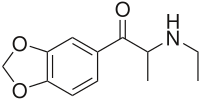
Ethylone
This is an old revision of this page, as edited by DMacks (talk | contribs) at 20:11, 19 June 2020 (Remove malformatted |molecular_weight= when infobox can autocalculate it, per Wikipedia talk:WikiProject Pharmacology#Molecular weights in drugboxes (via WP:JWB)). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral, nasal, IV |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C12H15NO3 |
| Molar mass | 221.256 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Ethylone, also known as 3,4-methylenedioxy-N-ethylcathinone (MDEC, βk-MDEA), is a recreational designer drug classified as an entactogen, stimulant, and psychedelic of the phenethylamine, amphetamine, and cathinone chemical classes. It is the β-keto analogue of MDEA ("Eve"). Ethylone has only a short history of human use and is reported to be less potent than its relative methylone.[citation needed] In the United States, it began to be found in cathinone products in late 2011.[1]
Very little data exists about the pharmacological properties, metabolism, and toxicity of ethylone, and although several ethylone-related deaths have been reported, but the cause of death was not due to ingestion of ethylone.[1]
Pharmacokinetics
Analysis of human and rat urine for the metabolites of bk-amphetamines suggested that ethylone was degraded in the following metabolic steps:[2]
- N-deethylation to the primary amine.
- Reduction of the keto moiety to the respective alcohol.
Legal Status
As of October 2015 Ethylone is a controlled substance in China.[3]
See also
References
- ^ a b Dayong Lee; Chris W. Chronister; Jennifer Hoyer; Bruce A. Goldberger (September 2015). "Ethylone-Related Deaths: Toxicological Findings". Journal of Analytical Toxicology. 39 (7): 567–571. doi:10.1093/jat/bkv053. PMID 26025164.
- ^ Meyer, Markus R; Jens Wilhelm; Frank T. Peters; Hans H. Maurer (June 2010). "Beta-keto amphetamines: studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography–mass spectrometry". Analytical and Bioanalytical Chemistry. 397 (3): 1225–1233. doi:10.1007/s00216-010-3636-5. PMID 20333362.
- ^ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Retrieved 1 October 2015.
External links
| Phenylalkyl- amines (other than cathinones) |
|
|---|---|
| Cyclized phenyl- alkylamines | |
| Cathinones | |
| Tryptamines | |
| Chemical classes | |
| Adamantanes | |
|---|---|
| Adenosine antagonists | |
| Alkylamines | |
| Ampakines | |
| Arylcyclohexylamines | |
| Benzazepines | |
| Cathinones |
|
| Cholinergics |
|
| Convulsants | |
| Eugeroics | |
| Oxazolines | |
| Phenethylamines |
|
| Phenylmorpholines | |
| Piperazines | |
| Piperidines |
|
| Pyrrolidines | |
| Racetams | |
| Tropanes |
|
| Tryptamines | |
| Others |
|
| DRAsTooltip Dopamine releasing agents |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRAsTooltip Norepinephrine releasing agents |
| ||||||||||||||
| SRAsTooltip Serotonin releasing agents |
| ||||||||||||||
| Others |
| ||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |
- CS1 Chinese-language sources (zh)
- Articles with changed CASNo identifier
- Articles with changed ChemSpider identifier
- Articles with changed InChI identifier
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- All articles with unsourced statements
- Articles with unsourced statements from May 2015
- All stub articles
