Ketotifen: Difference between revisions
IbnMostafa (talk | contribs) removed unreferenced template. |
No edit summary |
||
| Line 4: | Line 4: | ||
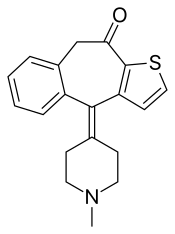
| IUPAC_name = 4-(1-Methylpiperidin-4-ylidene)-4,9-dihydro-10''H''-benzo[4,5]cyclohepta[1,2-''b'']thiophen-10-one |
| IUPAC_name = 4-(1-Methylpiperidin-4-ylidene)-4,9-dihydro-10''H''-benzo[4,5]cyclohepta[1,2-''b'']thiophen-10-one |
||
| image = Ketotifen.svg |
| image = Ketotifen.svg |
||
| width = |
| width = 175px |
||
<!--Clinical data--> |
<!--Clinical data--> |
||
| tradename = |
| tradename = Zaditor<ref>http://www.webmd.com/drugs/2/drug-17484/zaditor-opht/details{{full}}</ref> |
||
| Drugs.com = {{drugs.com|CONS|ketotifen}} |
| Drugs.com = {{drugs.com|CONS|ketotifen}} |
||
| MedlinePlus = a604033 |
| MedlinePlus = a604033 |
||
| pregnancy_US = C |
| pregnancy_US = C |
||
| legal_US = |
| legal_US = |
||
| legal_US_comment = |
| legal_US_comment = Oral — withdrawn, was ℞-only; Eye drops — over-the-counter |
||
| legal_status = |
| legal_status = |
||
| routes_of_administration = Oral ([[Tablet (pharmacy)|tablets]]), ophthalmic solution |
| routes_of_administration = Oral ([[Tablet (pharmacy)|tablets]]), ophthalmic solution |
||
| Line 28: | Line 28: | ||
| ATC_prefix = R06 |
| ATC_prefix = R06 |
||
| ATC_suffix = AX17 |
| ATC_suffix = AX17 |
||
| ATC_supplemental = |
| ATC_supplemental = {{ATC|S01|GX08}} |
||
| PubChem = 3827 |
| PubChem = 3827 |
||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
||
| Line 44: | Line 44: | ||
| C=19 | H=19 | N=1 | O=1 | S=1 |
| C=19 | H=19 | N=1 | O=1 | S=1 |
||
| molecular_weight = 309.426 g/mol |
| molecular_weight = 309.426 g/mol |
||
| |
| SMILES = O=C3c1sccc1C(\c2c(cccc2)C3)=C4/CCN(C)CC4 |
||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChI = 1S/C19H19NOS/c1-20-9-6-13(7-10-20)18-15-5-3-2-4-14(15)12-17(21)19-16(18)8-11-22-19/h2-5,8,11H,6-7,9-10,12H2,1H3 |
| StdInChI = 1S/C19H19NOS/c1-20-9-6-13(7-10-20)18-15-5-3-2-4-14(15)12-17(21)19-16(18)8-11-22-19/h2-5,8,11H,6-7,9-10,12H2,1H3 |
||
| Line 51: | Line 51: | ||
}} |
}} |
||
'''Ketotifen''' is a first-generation noncompetitive H<sub>1</sub>-[[antihistamine]] and [[mast cell stabilizer]]. It is most commonly sold as a [[salt (chemistry)|salt]] with [[fumaric acid]], '''ketotifen fumarate''', and is available in two forms. |
'''Ketotifen''' is a first-generation noncompetitive H<sub>1</sub>-[[antihistamine]] and [[mast cell stabilizer]]. It is most commonly sold as a [[salt (chemistry)|salt]] with [[fumaric acid]], '''ketotifen fumarate''', and is available in two forms. In its [[Ophthalmology|ophthalmic]] form, it is used to treat [[allergic conjunctivitis]].<ref name="Zaditor_pi">[http://www.pharma.us.novartis.com/product/pi/pdf/zaditor.pdf Zaditor prescribing information] Novartis</ref> In its [[Oral administration|oral]] form, it is used to prevent [[asthma]] attacks or Anaphylaxis, as well as various mast cell, allergic-type disorders.<ref>{{cite journal |doi=10.1016/j.anai.2013.10.003 |pmid=24267353 |pmc=4309375 |title=Ketotifen in the management of chronic urticaria: Resurrection of an old drug |journal=Annals of Allergy, Asthma & Immunology |volume=111 |issue=6 |pages=433–6 |year=2013 |last1=Sokol |first1=Kristin C. |last2=Amar |first2=Neil K. |last3=Starkey |first3=Jonathan |last4=Grant |first4=J. Andrew }}</ref><ref>{{cite journal |doi=10.1016/j.ejmhg.2015.04.003 |title=The relation between antihistamine medication during early pregnancy & birth defects |journal=Egyptian Journal of Medical Human Genetics |volume=16 |issue=4 |pages=287–90 |year=2015 |last1=Shawky |first1=Rabah