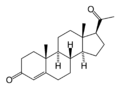
17α-Hydroxyprogesterone
 | |
 | |
| Clinical data | |
|---|---|
| License data | |
| Pregnancy category |
|
| Routes of administration | Intramuscular |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver |
| Elimination half-life | 10 days (17-OHPC)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.636 |
| Chemical and physical data | |
| Formula | C21H30O3 |
| Molar mass | 330.46 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 219.5 °C (427.1 °F) |
| |
| |
| (verify) | |
17-Hydroxyprogesterone (17-OH progesterone or 17OHP) is a C-21 steroid hormone produced during the synthesis of glucocorticoids and sex steroids.
As a hormone, 17OHP also interacts with the progesterone receptor.
Production
It is derived from progesterone via 17-hydroxylase, a P450c17 enzyme, or from 17-hydroxypregnenolone via 3β-hydroxysteroid dehydrogenase/Δ5-4 isomerase.
17-Hydroxyprogesterone is a natural progestogen, and in pregnancy increases in the third trimester primarily due to fetal adrenal production.
This hormone is primarily produced in the adrenal glands and to some degree in the gonads, specifically the corpus luteum of the ovary. Normal levels are 3-90 ng/dl in children, and in women, 20-100 ng/dl prior to ovulation, and 100-500 ng/dl during the luteal phase.[2][3]
17-Hydroxyprogesterone caproate
17-Hydroxyprogesterone (17OHP) is not the same compound as 17-hydroxyprogesterone caproate (17OHPC), the latter being the caproic acid of 17-Hydroxyprogesterone that was first clinically introduced as an injectable version of the steroid under the name of Delalutin in 1956.
The use of 17-hydroxyprogesterone caproate in pregnancy to prevent preterm birth has a long history. A 2006 Cochrane Review concluded "...important maternal and infant outcomes have been poorly reported to date... information regarding the potential harms of progesterone therapy to prevent preterm birth is limited".[4] There was a similar conclusion from a review by Marc Keirse.[5] Three clinical studies of 250 mg/week of i.m. 17-hydroxyprogesterone caproate showed a trend for an increase in pregnancy loss due to miscarriage compared to placebo.[6][7][8][9] There had been speculation that the castor oil in some 17-hydroxyprogesterone caproate formulations may not be beneficial for pregnancy.[10][11] In contrast, a large NIH study in 2003 looked at the effect of 17OHPC injections in women at risk for repeat premature birth and found that the treated group experienced premature birth in 37% versus 55% in the controls.[12] A follow-up study of the offspring showed no evidence that 17OHPC affected the children in the first years of life.[13] Based on these NIH data, 17 OHPC was approved by the FDA in 2011 as a drug to reduce the risk of premature birth in selected patients at risk. (v.i.)
17-hydroxyprogesterone caproate is a category B drug according to the FDA.
Clinical use
Measurement
Measurements of levels of 17-hydroxyprogesterone are useful in the evaluation of patients with suspected congenital adrenal hyperplasia as the typical enzymes that are defective, namely 21-hydroxylase and 11β-hydroxylase, lead to a build-up of 17OHP. In contrast, the rare patient with 17α-hydroxylase deficiency will have very low or undetectable levels of 17OHP. 17OHP levels can also be used to measure contribution of progestational activity of the corpus luteum during pregnancy as progesterone but not 17OHP is also contributed by the placenta.
Medication
17OHPC was first introduced in 1956 as Delalutin to reduce pregnancy loss. After decades of use, Squibb, the manufacturer, voluntarily withdrew the brand, however, physicians continued to use 17OHPC "off label". Renewed interest was sparked with a large NIH-sponsored study in 2003 that found that 17OHPC reduced the risk of premature birth in selected at-risk pregnant women.[12] With follow-up data showing no evidence of harmful effects on the offspring, the FDA approved the drug, as sponsored by KV Pharmaceutical as Makena, as an orphan drug in February 2011 to reduce the risk of premature birth in women prior to 37 weeks gestation with a single fetus who had at least one previous premature birth.[14] The drug is not effective in preventing premature birth in women with multiples. With the arrival of Makena as an orphan drug, the price of the drug was to increase from $15 to $1,500 per dose meaning a typical treatment would cost $25–30,000, - a pricing strategy that was strongly criticized. The FDA then announced that pharmacies could continue to compound the drug at their usual cost of $10~20 per dose without fear of legal reprisals.,[15] and KV reduced its price to $690 per dose.[16]

Determination
Earlier immunoassays like RIA (radioimmunoassay) or IRMA (immunoradiometric assay) were used to clinically determine 17-Hydroxyprogesterone. Today more sophisticated methods use gas or liquid chromatography and mass spectrometry (e.g. LC-MS/MS).
Additional images
References
- ^ Pharmacokinetics of 17-hydroxyprogesterone caproate in multifetal gestation.
- ^ Reference Values During Pregnancy
- ^ normal ranges for hormone tests in women
- ^ Dodd JM, Flenady V, Cincotta R, Crowther CA; The Cochrane Database of Systematic Reviews 2006 Issue 1
- ^ Keirse MJNC. Progesterone and Preterm: Seventy Years of "Deja vu" or "Still To Be Seen"?. Birth, 2004 September; 31:3.
- ^ Johnson JWC, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17 alpha-hydroxyprogesterone caproate in the prevention of premature labor. NEJM 1975 October. 293(14):675.
- ^ Yemini M, Borenstein R, Dreazen, et al. Prevention of premature labor by 17 alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 1985;151(5):574-7.
- ^ Meis PJ et al. Prevention of Recurrent Preterm Delivery by 17 Alpha-hydroxyprogesterone Caproate. NEJM, 2003: vol 348, no 24, pg 2379-2385.
- ^ Keirse MJNC, Progestogen administration in pregnancy may prevent preterm delivery. Br J Obstet Gynecol 1990 February; 97:149.
- ^ Duke University Medical Center, New England Journal of Medicine, correspondence, vol 349.
- ^ Hauth JC, Gilstrap LC, Brekken AL, Hauth JM. The effect of 17 alpha-hydroxyprogesterone caproate on pregnancy outcome in an active-duty military population. Am J Obstet Gynecol. 1983 May; 146(2): 187.
- ^ a b Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, Spong CY, Hauth JC, Miodovnik M, Varner MW, Leveno KJ, Caritis SN, Iams JD, Wapner RJ, Conway D, O'Sullivan MJ, Carpenter M, Mercer B, Ramin SM, Thorp JM, Peaceman AM, Gabbe S; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. (2003). "Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate". N Engl J Med. 348 (24): 2379–85. doi:10.1056/NEJMoa035140. PMID 12802023.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Northen AT, Norman GS, Anderson K, Moseley L, Divito M, Cotroneo M, Swain M, Bousleiman S, Johnson F, Dorman K, Milluzzi C, Tillinghast JA, Kerr M, Mallett G, Thom E, Pagliaro S, Anderson GD; National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. (2007). "Follow-up of children exposed in utero to 17 alpha-hydroxyprogesterone caproate compared with placebo". Obstet Gynecol. 110 (4): 865–72. doi:10.1097/01.AOG.0000281348.51499.bc. PMID 17906021.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ FDA press release regarding Makena approval
- ^ http://www.macleans.ca/article.jsp?content=w6411446
- ^ Associated Press (April 2, 2011). "Price of preterm birth medicine cut". Boston.com. Retrieved April 2, 2011.
External links