From Wikipedia, the free encyclopedia
Amiloride Trade names Midamor AHFS /Drugs.com Monograph Routes of Oral ATC code Legal status Bioavailability Readily absorbed, 15–25% Protein binding ~23% Metabolism Nil Onset of action 2 hours (peak at 6–10 hours, duration ~24 hours) Elimination half-life 6 to 9 hours Excretion Urine (20–50%), feces (40%)
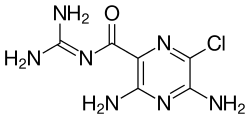
3,5-diamino-6-chloro-N -(diaminomethylene)pyrazine-2-carboxamide
CAS Number PubChem CID IUPHAR/BPS DrugBank ChemSpider UNII KEGG ChEBI ChEMBL CompTox Dashboard (EPA ) ECHA InfoCard 100.018.205 Formula C 6 H 8 Cl N 7 O Molar mass 229.627 g/mol g·mol−1 3D model (JSmol )
Clc1nc(C(=O)\N=C(/N)N)c(nc1N)N
InChI=1S/C6H8ClN7O/c7-2-4(9)13-3(8)1(12-2)5(15)14-6(10)11/h(H4,8,9,13)(H4,10,11,14,15)
Y Key:XSDQTOBWRPYKKA-UHFFFAOYSA-N
Y (verify)
Amiloride is a potassium-sparing diuretic , first approved for use in 1967 (then known as MK 870), used in the management of hypertension and congestive heart failure . Amiloride was also tested as treatment of cystic fibrosis , but it was revealed inefficient in vivo due to its short time of action, therefore longer-acting epithelial sodium channel (ENaC) inhibitors may prove more effective, e.g. benzamil .[1]
It is on the World Health Organization's List of Essential Medicines , a list of the most important medication needed in a basic health system .[2]
Structure Amiloride is a guanidinium group containing pyrazine derivative .
Contraindications Amiloride is contraindicated in patients with Addison's disease , hyperkalaemia and anuria .
Mechanism of action Amiloride works by directly blocking the epithelial sodium channel (ENaC) thereby inhibiting sodium reabsorption in the late distal convoluted tubules , connecting tubules, and collecting ducts in the kidneys (this mechanism is the same for triamterene ).[3] potassium . The drug is often used in conjunction with thiazide (e.g. co-amilozide ) or loop diuretics (e.g. co-amilofruse ). Due to its potassium-sparing capacities, hyperkalemia (high blood potassium levels) is occasionally observed in patients taking amiloride. The risk is high in concurrent use of ACE inhibitors or spironolactone . Patients are also advised not to use potassium-containing salt replacements.[4] acidosis .
A fraction of the effects of amiloride is inhibition of cyclic GMP-gated cation channels in the inner medullary collecting duct .[5]
Amiloride has a second action on the heart, blocking Na+ /H+ exchangers sodium–hydrogen antiporter 1 or NHE-1. This minimizes reperfusion injury in ischemic attacks.