M. |last2=Seifeldin |first2=Neveen S. }}</ref><ref name=Zuberbier2012>{{cite journal |doi=10.1186/1939-4551-5-S1-S1 |title=A Summary of the New International EAACI/GA2LEN/EDF/ WAO Guidelines in Urticaria |journal=World Allergy Organization Journal |volume=5 |issue=1 |pages=S1–S5 |year=2012 |last1=Zuberbier |first1=Torsten }}</ref><ref name="Zuberbier et al 2009">{{cite journal |doi=10.1111/j.1398-9995.2009.02178.x |pmid=19772513 |title=EAACI/GA²LEN/EDF/WAO guideline: Management of urticaria |journal=Allergy |volume=64 |issue=10 |pages=1427–43 |year=2009 |last1=Zuberbier |first1=T. |last2=Asero |first2=R. |last3=Bindslev-Jensen |first3=C. |last4=Walter Canonica |first4=G. |last5=Church |first5=M. K. |last6=Giménez-Arnau |first6=A. M. |last7=Grattan |first7=C. E. H. |last8=Kapp |first8=A. |last9=Maurer |first9=M. |last10=Merk |first10=H. F. |last11=Rogala |first11=B. |last12=Saini |first12=S. |last13=Sánchez-Borges |first13=M. |last14=Schmid-Grendelmeier |first14=P. |last15=Schünemann |first15=H. |last16=Staubach |first16=P. |last17=Vena |first17=G. A. |last18=Wedi |first18=B. }}</ref><ref>{{cite conference |first1=Zhenhong |last1=Li |first2=Jocelyn |last2=Celestin |date=February 23, 2015 |title=Ketotifen: A Role in the Treatment of Idiopathic Anaphylaxis |url=https://aaaai.confex.com/aaaai/2015/webprogram/Paper19998.html |conference=American Academy of Allergy, Asthma & Immunology Annual Meeting |location=Houston }}</ref> |
||
== |
==Medical uses== |
||
Ketotifen relieves and prevents eye itchiness and/or irritation associated with most [[allergen|seasonal allergies]]. |
Ketotifen relieves and prevents eye itchiness and/or irritation associated with most [[allergen|seasonal allergies]]. It starts working within minutes after administering the drops. The drug has not been studied in children under three.<ref name="Zaditor_pi" /> The mean elimination half life is 12 hours.<ref>{{cite journal |doi=10.1002/bdd.2510130404 |pmid=1600111 |title=Pharmacokinetics of ketotiffn after oral administration to healthy male subjects |journal=Biopharmaceutics & Drug Disposition |volume=13 |issue=4 |pages=255–62 |year=1992 |last1=Grahnén |first1=A. |last2=Lönnebo |first2=A. |last3=Beck |first3=O. |last4=Eckernäs |first4=S-Å |last5=Dahlström |first5=B. |last6=Lindström |first6=B. }}</ref> Besides its anti-histaminic activity, it is also a functional [[leukotriene]] antagonist and a [[phosphodiesterase inhibitor]]. |
||
"[O]ral ketotifen has been used in patients with asthma, allergic rhinitis, allergic conjunctivitis, atopic dermatitis, chronic urticaria, cold-induced urticaria, cholinergic urticaria, exercise-induced urticaria, [systemic mast cell disease including mastocytosis, MCAS, allergic and nonallergic anaphylaxis, angioedema], and food allergy in Canada, Europe, and Mexico." |
"[O]ral ketotifen has been used in patients with asthma, allergic rhinitis, allergic conjunctivitis, atopic dermatitis, chronic urticaria, cold-induced urticaria, cholinergic urticaria, exercise-induced urticaria, [systemic mast cell disease including mastocytosis, MCAS, allergic and nonallergic anaphylaxis, angioedema], and food allergy in Canada, Europe, and Mexico." Now available via prescription at US compounding pharmacies: "For adults and older children with asthma or allergic disease, the recommended dose of ketotifen is 1 mg twice daily." "FDA staff did recommend more extensive evaluations for management of urticaria."<ref name=Zuberbier2012/><ref name="Zuberbier et al 2009"/> |
||
