in Paris and had found a makeshift laboratory that contained flasks full of Clostridium botulinum, which makes botulinum toxin. Their reports were later found to be incorrect; no such lab was ever found.[109]
Brand names
The examples and perspective in this article deal primarily with the United States and do not represent a worldwide view of the subject. (April 2017) |
Botulinum toxin A is sold under the brand names Jeuveau, Botox, and Xeomin. Botulinum toxin B is sold under the brand name Myobloc.[5]
In the United States, botulinum toxin products are manufactured by a variety of companies, for both therapeutic and cosmetic use. A U.S. supplier reported in its company materials in 2011 that it could "supply the world's requirements for 25 indications approved by Government agencies around the world" with less than one gram of raw botulinum toxin.[110] Myobloc or Neurobloc, a botulinum toxin type B product, is produced by Solstice Neurosciences, a subsidiary of US WorldMeds. AbobotulinumtoxinA), a therapeutic formulation of the type A toxin manufactured by Galderma in the United Kingdom, is licensed for the treatment of focal dystonias and certain cosmetic uses in the U.S. and other countries.[60]
Besides the three primary U.S. manufacturers, numerous other botulinum toxin producers are known. Xeomin, manufactured in Germany by Merz, is also available for both therapeutic and cosmetic use in the U.S.[111] Lanzhou Institute of Biological Products in China manufactures a BTX-A product; as of 2014, it was the only BTX-A approved in China.[111] BTX-A is also sold as Lantox and Prosigne on the global market.[112] Neuronox, a BTX-A product, was introduced by Medy-Tox Inc. of South Korea in 2009.[113]
Toxin production
Botulism toxins are produced by bacteria of the genus Clostridium, namely C. botulinum, C. butyricum, C. baratii and C. argentinense,[114] which are widely distributed, including in soil and dust. Also, the bacteria can be found inside homes on floors, carpet, and countertops even after cleaning.[115] Food-borne botulism results, indirectly, from ingestion of food contaminated with Clostridium spores, where exposure to an anaerobic environment allows the spores to germinate, after which the bacteria can multiply and produce toxin.[115] Critically, ingestion of toxin rather than spores or vegetative bacteria causes botulism.[115] Botulism is nevertheless known to be transmitted through canned foods not cooked correctly before canning or after can opening, so is preventable.[115] Infant botulism arising from consumption of honey or any other food that can carry these spores can be prevented by eliminating these foods from diets of children less than 12 months old.[116]
Organism and toxin susceptibilities
This section needs expansion with: modern content and referencing on antibiotic susceptibilities. You can help by adding to it. (February 2015) |
Proper refrigeration at temperatures below 3 °C (38 °F) slows the growth of C. botulinum. The organism is also susceptible to high salt, high oxygen, and low pH levels.[16] The toxin itself is rapidly destroyed by heat, such as in thorough cooking.[117] The spores that produce the toxin are heat-tolerant and will survive boiling water for an extended period of time.[118]
The botulinum toxin is denatured and thus deactivated at temperatures greater than 85 °C (185 °F) for five minutes.[119] As a zinc metalloprotease (see below), the toxin's activity is also susceptible, post-exposure, to inhibition by protease inhibitors, e.g., zinc-coordinating hydroxamates.[69][120]
Research
Blepharospasm and strabismus
University-based ophthalmologists in the US and Canada further refined the use of botulinum toxin as a therapeutic agent. By 1985, a scientific protocol of injection sites and dosage had been empirically determined for treatment of blepharospasm and strabismus.[121] Side effects in treatment of this condition were deemed to be rare, mild and treatable.[122] The beneficial effects of the injection lasted only 4–6 months. Thus, blepharospasm patients required re-injection two or three times a year.[123]
In 1986, Scott's micromanufacturer and distributor of Botox was no longer able to supply the drug because of an inability to obtain product liability insurance. Patients became desperate, as supplies of Botox were gradually consumed, forcing him to abandon patients who would have been due for their next injection. For a period of four months, American blepharospasm patients had to arrange to have their injections performed by participating doctors at Canadian eye centers until the liability issues could be resolved.[84]
In December 1989, Botox was approved by the U.S. FDA for the treatment of strabismus, blepharospasm, and hemifacial spasm in patients over 12 years old.[85]
Botox has not been approved for any pediatric use.[60] It has, however, been used off-label by physicians for several conditions, including spastic conditions in pediatric patients with cerebral palsy, a therapeutic course that has resulted in patient deaths.[60] In the case of treatment of infantile esotropia in patients younger than 12 years of age, several studies have yielded differing results.[44][better source needed]
Cosmetic
The effect of BTX-A on reducing and eliminating forehead wrinkles was first described and published by Richard Clark, MD a plastic surgeon from Sacramento, California. In 1987 Clark was challenged with eliminating the disfigurement caused by only the right side of the forehead muscles functioning after the left side of the forehead was paralyzed during a facelift procedure. This patient had desired to look better from her facelift, but was experiencing bizarre unilateral right forehead eyebrow elevation while the left eyebrow drooped and she emoted with deep expressive right forehead wrinkles while the left side was perfectly smooth due to the paralysis. Clark was aware that Botulinum toxin was safely being used to treat babies with strabismus and he requested and was granted FDA approval to experiment with Botulinum toxin to paralyze the moving and wrinkling normal functioning right forehead muscles to make both sides of the forehead appear the same. This study and case report of the Cosmetic use of Botulinum toxin to treat a Cosmetic complication of a Cosmetic surgery was the first report on the specific treatment of wrinkles and was published in the journal Plastic and Reconstructive Surgery in 1989.[87] Editors of the journal of the American Society of Plastic Surgeons have clearly stated "the first described use of the toxin in aesthetic circumstances was by Clark and Berris in 1989."[88]
