Rimonabant
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Undetermined |
| Protein binding | Nearly 100% |
| Metabolism | Hepatic, CYP3A4 involved |
| Elimination half-life | Variable: 6 to 9 days with normal BMI 16 days if BMI >30 |
| Excretion | Fecal (86%) and renal (3%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.210.978 |
| Chemical and physical data | |
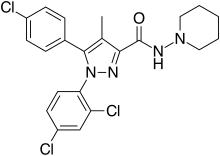
| Formula | C22H21Cl3N4O |
| Molar mass | 463.79 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Rimonabant (also known as SR141716; trade names Acomplia, Zimulti) is an anorectic antiobesity drug that has been withdrawn from the market due to potentially serious side effects. It was approved for use in Europe and other countries, but never approved in the United States. Rimonabant is an inverse agonist for the cannabinoid receptor CB1.[3] It has also been shown to be a μ-opioid receptor antagonist[4] (possibly the contributing factor in its reported dysphoric qualities).[citation needed] Its main effect is reduction in appetite.
History
Rimonabant was the first selective CB1 receptor blocker to be approved for use anywhere in the world. In Europe, it was indicated for use in conjunction with diet and exercise for patients with a body mass index (BMI) greater than 30 kg/m², or patients with a BMI greater than 27 kg/m² with associated risk factors, such as type 2 diabetes or dyslipidaemia. In the UK, it was available beginning in July 2006. As of 2008, the drug was available in 56 countries.
On 21 June 2006, the European Commission approved the sale of rimonabant in the then-25-member European Union as a prescription drug. Pharmaceutical company Sanofi-Aventis announced rimonabant would be launched in the United Kingdom. Sales began in July 2006. Sanofi-Aventis also projected that the drug would be sold shortly thereafter in Denmark, Ireland, Germany, Finland, and Norway. It was expected in Belgium[5] and Sweden in 2007. Ordinary obesity would, according to official medical recommendations, not be enough to acquire the prescription in Sweden; there would be additional requirements concerning abnormal blood lipid levels.[6]
Rimonabant was submitted to the Food and Drug Administration (FDA) for approval in the United States. However, in 2007, the FDA's Endocrine and Metabolic Drugs Advisory Committee (EMDAC) concluded the French manufacturer Sanofi-Aventis failed to demonstrate the safety of rimonabant and voted against recommending the anti-obesity treatment for approval.[2] Subsequently, Sanofi-Aventis announced it was suspending the new drug application (NDA) for rimonabant, and that it would resubmit an application at some point in the future.
The EU's approval was not a blanket approval, nor did it approve Acomplia for nonobesity-related problems, such as smoking cessation, although off-label use of the drug was still possible. The approval was, in combination with diet and exercise, for the treatment of obese patients (BMI greater than or equal to 30), or overweight patients (BMI greater than 27) with associated risk factors, such as type 2 diabetes or dyslipidaemia. In 2007, a label warning against use in patients with depression was upgraded to complete contraindication by the European Medicines Agency.[7]
In October 2008, the European Medicines Agency recommended the suspension of Acomplia after the Committee for Medicinal Products for Human Use (CHMP) had determined that the risks of Acomplia outweighed its benefits due to the risk of serious psychiatric problems, and even suicide.[8] Sanofi-Aventis then suspended sale of the drug and its approval was withdrawn by the European Commission on 16 January 2009.[1][9]
India has prohibited the manufacture and sale of the drug.[10]
Research
In 2009 Moreira and Crippa[11] gathered experimental evidence pertaining to the way rimonabant enhances cannabinoid type-1(CB1) receptor. Information gathered derived from data bases up until February 2009. The conclusion of all the data indicated rimonabant has been used for treating diabetes, promoting smoking cessation and reducing alcohol consumption. Psychiatric side affects have been induced by the use of this drug: anxiety, depression, agitation, eating disorders, irritability, aggression, and insomnia. Nevertheless, it is thought that the scale used to determine all psychiatric affects might have not been able to detect all of the possible ones.
