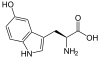
5-Hydroxytryptophan

| |

| |
| Names | |
|---|---|
| IUPAC name
2-Amino-3-(5-hydroxy-1H-indol-3-yl)propanoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.022.193 |
| KEGG | |
| MeSH | 5-Hydroxytryptophan |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H12N2O3 | |
| Molar mass | 220.228 g·mol−1 |
| Density | 1.484 g/mL |
| Melting point | 298 °C (568 °F; 571 K) |
| Boiling point | 520.6 °C (969.1 °F; 793.8 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
5-Hydroxytryptophan (5-HTP), also known as oxitriptan (INN), is a naturally occurring amino acid and chemical precursor as well as a metabolic intermediate in the biosynthesis of the neurotransmitters serotonin and melatonin from tryptophan.
5-HTP is sold over the counter in the United Kingdom, the United States and Canada as a dietary supplement for use as an antidepressant, appetite suppressant, and sleep aid, and is also marketed in many European countries for the indication of major depression under trade names like Cincofarm, Levothym, Levotonine, Oxyfan, Telesol, Tript-OH, and Triptum.[1][2] Several double-blind placebo-controlled clinical trials have demonstrated the effectiveness of 5-HTP in the treatment of depression,[1] though a lack of high quality studies has been noted.[3] More and larger studies are needed to determine if 5-HTP is truly effective in treating depression,[4] but funding for such studies is lacking due to its non-patentable status.[5]
Metabolism
5-HTP is decarboxylated to serotonin (5-hydroxytryptamine or 5-HT) by the enzyme aromatic-L-amino-acid decarboxylase with the help of vitamin B6.[6] This reaction occurs both in nervous tissue and in the liver.[7] 5-HTP crosses the blood–brain barrier,[8] while 5-HT does not. Excess 5-HTP, especially when administered with vitamin B6, is thought to be metabolized and excreted.[9][10]
| ||||||||||||||||||||
Pharmacology
The psychoactive action of 5-HTP is derived from its increase in production of serotonin in central nervous system tissue.[11]

Research shows that co-administration with carbidopa greatly increases plasma 5-HTP levels.[12] However, several studies have reported that 5-HTP is effective even without a peripheral decarboxylase inhibitor (e.g. carbidopa).[13] Other studies have indicated the risk of a scleroderma-like condition resulting from the combination of 5-HTP and carbidopa.[14]
Dietary sources
Though 5-HTP is found in food only in insignificant quantities, it is a chemical involved intermediately in the metabolism of tryptophan, an amino acid found in milk, meat, potatoes, pumpkin, and various greens.[15] See also the Dietary sources section of the article on L-tryptophan.
Therapeutic use
5-HTP is sold over-the-counter in the United States, the United Kingdom, and Canada as a dietary supplement for use as an antidepressant, appetite suppressant, and sleep aid. 5-HTP in supplement form is typically sold in 50 mg or 100 mg gelatin or vegetarian capsules, however in the United Kingdom 5-HTP became available in transdermal patch form in early 2014.[16] It is usually sourced from the seeds of Griffonia simplicifolia.
In 2001 a Cochrane Review of the effect of 5-HTP and tryptophan on depression was published. The authors included only studies of a high rigor and included both 5-HTP and tryptophan in their review because of the limited data on either. Of 108 studies of 5-HTP and tryptophan on depression published between 1966 and 2000, only two met the authors' quality standards for inclusion, totaling 64 study participants. The substances were more effective than placebo in the two studies included but the authors state that, "the evidence was of insufficient quality to be conclusive," and note, "because alternative antidepressants exist which have been proven to be effective and safe, the clinical usefulness of 5-HTP and tryptophan is limited at present."[3]
5-HTP is often taken by people coming down from MDMA to relieve post-MDMA dysphoria. The basis for doing this is that 5-HTP is a necessary precursor for the brain to produce more serotonin, and MDMA use depletes a person's natural serotonin levels, thus taking 5-HTP after consuming MDMA is speculated as helping improve serotonin production. While the practice is common, the theory is physiologically reasonable, and anecdotal evidence is widespread,[17] no scientifically verifiable evidence can currently be found to confirm whether the practice actually works. Concurrent use of 5-HTP and monoamine oxidase inhibitors may increase risk of serotonin syndrome. Due to the rate-limiting nature of the decarboxylase enzyme which converts 5-HTP into serotonin, the risk of serotonin syndrome is thought to be quite low unless both monoamine oxidase inhibitors and 5-htp are taken in large quantities. No conclusive evidence suggests coadministration of 5-HTP and serotonin releasing agents such as MDMA carry increased risk of serotonin syndrome, and anecdotal evidence suggests coadministration may decrease some of the neurotoxic metabolites produced as degradation products of MDMA (see Alpha-Methyldopamine)
Possible risks or side effects
Because 5-HTP has not been thoroughly studied in a clinical setting, possible side effects and interactions with other drugs are not well known. However, it is noteworthy that no published reports of serious side effects (from non-contaminated 5-HTP) exist, despite that 5-HTP is freely available as a nutraceutical.[18][19][20] This could indicate that serious side effects are relatively rare with 5-HTP, at least in moderate doses[quantify]. On the other hand, acute moderate gastrointestinal effects, such as diarrhea and vomiting, are common upon administration of 5-HTP, probably due to rapid formation of serotonin in the upper intestinal tract.[18][21][22]
Oral 5-HTP results in an increase in urinary 5-HIAA, a serotonin metabolite, indicating that 5-HTP is peripherally metabolized to serotonin, which is then metabolized. This might cause a false positive test in tests looking for carcinoid syndrome.[23]
Known drug interactions:
- When combined with antidepressants of the MAOI or SSRI class, high dose 5-HTP can cause acute serotonin syndrome in rats.[24][25]
In humans 5-HTP has never been clinically associated with serotonin syndrome.