The drug may also help relieve the symptoms of [[irritable bowel syndrome]].<ref>{{cite journal |doi=10.1136/gut.2010.213108 |pmid=20650926 |title=The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome |journal=Gut |volume=59 |issue=9 |pages=1213–21 |year=2010 |last1=Klooker |first1=T. K. |last2=Braak |first2=B. |last3=Koopman |first3=K. E. |last4=Welting |first4=O. |last5=Wouters |first5=M. M. |last6=Van Der Heide |first6=S. |last7=Schemann |first7=M. |last8=Bischoff |first8=S. C. |last9=Van Den Wijngaard |first9=R. M. |last10=Boeckxstaens |first10=G. E. }}</ref> |
The drug may also help relieve the symptoms of [[irritable bowel syndrome]].<ref>{{cite journal |doi=10.1136/gut.2010.213108 |pmid=20650926 |title=The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome |journal=Gut |volume=59 |issue=9 |pages=1213–21 |year=2010 |last1=Klooker |first1=T. K. |last2=Braak |first2=B. |last3=Koopman |first3=K. E. |last4=Welting |first4=O. |last5=Wouters |first5=M. M. |last6=Van Der Heide |first6=S. |last7=Schemann |first7=M. |last8=Bischoff |first8=S. C. |last9=Van Den Wijngaard |first9=R. M. |last10=Boeckxstaens |first10=G. E. }}</ref> |
||
==Side effects== |
==Side effects== |
||
Side effects include drowsiness, weight gain, dry mouth, irritability, and increased nosebleeds.<ref>http://www.mims.co.uk/drugs/allergic-disorders/allergic-rhinitis-urticaria-other-allergies/zaditen</ref> |
Side effects include drowsiness, weight gain, dry mouth, irritability, and increased nosebleeds.<ref>http://www.mims.co.uk/drugs/allergic-disorders/allergic-rhinitis-urticaria-other-allergies/zaditen</ref> |
||
== |
==Pharmacology== |
||
Ketotifen is a [[binding selectivity|selective]] [[antihistamine]] – that is, an [[inverse agonist]] of the [[histamine]] [[H1 receptor|H<sub>1</sub> receptor]] (K<sub>i</sub> = 0.166 nM)<ref name="pmid9165365">{{cite journal | vauthors = Kakiuchi M, Ohashi T, Musoh K, Kawamura K, Morikawa K, Kato H | title = Studies on the novel antiallergic agent HSR-609: its penetration into the central nervous system in mice and guinea pigs and its selectivity for the histamine H1-receptor | journal = Jpn. J. Pharmacol. | volume = 73 | issue = 4 | pages = 291–8 | year = 1997 | pmid = 9165365 | doi = | url = }}</ref> – and [[mast cell stabilizer]].<ref name="LemkeWilliams2008">{{cite book|author1=Thomas L. Lemke|author2=David A. Williams|title=Foye's Principles of Medicinal Chemistry|url=https://books.google.com/books?id=R0W1ErpsQpkC&pg=PA1019|year=2008|publisher=Lippincott Williams & Wilkins|isbn=978-0-7817-6879-5|pages=1019–}}</ref> In addition, ketotifen has weak [[anticholinergic]] (K<sub>i</sub> = 204 nM for {{abbrlink|mACh|muscarinic acetylcholine receptor}}) and [[antiserotonergic]] (K<sub>i</sub> = 39.9 nM for [[5-HT2A receptor|5-HT<sub>2A</sub>]]) activity.<ref name="Alagarsamy2012">{{cite book|author=V Alagarsamy|title=Textbook of Medicinal Chemistry Vol II - E-Book|url=https://books.google.com/books?id=0FPCuckMacAC&pg=PA38|date=16 June 2012|publisher=Elsevier Health Sciences|isbn=81-312-3259-X|pages=38–}}</ref> However, at the dosages in which it is typically used clinically, both the anticholinergic and antiserotonergic activity of ketotifen are said not to be appreciable.<ref name="Drews2012">{{cite book|author=Jürgen Drews|title=Immunopharmacology: Principles and Perspectives|url=https://books.google.com/books?id=mIN9CAAAQBAJ&pg=PT282|date=6 December 2012|publisher=Springer Science & Business Media|isbn=978-3-642-75561-3|pages=282–}}</ref> |
|||
| ⚫ | |||
== |
==Society and culture== |
||
*[[Alcaftadine]] |
|||
===Brand names=== |
|||
{{clear}} |
|||
| ⚫ | |||
==References== |
==References== |
||
| Line 79: | Line 79: | ||
{{Antihistamines}} |
{{Antihistamines}} |
||