Uses/potential uses
Obesity
In a 2006 (2 year) study reported in JAMA, "Compared with the placebo group, the 20 mg of rimonabant group produced greater mean (SEM) reductions in weight (-6.3 [0.2] kg vs -1.6 [0.2] kg; P<.001), waist circumference (-6.1 [0.2] cm vs -2.5 [0.3] cm; P<.001), and level of triglycerides (percentage change, -5.3 [1.2] vs 7.9 [2.0]; P<.001) and a greater increase in level of high-density lipoprotein cholesterol (percentage change, 12.6 [0.5] vs 5.4 [0.7]; P<.001)." [12]
Smoking cessation
Rimonabant may also be found to be effective in assisting some smokers to quit smoking. Sanofi is currently conducting studies to determine the possible value of rimonabant in smoking-cessation therapy. The Studies with Rimonabant and Tobacco Use (STRATUS) program involves more than 6,000 subjects. STRATUS is designed to explore two smoking-related therapies: first, to use rimonabant directly to aid in smoking cessation; second, to help prevent weight gain in former smokers. Initial results apparently suggest rimonabant is effective for both uses. However, the FDA has explicitly stated to Sanofi that, without additional studies, rimonabant cannot be approved in the United States for smoking cessation therapy. According to a Cochrane Collaboration review in 2007, rimonabant "may increase the odds of quitting approximately 11/2-fold".[13][needs update]
Addiction behaviors
Rimonabant reduced resumption of cocaine-seeking responses triggered by two of the three most common triggers of relapse in humans: priming and cues. It may also reduce ethanol and opiate-seeking behavior.[14]
Short-term memory
Tetrahydrocannabinol (THC) is known to impair short-term memory. It was therefore hypothesised that rimonabant may reduce or inhibit the atrophic effects of cannabinoids. Indeed, in animal studies, it significantly improved the ability of rats to encode information into short-term memory.[15]
Blockage of cannabis effects
Rimonabant blocks the psychoactive and some of the cardiovascular effects of Δ9-tetrahydrocannabinol (THC) in humans without affecting the pharmacokinetics.[16] Rimonabant has been described colloquially as "reverse marijuana", having a depressing effect on appetite inverse to the increased appetite created by cannabinoids.[17]
Other effects
Rimonabant reduces voluntary wheel running in laboratory mice.[18]
Rimonabant significantly increased human sperm motility and viability in vitro.[19]
Rimonabant has been used as a treatment for patients with diabetes.[11]
Negative side effects
Shortly after market introduction, press reports and independent studies suggested that side effects occurred more intensely and more commonly than had been found by the manufacturer in their clinical studies. Reports of severe depression and suicidal thoughts were frequent.[20] As the drug's target CB1 receptors are fairly ubiquitous throughout the central nervous system, it is not currently understood where the inverse agonist is acting to cause these side-effects.
In 2007, it was reported that the committee advising the U.S. FDA had voted not to recommend the drug's approval because of concerns over suicide, depression, and other related side effects associated with use of the drug.[21]
Additional side effects that have been observed by data collection over many years resulted in patients exhibiting: depressive mood, major depression, dysthymia and depressive symptoms, which although they sound very similar they are different in severity and symptoms.[11]
Preparation
The chemical synthesis of rimonabant is described as follows:[22]
Brand names
Brand names for rimonabant include Acomplia, Bethin, Monaslim, Remonabent, Riobant, Slimona, Rimoslim and Redufast. The proposed brand name if it had been approved for use in the United States was Zimulti.
References
- ^ a b "Anti-obesity drug use suspended". BBC News. 23 October 2008. Retrieved 4 March 2010.
- ^ a b "Zimulti Acomplia Report - Diet Drug Acomplia / Zimulti Gets Thumbs Down From FDA Panel". Acompliareport.com. 2007-06-13. Retrieved 2010-03-19.
- ^ Fong TM, Heymsfield SB (September 2009). "Cannabinoid-1 receptor inverse agonists: current understanding of mechanism of action and unanswered questions". Int J Obes (Lond). 33 (9): 947–55. doi:10.1038/ijo.2009.132. PMID 19597516.