JD and JA Carruthers also studied and reported in 1992 the use of BTX-A as a cosmetic treatment.[78] They conducted a study of patients whose only concern was their glabellar forehead wrinkle or furrow. Study participants were otherwise normal. Sixteen of seventeen patients available for follow-up demonstrated a cosmetic improvement. This study was reported at a meeting in 1991. The study for the treatment of glabellar frown lines was published in 1992.[90] This result was subsequently confirmed by other groups (Brin, and the Columbia University group under Monte Keen[124]). The FDA announced regulatory approval of botulinum toxin type A (Botox Cosmetic) to temporarily improve the appearance of moderate-to-severe frown lines between the eyebrows (glabellar lines) in 2002 after extensive clinical trials.[125] Well before this, the cosmetic use of botulinum toxin type A became widespread.[126] The results of Botox Cosmetic can last up to four months and may vary with each patient.[127] The U.S. Food and Drug Administration (FDA) approved an alternative product-safety testing method in response to increasing public concern that LD50 testing was required for each batch sold in the market.[128][129]
BTX-A has also been used in the treatment of gummy smiles,[130] the material is injected into the hyperactive muscles of upper lip, which causes a reduction in the upward movement of lip thus resulting in a smile with a less exposure of gingiva.[131] Botox is usually injected in the three lip elevator muscles that converge on the lateral side of the ala of the nose; the levator labii superioris (LLS), the levator labii superioris alaeque nasi muscle (LLSAN), and the zygomaticus minor (ZMi).[132][133]
Upper motor neuron syndrome
BTX-A is now a common treatment for muscles affected by the upper motor neuron syndrome (UMNS), such as cerebral palsy,[13] for muscles with an impaired ability to effectively lengthen. Muscles affected by UMNS frequently are limited by weakness, loss of reciprocal inhibition, decreased movement control, and hypertonicity (including spasticity). In January 2014, Botulinum toxin was approved by UK's Medicines and Healthcare products Regulatory Agency (MHRA) for the treatment of ankle disability due to lower limb spasticity associated with stroke in adults.[38] Joint motion may be restricted by severe muscle imbalance related to the syndrome, when some muscles are markedly hypertonic, and lack effective active lengthening. Injecting an overactive muscle to decrease its level of contraction can allow improved reciprocal motion, so improved ability to move and exercise.[13]
Sialorrhea
Sialorrhea is a condition where oral secretions are unable to be eliminated, causing pooling of saliva in the mouth. This condition can be caused by various neurological syndromes such as Bell's_palsy, intellectual disability, and cerebral palsy. Injection of BTX-A into salivary glands is useful in reducing the secretions.[134]
Cervical dystonia
BTX-A is commonly used to treat cervical dystonia, but it can become ineffective after a time. Botulinum toxin type B (BTX-B) received FDA approval for treatment of cervical dystonia on 21 December 2000. Brand names for BTX-B are Myobloc in the United States, and Neurobloc in the European Union.[111]
Chronic migraine
Onabotulinumtoxin A (trade name Botox) received FDA approval for treatment of chronic migraines on 15 October 2010. The toxin is injected into the head and neck to treat these chronic headaches. Approval followed evidence presented to the agency from two studies funded by Allergan showing a very slight improvement in incidence of chronic migraines for those with migraines undergoing the Botox treatment.[135][136]
Since then, several randomized control trials have shown botulinum toxin type A to improve headache symptoms and quality of life when used prophylactically for patients with chronic migraine[137] who exhibit headache characteristics consistent with: pressure perceived from outside source, shorter total duration of chronic migraines (<30 years), "detoxification" of patients with coexisting chronic daily headache due to medication overuse, and no current history of other preventive headache medications.[138]
Depression
A few small trials have found benefits in people with depression.[139][140] Research is based on the facial feedback hypothesis.[141]
Premature ejaculation
The drug for the treatment of premature ejaculation has been under development since 7 August 2013, and is in Phase II of the FDA trials.[140][142]
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Regulatory Decision Summary - Botox". Health Canada. 23 October 2014. Archived from the original on 12 June 2022. Retrieved 12 June 2022.
- ^ "Regulatory Decision Summary - Nuceiva". Health Canada. 23 October 2014. Archived from the original on 7 June 2022. Retrieved 11 June 2022.
- ^ "Botox- onabotulinumtoxina injection, powder, lyophilized, for solution". DailyMed. 30 July 2021. Archived from the original on 2 June 2022. Retrieved 12 June 2022.
- ^ a b "Myobloc- rimabotulinumtoxinb injection, solution". DailyMed. 22 March 2021. Archived from the original on 2 June 2022. Retrieved 12 June 2022.
- ^ "Dysport- botulinum toxin type a injection, powder, lyophilized, for solution". DailyMed. 28 February 2022. Archived from the original on 2 June 2022. Retrieved 12 June 2022.
- ^ a b c d e f g Montecucco C, Molgó J (June 2005). "Botulinal neurotoxins: revival of an old killer". Current Opinion in Pharmacology. 5 (3): 274–279. doi:10.1016/j.coph.2004.12.006. PMID 15907915.
- ^ Figgitt DP, Noble S (2002). "Botulinum toxin B: a review of its therapeutic potential in the management of cervical dystonia". Drugs. 62 (4): 705–722. doi:10.2165/00003495-200262040-00011. PMID 11893235. S2CID 46981635.
- ^ Janes LE, Connor LM, Moradi A, Alghoul M (April 2021). "Current Use of Cosmetic Toxins to Improve Facial Aesthetics". Plastic and Reconstructive Surgery. 147 (4): 644e–657e. doi:10.1097/PRS.0000000000007762. PMID 33776040. S2CID 232408799.