- When combined with carbidopa (as a treatment for symptoms of Parkinson's disease), 5-HTP causes nausea and vomiting; however this can be alleviated via administration of granisetron.[26] As mentioned above under pharmacology, cases of scleroderma-like illness have been reported in patients using carbidopa and 5-HTP.[27]
It has been suggested by the pharmaceutical industry that 5-HTP may cause eosinophilia-myalgia syndrome (EMS), a serious condition which results in extreme muscle tenderness, myalgia, and blood abnormalities. However, there is evidence to show that EMS was caused by a contaminant in early 5-HTP supplements, before the introduction of the current Good Manufacturing Practices by the United States FDA in 2007. Many countries now employ similar regulation.[28]
See also
References
- ^ a b Turner EH, Blackwell AD (2005). "5-Hydroxytryptophan plus SSRIs for interferon-induced depression: synergistic mechanisms for normalizing synaptic serotonin". Medical Hypotheses. 65 (1): 138–44. doi:10.1016/j.mehy.2005.01.026. PMID 15893130.
- ^ Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. ISBN 3-88763-075-0.
- ^ a b Shaw K, Turner J, Del Mar C (2002). Shaw, Kelly A (ed.). "Tryptophan and 5-hydroxytryptophan for depression". Cochrane Database of Systematic Reviews (Online) (1): CD003198. doi:10.1002/14651858.CD003198. PMID 11869656.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ 5-Hydroxytryptophan (5-HTP) University of Maryland Medical Center. 2011. Accessed: 9 January 2012.
- ^ Baker, Dean, Financing Drug Research: What are the Issues?. 2008 Industry Studies Conference Paper. Available at SSRN: http://ssrn.com/abstract=1134983 or http://dx.doi.org/10.2139/ssrn.1134983
- ^ Rahman MK, Nagatsu T, Sakurai T, Hori S, Abe M, Matsuda M (1982). "Effect of pyridoxal phosphate deficiency on aromatic L-amino acid decarboxylase activity with L-DOPA and L-5-hydroxytryptophan as substrates in rats". Jpn. J. Pharmacol. 32 (5): 803–11. doi:10.1254/jjp.32.803. PMID 6983619.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bouchard, S; Bousquet, C; Roberge, AG (1981). "Characteristics of dihydroxyphenylalanine/5-hydroxytryptophan decarboxylase activity in brain and liver of cat". Journal of Neurochemistry. 37 (3): 781–7. doi:10.1111/j.1471-4159.1982.tb12555.x. PMID 6974228.
- ^ Gomes P, Soares-da-Silva P. (1999). "L-DOPA transport properties in an immortalised cell line of rat capillary cerebral endothelial cells, RBE 4". Brain Res. 829 (1–2): 143–150. doi:10.1016/S0006-8993(99)01387-6. PMID 18445233.
- ^ Bouchard S, Roberge AG (1979). "Biochemical properties and kinetic parameters of dihydroxyphenylalanine--5-hydroxytryptophan decarboxylase in brain, liver, and adrenals of cat". Can. J. Biochem. 57 (7): 1014–8. doi:10.1139/o79-126. PMID 39668.
- ^ Amamoto T, Sarai K (1976). "On the tryptophan-serotonin metabolism in manic-depressive disorders. Changes in plasma 5-HT and 5-HIAA levels and urinary 5-HIAA excretion following oral loading of L-5HTP in patients with depression". Hiroshima J. Med. Sci. 25 (2–3): 135–40. PMID 1088369.
- ^ "5-HTP: Uses, Side Effects, Interactions and Warnings - WebMD". Archived from the original on 16 November 2009. Retrieved 2009-10-05.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Magnussen I, Jensen TS, Rand JH, Van Woert MH (1981). "Plasma accumulation of metabolism of orally administered single dose L-5-hydroxytryptophan in man". Acta pharmacologica et toxicologica. 49 (3): 184–9. doi:10.1111/j.1600-0773.1981.tb00890.x. PMID 6175178.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Birdsall TC (1998). "5-Hydroxytryptophan: a clinically-effective serotonin precursor". Alternative medicine review : a journal of clinical therapeutic. 3 (4): 271–80. PMID 9727088.