Amiloride also blocks the Na+ /H+ antiporter on the apical surface of the proximal tubule cells, in the kidney, abolishing more than 80% of the action of angiotensin II on the secretion of hydrogen ions in proximal tubule cells.[6]
Acid-sensing ion channels (ASICs) are also sensitive to inhibition by amiloride. ASICs are involved in nociceptor responses to pH.[7]
Formulations and trade names See also References
^ (Review)Pharmacological treatment of the biochemical defect in cystic fibrosis airways, H.C. Rodgers, A.J. Knoxhttp://erj.ersjournals.com/content/17/6/1314.full.pdf+html
^ "WHO Model List of EssentialMedicines" (PDF) . World Health Organization . October 2013. Retrieved 22 April 2014 .^ Loffing, Johannes; Kaissling, Brigitte (2003). "Sodium and calcium transport pathways along the mammalian distal nephron: from rabbit to human". Am J Physiol Renal Physiol . 284 (4): F628–F643. doi :10.1152/ajprenal.00217.2002 . PMID 12620920 . ^ LoSalt Advisory Statement (PDF)
^ Walter F. Boron. Medical Physiology: A Cellular And Molecular Approaoch . Elsevier/Saunders. ISBN 1-4160-2328-3 ^ M G Cogan, Angiotensin II: a powerful controller of sodium transport in the early proximal tubule, Hypertension. 1990;15:451-458, doi: 10.1161/01.HYP.15.5.451, http://hyper.ahajournals.org/content/15/5/451
^ Hunt and Koltzenburg 2005 'The neurobiology of pain'
Calcium
VDCCs Tooltip Voltage-dependent calcium channels
Potassium
VGKCs Tooltip Voltage-gated potassium channels
IRKs Tooltip Inwardly rectifying potassium channel
KCa Tooltip Calcium-activated potassium channel
K2Ps Tooltip Tandem pore domain potassium channel
Sodium
VGSCs Tooltip Voltage-gated sodium channels
ENaC Tooltip Epithelial sodium channel
ASICs Tooltip Acid-sensing ion channel
Chloride
CaCCs Tooltip Calcium-activated chloride channel
CFTR Tooltip Cystic fibrosis transmembrane conductance regulator
Unsorted
Others
TRPs Tooltip Transient receptor potential channels LGICs Tooltip Ligand gated ion channels
Ionotropic
GABAA Tooltip γ-Aminobutyric acid A receptor
Positive modulators (abridged; see here for a full list): α-EMTBL Alcohols (e.g., drinking alcohol , 2M2B )Anabolic steroids Avermectins (e.g., ivermectin )Barbiturates (e.g., phenobarbital )Benzodiazepines (e.g., diazepam )Bromide compounds (e.g., potassium bromide )Carbamates (e.g., meprobamate )Carbamazepine Chloralose Chlormezanone Clomethiazole Dihydroergolines (e.g., ergoloid (dihydroergotoxine) )Etazepine Etifoxine Fenamates (e.g., mefenamic acid )Flavonoids (e.g., apigenin , hispidulin )Fluoxetine Flupirtine Imidazoles (e.g., etomidate )Kava constituents (e.g., kavain )Lanthanum Loreclezole Monastrol Neuroactive steroids (e.g., allopregnanolone , cholesterol , THDOC )Niacin Niacinamide Nonbenzodiazepines (e.g., β-carbolines (e.g., abecarnil ), cyclopyrrolones (e.g., zopiclone ), imidazopyridines (e.g., zolpidem ), pyrazolopyrimidines (e.g., zaleplon ))Norfluoxetine Petrichloral Phenols (e.g., propofol )Phenytoin Piperidinediones (e.g., glutethimide )Propanidid Pyrazolopyridines (e.g., etazolate )Quinazolinones (e.g., methaqualone )Retigabine (ezogabine) ROD-188 Skullcap constituents (e.g., baicalin )Stiripentol Sulfonylalkanes (e.g., sulfonmethane (sulfonal) )Topiramate Valerian constituents (e.g., valerenic acid )Volatiles /gases (e.g., chloral hydrate , chloroform , diethyl ether , paraldehyde , sevoflurane )Negative modulators: 1,3M1B 3M2B 11-Ketoprogesterone 17-Phenylandrostenol α3IA α5IA (LS-193,268) β-CCB β-CCE β-CCM β-CCP β-EMGBL Anabolic steroids Amiloride Anisatin β-Lactams (e.g., penicillins , cephalosporins , carbapenems )Basmisanil Bemegride Bicyclic