- ^ AM-251 and rimonabant act as direct antagonists at mu-opioid receptors: implications for opioid/cannabinoid interaction studies. Neuropharmacology. 2012 Oct;63(5):905-15. doi: 10.1016/j.neuropharm.2012.06.046. Epub 2012 Jul 4. PMID 22771770 PMCID: PMC3408547
- ^ Auteur: Femke Gebruers. "Article from the Belgian newspaper De Standaard". Standaard.be. Retrieved 2010-03-19.
- ^ "Article from the Swedish TV station TV 4 website". Tv4.se. 2008-03-06. Retrieved 2010-03-19. [dead link]
- ^ "European Medicines Agency recommends Acomplia must not be used in patients on antidepressants or with major depression". European Medicines Agency. 19 July 2007. Retrieved 18 January 2016.
- ^ "The European Medicines Agency recommends suspension of the marketing authorisation of Acomplia". European Medicines Agency. 23 October 2008. Retrieved 18 January 2016.
- ^ "PUBLIC STATEMENT ON Acomplia (rimonabant) WITHDRAWAL OF THE MARKETING AUTHORISATION IN THE EUROPEAN UNION" (PDF). European Medicines Agency. 30 January 2009. Retrieved 18 January 2016.
- ^ "Drugs banned in India". Central Drugs Standard Control Organization, Dte.GHS, Ministry of Health and Family Welfare, Government of India. Retrieved 2013-09-17.
- ^ a b c Moreira, F. A., & Crippa, J. A. (2009). The psychiatric side-effects of rimonabant. Rev. Bras. Psiquiatr. Revista Brasileira De Psiquiatria, 31(2), 145-153. doi:10.1590/s1516-44462009000200012
- ^ JAMA. 2006 Feb 15;295(7):761-75. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J; RIO-North America Study Group. PMID 16478899
- ^ Cahill K, Ussher M (2007). Cahill, Kate (ed.). "Cannabinoid type 1 receptor antagonists (rimonabant) for smoking cessation". Cochrane database of systematic reviews (Online) (4): CD005353. doi:10.1002/14651858.CD005353.pub3. PMID 17943852.
- ^ Maldonado R, Valverde O, Berrendero F (2006). "Involvement of the endocannabinoid system in drug addiction". Trends Neurosci. 29 (4): 225–32. doi:10.1016/j.tins.2006.01.008. PMID 16483675.
- ^ Deadwyler SA, Goonawardena AV, Hampson RE (2007). "Short-term memory is modulated by the spontaneous release of endocannabinoids: evidence from hippocampal population codes". Behavioural Pharmacology. 18 (5–6): 571–80. doi:10.1097/FBP.0b013e3282ee2adb. PMID 17762525.
- ^ Huestis MA, et al. (2001). "Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716". Arch. Gen. Psychiatry. 58 (4): 322–8. doi:10.1001/archpsyc.58.4.322. PMID 11296091.
- ^ Stephan Guyenet, PhD (9 March 2012) Seduced by Food: Obesity and the Human Brain Boing Boing
- ^ Keeney BK, et al. (2008). "Differential response to a selective cannabinoid receptor antagonist (SR141716: rimonabant) in female mice from lines selectively bred for high voluntary wheel-running behavior". Behavioural Pharmacology. 19 (8): 812–820. doi:10.1097/FBP.0b013e32831c3b6b. PMID 19020416.
- ^ Aguila S, et al. (2010). "Rimonabant (SR141716) induces metabolism and acquisition of fertilizing ability in human sperm". Br J Pharmacol. 159 (4): 831–41. doi:10.1111/j.1476-5381.2009.00570.x. PMC 2829209. PMID 20067470.
- ^ "Kassen müssen nicht für "Acomplia" zahlen". tagesschau.de. 2006-10-17. Retrieved 2007-06-13.
- ^ "Suicide risk fears over diet pill". BBC News. 15 June 2007. Retrieved 4 March 2010.
- ^ Yoshioka, T.; et al. (1989). "Studies on hindered phenols and analogs. 1. Hypolipidemic and hypoglycemic agents with ability to inhibit lipid peroxidation". Journal of Medicinal Chemistry. 32 (2): 421–8. doi:10.1021/jm00122a022. PMID 2913302.