- ^ Rosales RL, Bigalke H, Dressler D (February 2006). "Pharmacology of botulinum toxin: differences between type A preparations". European Journal of Neurology. 13 (Suppl 1): 2–10. doi:10.1111/j.1468-1331.2006.01438.x. PMID 16417591. S2CID 32387953.
- ^ a b "Botulism toxin X: Time to update the textbooks, thanks to genomic sequencing". Boston Children's Hospital. 7 August 2017. Archived from the original on 14 September 2021. Retrieved 28 October 2019.
- ^ a b c "Study: Novel botulinum toxin less dangerous than thought". CIDRAP. University of Minnesota. 17 June 2015. Archived from the original on 28 October 2019. Retrieved 28 October 2019.
- ^ a b c d e f Farag SM, Mohammed MO, El-Sobky TA, ElKadery NA, ElZohiery AK (March 2020). "Botulinum Toxin A Injection in Treatment of Upper Limb Spasticity in Children with Cerebral Palsy: A Systematic Review of Randomized Controlled Trials". JBJS Reviews. 8 (3): e0119. doi:10.2106/JBJS.RVW.19.00119. PMC 7161716. PMID 32224633.
- ^ a b c d Blumetti FC, Belloti JC, Tamaoki MJ, Pinto JA (October 2019). "Botulinum toxin type A in the treatment of lower limb spasticity in children with cerebral palsy". The Cochrane Database of Systematic Reviews. 2019 (10): CD001408. doi:10.1002/14651858.CD001408.pub2. PMC 6779591. PMID 31591703.
- ^ American Society of Health-System Pharmacists (27 October 2011). "OnabotulinumtoxinA (Botulinum Toxin Type A) Monograph for Professionals". drugs.com. Archived from the original on 6 September 2015. Retrieved 4 March 2015.
- ^ a b c "Botulism: Key facts". World Health Organization. Archived from the original on 20 December 2017. Retrieved 22 January 2022.
- ^ Košenina S, Masuyer G, Zhang S, Dong M, Stenmark P (June 2019). "Crystal structure of the catalytic domain of the Weissella oryzae botulinum-like toxin". FEBS Letters. 593 (12): 1403–1410. doi:10.1002/1873-3468.13446. PMID 31111466.
- ^ Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, et al. (February 2001). "Botulinum toxin as a biological weapon: medical and public health management". JAMA. 285 (8): 1059–1070. doi:10.1001/jama.285.8.1059. PMID 11209178.
- ^ "Drug Approval Package: Dysport (abobotulinumtoxin) NDA #125274s000". U.S. Food and Drug Administration (FDA). 17 August 2011. Archived from the original on 24 November 2019. Retrieved 23 November 2019.
- ^ "Drug Approval Package: Brand Name (Generic Name) NDA #". accessdata.fda.gov. 24 December 1999. Archived from the original on 27 July 2020. Retrieved 23 November 2019.
- ^ "Drug Approval Package: Jeuveau". U.S. Food and Drug Administration (FDA). 5 March 2019. Archived from the original on 23 November 2019. Retrieved 22 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b Krause R (10 June 2019). "Jeuveau, The Most Affordable Wrinkle Injectable". refinery29.com. Archived from the original on 18 March 2021. Retrieved 9 July 2019.
- ^ Al-Ghamdi AS, Alghanemy N, Joharji H, Al-Qahtani D, Alghamdi H (January 2015). "Botulinum toxin: Non cosmetic and off-label dermatological uses". Journal of Dermatology & Dermatologic Surgery. 19 (1): 1–8. doi:10.1016/j.jdds.2014.06.002.
- ^ Ozcakir S, Sivrioglu K (June 2007). "Botulinum toxin in poststroke spasticity". Clinical Medicine & Research. 5 (2): 132–138. doi:10.3121/cmr.2007.716. PMC 1905930. PMID 17607049.
- ^ Yan X, Lan J, Liu Y, Miao J (November 2018). "Efficacy and Safety of Botulinum Toxin Type A in Spasticity Caused by Spinal Cord Injury: A Randomized, Controlled Trial". Medical Science Monitor. 24: 8160–8171. doi:10.12659/MSM.911296. PMC 6243868. PMID 30423587.
- ^ "Cervical dystonia - Symptoms and causes". Mayo Clinic. 28 January 2014. Archived from the original on 12 December 2018. Retrieved 14 October 2015.
- ^ a b Shukla HD, Sharma SK (2005). "Clostridium botulinum: a bug with beauty and weapon". Critical Reviews in Microbiology. 31 (1): 11–18. doi:10.1080/10408410590912952. PMID 15839401. S2CID 2855356.
- ^ Pacik PT (December 2009). "Botox treatment for vaginismus". Plastic and Reconstructive Surgery. 124 (6): 455e–456e. doi:10.1097/PRS.0b013e3181bf7f11. PMID 19952618.
- ^ a b c d Felber ES (October 2006). "Botulinum toxin in primary care medicine". The Journal of the American Osteopathic Association. 106 (10): 609–614. doi:10.7556/jaoa. PMID 17122031. S2CID 245177279.
- ^ Stavropoulos SN, Friedel D, Modayil R, Iqbal S, Grendell JH (March 2013). "Endoscopic approaches to treatment of achalasia". Therapeutic Advances in Gastroenterology. 6 (2): 115–135. doi:10.1177/1756283X12468039. PMC 3589133. PMID 23503707.
- ^ Long H, Liao Z, Wang Y, Liao L, Lai W (February 2012). "Efficacy of botulinum toxins on bruxism: an evidence-based review". International Dental Journal. 62 (1): 1–5. doi:10.1111/j.1875-595X.2011.00085.x. PMC 9374973. PMID 22251031.