{{Acetylcholine receptor modulators}} |
|||
{{Histaminergics}} |
|||
{{Histamine receptor modulators}} |
|||
{{Serotonin receptor modulators}} |
|||
{{Tricyclics}} |
{{Tricyclics}} |
||
[[Category:Benzocycloheptathiophenes]] |
|||
[[Category:H1 receptor antagonists]] |
[[Category:H1 receptor antagonists]] |
||
[[Category:Muscarinic antagonists]] |
|||
[[Category:Piperidines]] |
[[Category:Piperidines]] |
||
[[Category: |
[[Category:Serotonin antagonists]] |
||
Revision as of 14:23, 24 August 2017
 | |
| Clinical data | |
|---|---|
| Trade names | Zaditor[1] |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a604033 |
| Routes of administration | Oral (tablets), ophthalmic solution |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 60% |
| Protein binding | 75% |
| Metabolism | Hepatic |
| Elimination half-life | 12 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.047.348 |
| Chemical and physical data | |
| Formula | C19H19NOS |
| Molar mass | 309.426 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Ketotifen is a first-generation noncompetitive H1-antihistamine and mast cell stabilizer. It is most commonly sold as a salt with fumaric acid, ketotifen fumarate, and is available in two forms. In its ophthalmic form, it is used to treat allergic conjunctivitis.[2] In its oral form, it is used to prevent asthma attacks or Anaphylaxis, as well as various mast cell, allergic-type disorders.[3][4][5][6][7]
Medical uses
Ketotifen relieves and prevents eye itchiness and/or irritation associated with most seasonal allergies. It starts working within minutes after administering the drops. The drug has not been studied in children under three.[2] The mean elimination half life is 12 hours.[8] Besides its anti-histaminic activity, it is also a functional leukotriene antagonist and a phosphodiesterase inhibitor.
"[O]ral ketotifen has been used in patients with asthma, allergic rhinitis, allergic conjunctivitis, atopic dermatitis, chronic urticaria, cold-induced urticaria, cholinergic urticaria, exercise-induced urticaria, [systemic mast cell disease including mastocytosis, MCAS, allergic and nonallergic anaphylaxis, angioedema], and food allergy in Canada, Europe, and Mexico." Now available via prescription at US compounding pharmacies: "For adults and older children with asthma or allergic disease, the recommended dose of ketotifen is 1 mg twice daily." "FDA staff did recommend more extensive evaluations for management of urticaria."[5][6]
The drug may also help relieve the symptoms of irritable bowel syndrome.[9]
Side effects
Side effects include drowsiness, weight gain, dry mouth, irritability, and increased nosebleeds.[10]
Pharmacology
Ketotifen is a selective antihistamine – that is, an inverse agonist of the histamine H1 receptor (Ki = 0.166 nM)[11] – and mast cell stabilizer.[12] In addition, ketotifen has weak anticholinergic (Ki = 204 nM for mACh) and antiserotonergic (Ki = 39.9 nM for 5-HT2A) activity.[13] However, at the dosages in which it is typically used clinically, both the anticholinergic and antiserotonergic activity of ketotifen are said not to be appreciable.[14]
Society and culture
Brand names
Ketotifen is marketed under many brand names worldwide.[15]
References
- ^ http://www.webmd.com/drugs/2/drug-17484/zaditor-opht/details[full citation needed]
- ^ a b Zaditor prescribing information Novartis
- ^ Sokol, Kristin C.; Amar, Neil K.; Starkey, Jonathan; Grant, J. Andrew (2013). "Ketotifen in the management of chronic urticaria: Resurrection of an old drug". Annals of Allergy, Asthma & Immunology. 111 (6): 433–6. doi:10.1016/j.anai.2013.10.003. PMC 4309375. PMID 24267353.