- ^ Sternberg EM, Van Woert MH, Young SN; et al. (1980). "Development of a scleroderma-like illness during therapy with L-5-hydroxytryptophan and carbidopa". N. Engl. J. Med. 303 (14): 782–7. doi:10.1056/NEJM198010023031403. PMID 6997735.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ "5-Hydroxytryptophan". University of Maryland Medical Center. Archived from the original on 6 January 2010. Retrieved 2010-01-21.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ ""Revolutionary" 5-HTP transdermal patch launches in United Kingdom". 5htppatch.co.uk. Retrieved 2014-06-18.
- ^ "Ecstasy and Depression". DanceSafe.Org. Retrieved 2014-01-09.
- ^ a b Turner EH, Loftis JM, Blackwell AD. Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan. Pharmacol Ther. 2006 Mar;109(3):325-38. Epub 2005 Jul 14. Review. PubMed PMID 16023217.
- ^ Byerley WF, Judd LL, Reimherr FW, Grosser BI. 5-Hydroxytryptophan: a review of its antidepressant efficiency and adverse effects. J Clin Psychopharmacol. 1987 Jun;7(3):127-37. Review. PubMed PMID 3298325.
- ^ van Hiele LJ. l-5-Hydroxytryptophan in depression: the first substitution therapy in psychiatry? The treatment of 99 out-patients with 'therapy-resistant' depressions. Neuropsychobiology. 1980;6(4):230-40. PubMed PMID 6967194.
- ^ Gijsman HJ, van Gerven JM, de Kam ML, Schoemaker RC, Pieters MS, Weemaes M, de Rijk R, van der Post J, Cohen AF. Placebo-controlled comparison of three dose-regimens of 5-hydroxytryptophan challenge test in healthy volunteers. J Clin Psychopharmacol. 2002 Apr;22(2):183-9. PubMed PMID 11910264.
- ^ 1: Lowe SL, Yeo KP, Teng L, Soon DK, Pan A, Wise SD, Peck RW. L-5-Hydroxytryptophan augments the neuroendocrine response to a SSRI. Psychoneuroendocrinology. 2006 May;31(4):473-84. Epub 2005 Dec 27. PubMed PMID 16378695.
- ^ Joy T, Walsh G, Tokmakejian S, Van Uum SH (January 2008). "Increase of urinary 5-hydroxyindoleacetic acid excretion but not serum chromogranin A following over-the-counter 5-hydroxytryptophan intake". Can. J. Gastroenterol. 22 (1): 49–53. PMC 2659120. PMID 18209781.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ma Z, Zhang G, Jenney C, Krishnamoorthy S, Tao R. (July 2008). "Characterization of serotonin-toxicity syndrome (toxidrome) elicited by 5-hydroxy-l-tryptophan in clorgyline-pretreated rats". Eur J Pharmacol. 588 (2–3): 198–206. doi:10.1016/j.ejphar.2008.04.004. PMID 18499101.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Izumi T, Iwamoto N, Kitaichi Y, Kato A, Inoue T, Koyama T. (2006). "Effects of co-administration of a selective serotonin reuptake inhibitor and monoamine oxidase inhibitors on 5-HT-related behavior in rats". Eur J Pharmacol. 532 (3): 258–264. doi:10.1016/j.ejphar.2005.12.075. PMID 16488409.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jacobs G, Kamerling I, de Kam M; et al. (Nov 2008). "Enhanced tolerability of the 5-hydroxytryptophane challenge test combined with granisetron". J Psychopharmacol. (Oxford). 24 (1): 65–72. doi:10.1177/0269881108094299. PMID 18719048.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ "Carbidopa/Levodopa". Truestarhealth.com. Retrieved 2014-01-09.
- ^ Michelson, D; Page, SW, Casey, R, Trucksess, MW, Love, LA, Milstien, S, Wilson, C, Massaquoi, SG, Crofford, LJ, Hallett, M (December 1994). "An eosinophilia-myalgia syndrome related disorder associated with exposure to L-5-hydroxytryptophan". The Journal of rheumatology. 21 (12): 2261–5. PMID 7699627.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Further reading
- den Boer JA, Westenberg HG (1990). "Behavioral, neuroendocrine, and biochemical effects of 5-hydroxytryptophan administration in panic disorder". Psychiatry Research. 31 (3): 267–78. doi:10.1016/0165-1781(90)90096-N. PMID 2139731.
- Angst J, Woggon B, Schoepf J (1977). "The treatment of depression with L-5-hydroxytryptophan versus imipramine. Results of two open and one double-blind study". Archiv für Psychiatrie und Nervenkrankheiten. 224 (2): 175–86. PMID 336002.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - article at Psychology Today
- Turner EH, Loftis JM, Blackwell AD (2006). "Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan". Pharmacol. Ther. 109 (3): 325–38. doi:10.1016/j.pharmthera.2005.06.004. PMID 16023217.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - 5-Hydroxytryptophan (5-HTP) Supplement Information at University of Maryland Medical Center
- 5- HTP- Myth Or Miracle? at Vanderbilt University · Nashville, Tennessee
- Ron Sturtz (2009). "what is the difference between L-Tryptophan and 5-HTP?". Neuropsychopharmacology: 1.