- ^ Mangera A, Andersson KE, Apostolidis A, Chapple C, Dasgupta P, Giannantoni A, et al. (October 2011). "Contemporary management of lower urinary tract disease with botulinum toxin A: a systematic review of botox (onabotulinumtoxinA) and dysport (abobotulinumtoxinA)". European Urology. 60 (4): 784–795. doi:10.1016/j.eururo.2011.07.001. PMID 21782318.
- ^ Villalba H, Villalba S, Abbas MA (2007). "Anal fissure: a common cause of anal pain". The Permanente Journal. 11 (4): 62–65. doi:10.7812/tpp/07-072. PMC 3048443. PMID 21412485.
- ^ Duthie JB, Vincent M, Herbison GP, Wilson DI, Wilson D (December 2011). Duthie JB (ed.). "Botulinum toxin injections for adults with overactive bladder syndrome". The Cochrane Database of Systematic Reviews (12): CD005493. doi:10.1002/14651858.CD005493.pub3. PMID 22161392.
- ^ Scott AB (1994). "Change of eye muscle sarcomeres according to eye position". Journal of Pediatric Ophthalmology and Strabismus. 31 (2): 85–88. doi:10.3928/0191-3913-19940301-05. PMID 8014792.
- ^ Simpson L (2 December 2012). Botulinum Neurotoxin and Tetanus Toxin. Elsevier. ISBN 978-0-323-14160-4. Archived from the original on 28 August 2021. Retrieved 1 October 2020.
- ^ "Could my child's crossed eye be corrected with Botox? - Boston Children's Hospital". Thriving Blog. 14 April 2017. Archived from the original on 25 January 2021. Retrieved 13 March 2020.
- ^ a b UK Approves New Botox Use Archived 22 February 2014 at the Wayback Machine. dddmag.com. 4 February 2014
- ^ "UK's MHRA approves Botox for treatment of ankle disability in stroke survivors". www.thepharmaletter.com. Archived from the original on 27 July 2020. Retrieved 16 March 2020.
- ^ "FDA Approved Drug Products – Dysport". U.S. Food and Drug Administration (FDA). Archived from the original on 8 November 2016. Retrieved 7 November 2016.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Pavone V, Testa G, Restivo DA, Cannavò L, Condorelli G, Portinaro NM, Sessa G (19 February 2016). "Botulinum Toxin Treatment for Limb Spasticity in Childhood Cerebral Palsy". Frontiers in Pharmacology. 7: 29. doi:10.3389/fphar.2016.00029. PMC 4759702. PMID 26924985.
- ^ Syed YY (August 2017). "AbobotulinumtoxinA: A Review in Pediatric Lower Limb Spasticity". Paediatric Drugs. 19 (4): 367–373. doi:10.1007/s40272-017-0242-4. PMID 28623614. S2CID 24857218.
- ^ Wittich CM, Burkle CM, Lanier WL (October 2012). "Ten common questions (and their answers) about off-label drug use". Mayo Clinic Proceedings. 87 (10): 982–990. doi:10.1016/j.mayocp.2012.04.017. PMC 3538391. PMID 22877654.
- ^ a b Ocampo VV, Foster CS (30 May 2012). "Infantile Esotropia Treatment & Management". Medscape. Archived from the original on 28 November 2014. Retrieved 6 April 2014.
- ^ a b Eisenach JH, Atkinson JL, Fealey RD (May 2005). "Hyperhidrosis: evolving therapies for a well-established phenomenon". Mayo Clinic Proceedings. 80 (5): 657–666. doi:10.4065/80.5.657. PMID 15887434.
- ^ "FDA Approves Botox to Treat Chronic Migraines". WebMD. Archived from the original on 5 May 2017. Retrieved 12 May 2017.
- ^ Satriyasa BK (10 April 2019). "Botulinum toxin (Botox) A for reducing the appearance of facial wrinkles: a literature review of clinical use and pharmacological aspect". Clinical, Cosmetic and Investigational Dermatology. 12: 223–228. doi:10.2147/CCID.S202919. PMC 6489637. PMID 31114283.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d e Small R (August 2014). "Botulinum toxin injection for facial wrinkles". American Family Physician. 90 (3): 168–175. PMID 25077722.
- ^ Mittal SO, Safarpour D, Jabbari B (February 2016). "Botulinum Toxin Treatment of Neuropathic Pain". Seminars in Neurology. 36 (1): 73–83. doi:10.1055/s-0036-1571953. PMID 26866499.
- ^ Charles PD (November 2004). "Botulinum neurotoxin serotype A: a clinical update on non-cosmetic uses". American Journal of Health-System Pharmacy. 61 (22 Suppl 6): S11–S23. doi:10.1093/ajhp/61.suppl_6.S11. PMID 15598005.
- ^ Singh JA, Fitzgerald PM (September 2010). "Botulinum toxin for shoulder pain". The Cochrane Database of Systematic Reviews (9): CD008271. doi:10.1002/14651858.cd008271.pub2. PMID 20824874.
- ^ a b Nigam PK, Nigam A (2010). "Botulinum toxin". Indian Journal of Dermatology. 55 (1): 8–14. doi:10.4103/0019-5154.60343. PMC 2856357. PMID 20418969.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d Coté TR, Mohan AK, Polder JA, Walton MK, Braun MM (September 2005). "Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases". Journal of the American Academy of Dermatology. 53 (3): 407–415. doi:10.1016/j.jaad.2005.06.011. PMID 16112345. Archived from the original on 23 May 2022. Retrieved 29 December 2021.
- ^ Witmanowski H, Błochowiak K (December 2020). "The whole truth about botulinum toxin - a review". Postepy Dermatologii I Alergologii. 37 (6): 853–861. doi:10.5114/ada.2019.82795. PMC 7874868. PMID 33603602.