- ^ Shawky, Rabah M.; Seifeldin, Neveen S. (2015). "The relation between antihistamine medication during early pregnancy & birth defects". Egyptian Journal of Medical Human Genetics. 16 (4): 287–90. doi:10.1016/j.ejmhg.2015.04.003.
- ^ a b Zuberbier, Torsten (2012). "A Summary of the New International EAACI/GA2LEN/EDF/ WAO Guidelines in Urticaria". World Allergy Organization Journal. 5 (1): S1–S5. doi:10.1186/1939-4551-5-S1-S1.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Zuberbier, T.; Asero, R.; Bindslev-Jensen, C.; Walter Canonica, G.; Church, M. K.; Giménez-Arnau, A. M.; Grattan, C. E. H.; Kapp, A.; Maurer, M.; Merk, H. F.; Rogala, B.; Saini, S.; Sánchez-Borges, M.; Schmid-Grendelmeier, P.; Schünemann, H.; Staubach, P.; Vena, G. A.; Wedi, B. (2009). "EAACI/GA²LEN/EDF/WAO guideline: Management of urticaria". Allergy. 64 (10): 1427–43. doi:10.1111/j.1398-9995.2009.02178.x. PMID 19772513.
- ^ Li, Zhenhong; Celestin, Jocelyn (February 23, 2015). Ketotifen: A Role in the Treatment of Idiopathic Anaphylaxis. American Academy of Allergy, Asthma & Immunology Annual Meeting. Houston.
- ^ Grahnén, A.; Lönnebo, A.; Beck, O.; Eckernäs, S-Å; Dahlström, B.; Lindström, B. (1992). "Pharmacokinetics of ketotiffn after oral administration to healthy male subjects". Biopharmaceutics & Drug Disposition. 13 (4): 255–62. doi:10.1002/bdd.2510130404. PMID 1600111.
- ^ Klooker, T. K.; Braak, B.; Koopman, K. E.; Welting, O.; Wouters, M. M.; Van Der Heide, S.; Schemann, M.; Bischoff, S. C.; Van Den Wijngaard, R. M.; Boeckxstaens, G. E. (2010). "The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome". Gut. 59 (9): 1213–21. doi:10.1136/gut.2010.213108. PMID 20650926.
- ^ http://www.mims.co.uk/drugs/allergic-disorders/allergic-rhinitis-urticaria-other-allergies/zaditen
- ^ Kakiuchi M, Ohashi T, Musoh K, Kawamura K, Morikawa K, Kato H (1997). "Studies on the novel antiallergic agent HSR-609: its penetration into the central nervous system in mice and guinea pigs and its selectivity for the histamine H1-receptor". Jpn. J. Pharmacol. 73 (4): 291–8. PMID 9165365.
- ^ Thomas L. Lemke; David A. Williams (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1019–. ISBN 978-0-7817-6879-5.
- ^ V Alagarsamy (16 June 2012). Textbook of Medicinal Chemistry Vol II - E-Book. Elsevier Health Sciences. pp. 38–. ISBN 81-312-3259-X.
- ^ Jürgen Drews (6 December 2012). Immunopharmacology: Principles and Perspectives. Springer Science & Business Media. pp. 282–. ISBN 978-3-642-75561-3.
- ^ drugs.com International availability of ketofin Page accessed April 21, 2015