phosphates (TBPS , TBPO , IPTBO )BIDN Bilobalide Bupropion CHEB Chlorophenylsilatrane Cicutoxin Cloflubicyne Cyclothiazide DHEA DHEA-S Dieldrin (+)-DMBB DMCM DMPC EBOB Etbicyphat FG-7142 (ZK-31906) Fiproles (e.g., fipronil )Flavonoids (e.g., amentoflavone , oroxylin A )Flumazenil Fluoroquinolones (e.g., ciprofloxacin )Flurothyl Furosemide Golexanolone Iomazenil (123 I) IPTBO Isopregnanolone (sepranolone) L-655,708 Laudanosine Lindane MaxiPost Morphine Morphine-3-glucuronide MRK-016 Naloxone Naltrexone Nicardipine Nonsteroidal antiandrogens (e.g., apalutamide , bicalutamide , enzalutamide , flutamide , nilutamide )Oenanthotoxin Pentylenetetrazol (pentetrazol) Phenylsilatrane Picrotoxin (i.e., picrotin , picrotoxinin and dihydropicrotoxinin )Pregnenolone sulfate Propybicyphat PWZ-029 Radequinil Ro 15-4513 Ro 19-4603 RO4882224 RO4938581 Sarmazenil SCS Suritozole TB-21007 TBOB TBPS TCS-1105 Terbequinil TETS Thujone U-93631 Zinc ZK-93426 GABAA -ρ Tooltip γ-Aminobutyric acid A-rho receptor
Metabotropic
GABAB Tooltip γ-Aminobutyric acid B receptor
Receptor (ligands )
GlyR Tooltip Glycine receptor
Positive modulators: Alcohols (e.g., brometone , chlorobutanol (chloretone) , ethanol (alcohol) , tert -butanol (2M2P)tribromoethanol , trichloroethanol , trifluoroethanol )Alkylbenzene sulfonate Anandamide Barbiturates (e.g., pentobarbital , sodium thiopental )Chlormethiazole D12-116 Dihydropyridines (e.g., nicardipine )Etomidate Ginseng constituents (e.g., ginsenosides (e.g., ginsenoside-Rf ))Glutamic acid (glutamate) Ivermectin Ketamine Neuroactive steroids (e.g., alfaxolone , pregnenolone (eltanolone) , pregnenolone acetate , minaxolone , ORG-20599 )Nitrous oxide Penicillin G Propofol Tamoxifen Tetrahydrocannabinol Triclofos Tropeines (e.g., atropine , bemesetron , cocaine , LY-278584 , tropisetron , zatosetron )Volatiles /gases (e.g., chloral hydrate , chloroform , desflurane , diethyl ether (ether) , enflurane , halothane , isoflurane , methoxyflurane , sevoflurane , toluene , trichloroethane (methyl chloroform) , trichloroethylene )Xenon Zinc Antagonists: 2-Aminostrychnine 2-Nitrostrychnine 4-Phenyl-4-formyl-N-methylpiperidine αEMBTL Bicuculline Brucine Cacotheline Caffeine Colchicine Colubrine Cyanotriphenylborate Dendrobine Diaboline Endocannabinoids (e.g., 2-AG , anandamide (AEA) )Gaboxadol (THIP) Gelsemine iso-THAZ Isobutyric acid Isonipecotic acid Isostrychnine Laudanosine N-Methylbicuculline N-Methylstrychnine N,N-Dimethylmuscimol Nipecotic acid Pitrazepin Pseudostrychnine Quinolines (e.g., 4-hydroxyquinoline , 4-hydroxyquinoline-3-carboxylic acid , 5,7-CIQA , 7-CIQ , 7-TFQ , 7-TFQA )RU-5135 Sinomenine Strychnine Thiocolchicoside Tutin Negative modulators: Amiloride Benzodiazepines (e.g., bromazepam , clonazepam , diazepam , flunitrazepam , flurazepam )Corymine Cyanotriphenylborate Daidzein Dihydropyridines (e.g., nicardipine , nifedipine , nitrendipine )Furosemide Genistein Ginkgo constituents (e.g., bilobalide , ginkgolides (e.g., ginkgolide A , ginkgolide B , ginkgolide C , ginkgolide J , ginkgolide M ))Imipramine NBQX Neuroactive steroids (e.g., 3α-androsterone sulfate , 3β-androsterone sulfate , deoxycorticosterone , DHEA sulfate , pregnenolone sulfate , progesterone )Opioids (e.g., codeine , dextromethorphan , dextrorphan , levomethadone , levorphanol , morphine , oripavine , pethidine , thebaine )Picrotoxin (i.e., picrotin and picrotoxinin )PMBA Riluzole Tropeines (e.g., bemesetron , LY-278584 , tropisetron , zatosetron )Verapamil Zinc NMDAR Tooltip N-Methyl-D-aspartate receptor
Transporter (blockers )
GlyT1 Tooltip Glycine transporter 1 GlyT2 Tooltip Glycine transporter 2