- ^ Witmanowski H, Błochowiak K (December 2020). "The whole truth about botulinum toxin - a review". Postepy Dermatologii I Alergologii. 37 (6): 853–861. doi:10.5114/ada.2019.82795. PMC 7874868. PMID 33603602.
- ^ Hamman MS, Goldman MP (August 2013). "Minimizing bruising following fillers and other cosmetic injectables". The Journal of Clinical and Aesthetic Dermatology. 6 (8): 16–18. PMC 3760599. PMID 24003345.
- ^ Schiffer, Jessica (8 April 2021). "How Barely-There Botox Became the Norm". The New York Times. ISSN 0362-4331. Archived from the original on 28 December 2021. Retrieved 23 November 2021.
- ^ "FDA Notifies Public of Adverse Reactions Linked to Botox Use". U.S. Food and Drug Administration (FDA). 8 February 2008. Archived from the original on 2 March 2012. Retrieved 6 May 2012.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b "FDA Gives Update on Botulinum Toxin Safety Warnings; Established Names of Drugs Changed". Pharmaceutical Online. 4 August 2009. Archived from the original on 6 July 2019. Retrieved 16 July 2019.
- ^ a b c d "Information for Healthcare Professionals: OnabotulinumtoxinA (marketed as Botox/Botox Cosmetic), AbobotulinumtoxinA (marketed as Dysport) and RimabotulinumtoxinB (marketed as Myobloc)". U.S. Food and Drug Administration (FDA). 13 September 2015. Archived from the original on 13 September 2015. Retrieved 1 September 2015.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Botox chemical may spread, Health Canada confirms". CBC News. 13 January 2009. Archived from the original on 21 February 2009.
- ^ "Kinds of Botulism". Centers for Disease Control and Prevention. Archived from the original on 5 October 2016. Retrieved 4 October 2016.
- ^ a b c d "Botulism – Diagnosis and Treatment". Centers for Disease Control and Prevention. Archived from the original on 5 October 2016. Retrieved 5 October 2016.
- ^ Barash JR, Arnon SS (January 2014). "A novel strain of Clostridium botulinum that produces type B and type H botulinum toxins". The Journal of Infectious Diseases. 209 (2): 183–191. doi:10.1093/infdis/jit449. PMID 24106296.
- ^ a b Barr JR, Moura H, Boyer AE, Woolfitt AR, Kalb SR, Pavlopoulos A, et al. (October 2005). "Botulinum neurotoxin detection and differentiation by mass spectrometry". Emerging Infectious Diseases. 11 (10): 1578–1583. doi:10.3201/eid1110.041279. PMC 3366733. PMID 16318699.
- ^ Nigam, P. K. (2010). "BOTULINUM TOXIN". Indian Journal of Dermatology. 55 (1): 8–14. doi:10.4103/0019-5154.60343. PMC 2856357. PMID 20418969.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Dressler D, Saberi FA, Barbosa ER (March 2005). "Botulinum toxin: mechanisms of action". Arquivos de Neuro-Psiquiatria. 63 (1): 180–185. doi:10.1159/000083259. PMID 15830090. S2CID 16307223.
- ^ Dong M, Masuyer G, Stenmark P (June 2019). "Botulinum and Tetanus Neurotoxins". Annual Review of Biochemistry. 88 (1): 811–837. doi:10.1146/annurev-biochem-013118-111654. PMC 7539302. PMID 30388027.
- ^ a b c d e Li B, Peet NP, Butler MM, Burnett JC, Moir DT, Bowlin TL (December 2010). "Small molecule inhibitors as countermeasures for botulinum neurotoxin intoxication". Molecules. 16 (1): 202–220. doi:10.3390/molecules16010202. PMC 6259422. PMID 21193845.
- ^ Hill KK, Smith TJ (2013). Rummel A, Binz T (eds.). "Genetic diversity within Clostridium botulinum serotypes, botulinum neurotoxin gene clusters and toxin subtypes". Current Topics in Microbiology and Immunology. 364. Springer: 1–20. doi:10.1007/978-3-642-33570-9_1. ISBN 978-3-642-33569-3. PMID 23239346.
- ^ a b Davies JR, Liu SM, Acharya KR (October 2018). "Variations in the Botulinum Neurotoxin Binding Domain and the Potential for Novel Therapeutics". Toxins. 10 (10): 421. doi:10.3390/toxins10100421. PMC 6215321. PMID 30347838.
- ^ Brunt J, Carter AT, Stringer SC, Peck MW (February 2018). "Identification of a novel botulinum neurotoxin gene cluster in Enterococcus". FEBS Letters. 592 (3): 310–317. doi:10.1002/1873-3468.12969. PMC 5838542. PMID 29323697.
- ^ a b c d Erbguth FJ (March 2004). "Historical notes on botulism, Clostridium botulinum, botulinum toxin, and the idea of the therapeutic use of the toxin". Movement Disorders. 19 (Supplement 8): S2–S6. doi:10.1002/mds.20003. PMID 15027048. S2CID 8190807.
- ^ a b Erbguth FJ, Naumann M (November 1999). "Historical aspects of botulinum toxin: Justinus Kerner (1786-1862) and the "sausage poison"". Neurology. 53 (8): 1850–1853. doi:10.1212/wnl.53.8.1850. PMID 10563638. S2CID 46559225.
- ^ a b c Monheit GD, Pickett A (May 2017). "AbobotulinumtoxinA: A 25-Year History". Aesthetic Surgery Journal. 37 (suppl_1): S4–S11. doi:10.1093/asj/sjw284. PMC 5434488. PMID 28388718.
- ^ Pellett S (June 2012). "Learning from the past: historical aspects of bacterial toxins as pharmaceuticals". Current Opinion in Microbiology. 15 (3): 292–299. doi:10.1016/j.mib.2012.05.005. PMID 22651975.
