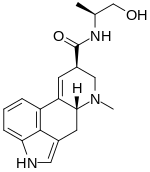
Ergometrine
| |||
| Clinical data | |||
|---|---|---|---|
| Other names | Ergonovine, lysergic acid beta- propanolamide | ||
| AHFS/Drugs.com | Monograph | ||
| Pregnancy category |
| ||
| Routes of administration | Oral | ||
| ATC code | |||
| Legal status | |||
| Legal status |
| ||
| Pharmacokinetic data | |||
| Metabolism | Liver | ||
| Excretion | Kidney | ||
| Identifiers | |||
| |||
| CAS Number | |||
| PubChem CID | |||
| IUPHAR/BPS | |||
| DrugBank | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEBI | |||
| ChEMBL | |||
| CompTox Dashboard (EPA) | |||
| ECHA InfoCard | 100.000.441 | ||
| Chemical and physical data | |||
| Formula | C19H23N3O2 | ||
| Molar mass | 325.41 g/mol g·mol−1 | ||
| 3D model (JSmol) | |||
| |||
| |||
| (verify) | |||
Ergometrine also known as ergonovine or d-lysergic acid beta-propanolamide, is a medication use to cause contractions of the uterus to treat heavy vaginal bleeding after childbirth.[1] It can be used either by mouth, by injection into a muscle, or injection into a vein. It begins working within 15 min when taken by mouth and is faster in onset when used by injection. Effects last between 45 and 180 minutes.[1]
Common side effect include high blood pressure, vomiting, seizures, headache, and low blood pressure. Other serious side effects include ergotism.[1] It was originally made from the rye ergot fungus but can also be made from lysergic acid.[2][3] Due to it being possible to make lysergic acid diethylamide (LSD) from ergometrine it is regulated.[4]
Ergometrine was discovered in 1932.[2] It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[5] The wholesale cost is between 0.12 and 0.41 USD for an injectable dose and 0.01 USD for a pill as of 2014.[6][7] In the United States it is about 1.75 USD per dose.[1]
Medical use
It has a medical use in obstetrics to facilitate delivery of the placenta and to prevent bleeding after childbirth by causing smooth muscle tissue in the blood vessel walls to narrow, thereby reducing blood flow. It is usually combined with oxytocin (Syntocinon) as syntometrine.
It can induce spasm of the coronary arteries.[8] It is used to diagnose variant (Prinzmetal's) angina.[9]
Side effects
Possible side effects include nausea, vomiting, abdominal pain, diarrhea, headache, dizziness, tinnitus, chest pain, palpitation, bradycardia, transient hypertension and other cardiac arrhythmias, dyspnea, rashes, and shock.[10] An overdose produces a characteristic poisoning, ergotism or "St. Anthony's fire": prolonged vasospasm resulting in gangrene and amputations; hallucinations and dementia; and abortions. Gastrointestinal disturbances such as diarrhea, nausea, and vomiting, are common.[11] The drug is contraindicated in pregnancy, vascular disease, and psychosis.
Mechanism of action
While it acts at alpha-adrenergic, dopaminergic, and serotonin receptors (the 5-HT2 receptor), it exerts on the uterus (and other smooth muscles) a powerful stimulant effect not clearly associated with a specific receptor type.
History
The pharmacological properties of ergot were known and had been utilised by midwives for centuries, but were not thoroughly researched and publicised until the early 20th century. However, its abortifacient effects and the danger of ergotism meant that it was only prescribed cautiously, as in the treatment of postpartum haemorrhage.[12]
Ergometrine was first isolated and obtained by the chemists C Moir and H W Dudley in 1935.[13] Caroline De Costa has argued that the adoption of ergometrine for prophylatic use and for treating haemorrhaging contributed to the decline in the maternal mortality rate in much of the West during the early 20th century.[12]
Legal status
Ergometrine is listed as Table I precursors under the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances, as possible precursor compound for LSD.[14] As an N-alkyl derivative of lysergamide, ergometrine is also covered by the Misuse of Drugs Act 1971, effectively rendering it illegal in the United Kingdom.
See also
Methylergometrine - a synthetic analogue
References
- ^ a b c d "Ergonovine Maleate". The American Society of Health-System Pharmacists. Retrieved Dec 2015.
{{cite web}}: Check date values in:|accessdate=(help) - ^ a b Ravina, Enrique (2011). The evolution of drug discovery : from traditional medicines to modern drugs (1. Aufl. ed.). Weinheim: Wiley-VCH. p. 245. ISBN 9783527326693.
- ^ Sneader, Walter (2005). Drug discovery : a history (Rev. and updated ed.). Chichester: Wiley. p. 349. ISBN 9780471899792.
- ^ King, L.A. (2009). Forensic chemistry of substance misuse : a guide to drug control. Cambridge, UK: Royal Society of Chemistry. p. 190. ISBN 9780854041787.
- ^ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ^ "Ergometrine Maleate". International Drug Price Indicator Guide. Retrieved 25 December 2015.
- ^ "Ergometrine Maleate". International Drug Price Indicator Guide. Retrieved 25 December 2015.
- ^ Romagnoli E, Niccoli G, Crea F (October 2005). "Images in cardiology: A coronary organic stenosis distal to severe, ergonovine induced spasm: decision making". Heart. 91 (10): 1310. doi:10.1136/hrt.2004.058560. PMC 1769140. PMID 16162623.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sunagawa O, Shinzato Y, Touma T, Tomori M, Fukiyama K (May 2000). "Differences between coronary hyperresponsiveness to ergonovine and vasospastic angina". Jpn Heart J. 41 (3): 257–68. doi:10.1536/jhj.41.257. PMID 10987346.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ergometrine drug information
- ^ McDonald S, Abbott JM, Higgins SP (2004). "Prophylactic ergometrine-oxytocin versus oxytocin for the third stage of labour". Cochrane Database Syst Rev. 1: CD000201. doi:10.1002/14651858.CD000201.pub2. PMID 14973949.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b De Costa, Caroline (May 2002). "St Anthony's fire and living ligatures: a short history of ergometrine". Lancet. 359 (9319): 1768–1770. doi:10.1016/S0140-6736(02)08658-0.
{{cite journal}}:|access-date=requires|url=(help) - ^ Dudley, H W; Moir, C (1935). "The substance responsible for the traditional clinical effect of ergot". BMJ. 1: 520–523. doi:10.1136/bmj.1.3871.520.
{{cite journal}}:|access-date=requires|url=(help) - ^ List Of Precursors And Chemicals Frequently Used In The Illicit Manufacture Of Narcotic Drugs And Psychotropic Substances Under International Control.