- ^ "Home Canning and Botulism". 24 June 2022.
- ^ Lamanna C, McELROY OE, Eklund HW (May 1946). "The purification and crystallization of Clostridium botulinum type A toxin". Science. 103 (2681): 613–614. Bibcode:1946Sci...103..613L. doi:10.1126/science.103.2681.613. PMID 21026141.
- ^ Burgen AS, Dickens F, Zatman LJ (August 1949). "The action of botulinum toxin on the neuro-muscular junction". The Journal of Physiology. 109 (1–2): 10–24. doi:10.1113/jphysiol.1949.sp004364. PMC 1392572. PMID 15394302.
- ^ Magoon E, Cruciger M, Scott AB, Jampolsky A (May 1982). "Diagnostic injection of Xylocaine into extraocular muscles". Ophthalmology. 89 (5): 489–491. doi:10.1016/s0161-6420(82)34764-8. PMID 7099568.
- ^ Drachman DB (August 1964). "Atrophy of Skeletal Muscle in Chick Embryos Treated with Botulinum Toxin". Science. 145 (3633): 719–721. Bibcode:1964Sci...145..719D. doi:10.1126/science.145.3633.719. PMID 14163805. S2CID 43093912.
- ^ Scott AB, Rosenbaum A, Collins CC (December 1973). "Pharmacologic weakening of extraocular muscles". Investigative Ophthalmology. 12 (12): 924–927. PMID 4203467.
- ^ Scott AB (October 1980). "Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery". Ophthalmology. 87 (10): 1044–1049. doi:10.1016/s0161-6420(80)35127-0. PMID 7243198.
- ^ a b Boffey PM (14 October 1986). "Loss Of Drug Relegates Many To Blindness Again". The New York Times. Archived from the original on 26 January 2011. Retrieved 14 July 2010.
- ^ a b United States Department of Health and Human Services (30 April 2009). "Re: Docket No. FDA-2008-P-0061" (PDF). U.S. Food and Drug Administration (FDA). Archived from the original (PDF) on 6 July 2010. Retrieved 26 July 2010.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Wellman-Labadie O, Zhou Y (May 2010). "The US Orphan Drug Act: rare disease research stimulator or commercial opportunity?". Health Policy. 95 (2–3): 216–228. doi:10.1016/j.healthpol.2009.12.001. PMID 20036435.
- ^ a b Clark RP, Berris CE (August 1989). "Botulinum toxin: a treatment for facial asymmetry caused by facial nerve paralysis". Plastic and Reconstructive Surgery. 84 (2): 353–355. doi:10.1097/01.prs.0000205566.47797.8d. PMID 2748749.
- ^ a b Rohrich RJ, Janis JE, Fagien S, Stuzin JM (October 2003). "The cosmetic use of botulinum toxin". Plastic and Reconstructive Surgery. 112 (5 Suppl): 177S–188S. doi:10.1097/01.prs.0000082208.37239.5b. PMID 14504502.
- ^ Carruthers A (November–December 2003). "History of the clinical use of botulinum toxin A and B". Clinics in Dermatology. 21 (6): 469–472. doi:10.1016/j.clindermatol.2003.11.003. PMID 14759577.
- ^ a b Carruthers JD, Carruthers JA (January 1992). "Treatment of glabellar frown lines with C. botulinum-A exotoxin". The Journal of Dermatologic Surgery and Oncology. 18 (1): 17–21. doi:10.1111/j.1524-4725.1992.tb03295.x. PMID 1740562.
- ^ Binder WJ, Brin MF, Blitzer A, Schoenrock LD, Pogoda JM (December 2000). "Botulinum toxin type A (BOTOX) for treatment of migraine headaches: an open-label study". Otolaryngology–Head and Neck Surgery. 123 (6): 669–676. doi:10.1067/mhn.2000.110960. PMID 11112955. S2CID 24406607.
- ^ Jackson JL, Kuriyama A, Hayashino Y (April 2012). "Botulinum toxin A for prophylactic treatment of migraine and tension headaches in adults: a meta-analysis". JAMA. 307 (16): 1736–1745. doi:10.1001/jama.2012.505. PMID 22535858.
- ^ Ramachandran R, Yaksh TL (September 2014). "Therapeutic use of botulinum toxin in migraine: mechanisms of action". British Journal of Pharmacology. 171 (18): 4177–4192. doi:10.1111/bph.12763. PMC 4241086. PMID 24819339.
- ^ "New plastic surgery statistics reveal trends toward body enhancement". Plastic Surgery. 11 March 2019. Archived from the original on 12 March 2019.
- ^ "Who Can Administer Botox® - Empire Medical Training Blog". Who Can Administer Botox® - Empire Medical Training Blog. Archived from the original on 28 July 2021. Retrieved 6 May 2022.
- ^ Chapman L (10 May 2012). "The global botox market forecast to reach $2.9 billion by 2018". Archived from the original on 6 August 2012. Retrieved 5 October 2012.
- ^ a b c "Botulinum Toxin Market". Fortune Business Insights. Archived from the original on 27 June 2021. Retrieved 22 May 2021.
- ^ "2020 National Plastic Surgery Statistics: Cosmetic Surgical Procedures" (PDF). American Society of Plastic Surgeons. Archived (PDF) from the original on 23 June 2021. Retrieved 22 May 2021.
- ^ "Medicare Guidelines for Botox Treatments". MedicareFAQ.com. 27 September 2021. Archived from the original on 23 May 2021. Retrieved 22 May 2021.
- ^ "BOTOX (onabotulinumtoxinA) for injection, for intramuscular, intradetrusor, or intradermal use" (PDF). Highlights of Prescribing Information. United States Food and Drug Administration. Archived (PDF) from the original on 28 March 2021. Retrieved 22 May 2021.
- ^ "Botox for Migraine". The American Migraine Foundation. Archived from the original on 19 June 2021. Retrieved 22 May 2021.
- ^ "BOTOX® Procedures: What is BOTOX® & How Does it Work". The American Academy of Facial Esthetics. Archived from the original on 22 May 2021. Retrieved 22 May 2021.
- ^ Koirala J, Basnet S (14 July 2004). "Botulism, Botulinum Toxin, and Bioterrorism: Review and Update". Medscape. Cliggott Publishing. Archived from the original on 1 June 2011. Retrieved 14 July 2010.
- ^ Public Health Agency of Canada (19 April 2011). "Pathogen Safety Data Sheets: Infectious Substances – Clostridium botulinum". Archived from the original on 24 January 2022. Retrieved 24 January 2022.
- ^ Fleisher LA, Roizen MF, Roizen J (31 May 2017). Essence of Anesthesia Practice E-Book. Elsevier Health Sciences. ISBN 978-0-323-39541-0. Archived from the original on 11 November 2021. Retrieved 10 June 2022.
- ^ "M8 Paper" (PDF). www.wmddetectorselector.army.mil. U.S. Army. Archived (PDF) from the original on 23 October 2020. Retrieved 16 September 2020.
M8 paper is a chemically-treated, dye-impregnated paper used to detect liquid substances for the presence of V- and G-type nerve agents and H- and L-type blister agents.
- ^ Baselt RC (2014). Disposition of toxic drugs and chemicals in man. Seal Beach, Ca.: Biomedical Publications. pp. 260–61. ISBN 978-0-9626523-9-4.
- ^ Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, et al. (Working Group on Civilian Biodefense) (February 2001). "Botulinum toxin as a biological weapon: medical and public health management". JAMA. 285 (8): 1059–1070. doi:10.1001/jama.285.8.1059. PMID 11209178.
- ^ McAdams D, Kornblet S (2011). "Baader-Meinhof Group (OR Baader-Meinhof Gang". In Pilch RF, Zilinskas RA (eds.). Encyclopedia of Bioterrorism Defense. Wiley-Liss. doi:10.1002/0471686786.ebd0012.pub2. ISBN 978-0-471-68678-1.
- ^ "2011 Allergan Annual Report" (PDF). Allergan. Archived (PDF) from the original on 15 November 2012. Retrieved 3 May 2012. See PDF p. 7.
- ^ a b c Walker TJ, Dayan SH (February 2014). "Comparison and overview of currently available neurotoxins". The Journal of Clinical and Aesthetic Dermatology. 7 (2): 31–39. PMC 3935649. PMID 24587850.
- ^ "Botulinum Toxin Type A". Hugh Source (International) Limited. Archived from the original on 24 July 2008. Retrieved 14 July 2010.
- ^ Petrou I (Spring 2009). "Medy-Tox Introduces Neuronox to the Botulinum Toxin Arena" (PDF). The European Aesthetic Guide. Archived from the original (PDF) on 20 March 2013. Retrieved 9 December 2009.
- ^ Schantz EJ, Johnson EA (March 1992). "Properties and use of botulinum toxin and other microbial neurotoxins in medicine". Microbiological Reviews. 56 (1): 80–99. doi:10.1128/MMBR.56.1.80-99.1992. PMC 372855. PMID 1579114.
- ^ a b c d "About Botulism | Botulism | CDC". www.cdc.gov. 9 October 2018. Archived from the original on 27 April 2020. Retrieved 13 May 2020.
- ^ "Botulism". Centers for Disease Control and Prevention (CDC). 19 August 2019. Archived from the original on 3 August 2016. Retrieved 28 August 2019.
- ^ Licciardello JJ, Nickerson JT, Ribich CA, Goldblith SA (March 1967). "Thermal inactivation of type E botulinum toxin". Applied Microbiology. 15 (2): 249–256. doi:10.1128/AEM.15.2.249-256.1967. PMC 546888. PMID 5339838.
- ^ Setlow P (April 2007). "I will survive: DNA protection in bacterial spores". Trends in Microbiology. 15 (4): 172–180. doi:10.1016/j.tim.2007.02.004. PMID 17336071.
- ^ "Fact sheets - Botulism". World Health Organization. 10 January 2018. Archived from the original on 23 March 2019. Retrieved 23 March 2019.
- ^ Capková K, Salzameda NT, Janda KD (October 2009). "Investigations into small molecule non-peptidic inhibitors of the botulinum neurotoxins". Toxicon. 54 (5): 575–582. doi:10.1016/j.toxicon.2009.03.016. PMC 2730986. PMID 19327377.
- ^ Flanders M, Tischler A, Wise J, Williams F, Beneish R, Auger N (June 1987). "Injection of type A botulinum toxin into extraocular muscles for correction of strabismus". Canadian Journal of Ophthalmology. Journal Canadien d'Ophtalmologie. 22 (4): 212–217. PMID 3607594.
- ^ "Botulinum toxin therapy of eye muscle disorders. Safety and effectiveness. American Academy of Ophthalmology". Ophthalmology. Suppl (Suppl 37-41): 37–41. September 1989. doi:10.1016/s0161-6420(89)32989-7. PMID 2779991.
- ^ Hellman A, Torres-Russotto D (March 2015). "Botulinum toxin in the management of blepharospasm: current evidence and recent developments". Therapeutic Advances in Neurological Disorders. 8 (2): 82–91. doi:10.1177/1756285614557475. PMC 4356659. PMID 25922620.
- ^ Keen M, Kopelman JE, Aviv JE, Binder W, Brin M, Blitzer A (April 1994). "Botulinum toxin A: a novel method to remove periorbital wrinkles". Facial Plastic Surgery. 10 (2): 141–146. doi:10.1055/s-2008-1064563. PMID 7995530.
- ^ "Botulinum Toxin Type A Product Approval Information – Licensing Action 4/12/02". U.S. Food and Drug Administration (FDA). 29 October 2009. Archived from the original on 8 March 2010. Retrieved 26 July 2010.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Giesler M (2012). "How Doppelgänger Brand Images Influence the Market Creation Process: Longitudinal Insights from the Rise of Botox Cosmetic". Journal of Marketing. 76 (6): 55–68. doi:10.1509/jm.10.0406. S2CID 167319134.
- ^ "Botox Cosmetic (onabotulinumtoxinA) Product Information". Allergan. 22 January 2014. Archived from the original on 21 July 2021. Retrieved 1 March 2018.
- ^ "Allergan Receives FDA Approval for First-of-Its-Kind, Fully in vitro, Cell-Based Assay for Botox and Botox Cosmetic (onabotulinumtoxinA)". Allergan. 24 June 2011. Archived from the original on 26 June 2011. Retrieved 26 June 2011.
- ^ "In U.S., Few Alternatives To Testing On Animals". The Washington Post. 12 April 2008. Archived from the original on 12 November 2012. Retrieved 26 June 2011.
- ^ Nayyar P, Kumar P, Nayyar PV, Singh A (December 2014). "BOTOX: Broadening the Horizon of Dentistry". Journal of Clinical and Diagnostic Research. 8 (12): ZE25–ZE29. doi:10.7860/JCDR/2014/11624.5341. PMC 4316364. PMID 25654058.
- ^ Hwang WS, Hur MS, Hu KS, Song WC, Koh KS, Baik HS, et al. (January 2009). "Surface anatomy of the lip elevator muscles for the treatment of gummy smile using botulinum toxin". The Angle Orthodontist. 79 (1): 70–77. doi:10.2319/091407-437.1. PMID 19123705.
- ^ Gracco A, Tracey S (May 2010). "Botox and the gummy smile". Progress in Orthodontics. 11 (1): 76–82. doi:10.1016/j.pio.2010.04.004. PMID 20529632.
- ^ Mazzuco R, Hexsel D (December 2010). "Gummy smile and botulinum toxin: a new approach based on the gingival exposure area". Journal of the American Academy of Dermatology. 63 (6): 1042–1051. doi:10.1016/j.jaad.2010.02.053. PMID 21093661.
- ^ Khan WU, Campisi P, Nadarajah S, Shakur YA, Khan N, Semenuk D, et al. (April 2011). "Botulinum toxin A for treatment of sialorrhea in children: an effective, minimally invasive approach". Archives of Otolaryngology–Head & Neck Surgery. 137 (4): 339–344. doi:10.1001/archoto.2010.240. PMID 21242533.
- ^ "FDA approves Botox to treat chronic migraine". U.S. Food and Drug Administration (FDA) (Press release). 19 October 2010. Archived from the original on 19 October 2010. Retrieved 23 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Watkins T (15 October 2010). "FDA approves Botox as migraine preventative". CNN. Archived from the original on 27 July 2020. Retrieved 16 October 2010.
- ^ Dodick DW, Turkel CC, DeGryse RE, Aurora SK, Silberstein SD, Lipton RB, et al. (June 2010). "OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program". Headache. 50 (6): 921–936. doi:10.1111/j.1526-4610.2010.01678.x. PMID 20487038. S2CID 9621285.
- ^ Ashkenazi A (March 2010). "Botulinum toxin type a for chronic migraine". Current Neurology and Neuroscience Reports. 10 (2): 140–146. doi:10.1007/s11910-010-0087-5. PMID 20425239. S2CID 32191932.
- ^ Magid M, Keeling BH, Reichenberg JS (November 2015). "Neurotoxins: Expanding Uses of Neuromodulators in Medicine--Major Depressive Disorder". Plastic and Reconstructive Surgery. 136 (5 Suppl): 111S–119S. doi:10.1097/PRS.0000000000001733. PMID 26441090. S2CID 24196194.
- ^ a b "Onabotulinum toxin A - Allergan - AdisInsight". Archived from the original on 30 October 2017. Retrieved 5 September 2017.
- ^ Finzi E, Rosenthal NE (May 2014). "Treatment of depression with onabotulinumtoxinA: a randomized, double-blind, placebo controlled trial". Journal of Psychiatric Research. 52: 1–6. doi:10.1016/j.jpsychires.2013.11.006. PMID 24345483.
- ^ Clinical trial number NCT01917006 for "An Exploratory Study of the Safety and Efficacy of Botox for the Treatment of Premature Ejaculation" at ClinicalTrials.gov
External links
- BotDB: extensive resources on BoNT structures, inhibitors, kinetics, and literature
- Overview of all the structural information available in the PDB for UniProt: P0DPI1 (Botulinum neurotoxin type A) at the PDBe-KB.
- Overview of all the structural information available in the PDB for UniProt: P10844 (Botulinum neurotoxin type B) at the PDBe-KB.
- Overview of all the structural information available in the PDB for UniProt: A0A0X1KH89 (Bontoxilysin A) at the PDBe-KB.
- "OnabotulinumtoxinA". Drug Information Portal. U.S. National Library of Medicine.
- "RimabotulinumtoxinB". Drug Information Portal. U.S. National Library of Medicine.
- "AbobotulinumtoxinA". Drug Information Portal. U.S. National Library of Medicine.
- "AbobotulinumtoxinA Injection". MedlinePlus.
- "IncobotulinumtoxinA Injection". MedlinePlus.
- "OnabotulinumtoxinA Injection". MedlinePlus.
- "PrabotulinumtoxinA-xvfs Injection". MedlinePlus.
- "RimabotulinumtoxinB Injection". MedlinePlus.
