GSK-3
| glycogen synthase kinase 3 alpha | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | GSK3A | ||||||
| NCBI gene | 2931 | ||||||
| HGNC | 4616 | ||||||
| OMIM | 606784 | ||||||
| RefSeq | NM_019884 | ||||||
| UniProt | P49840 | ||||||
| Other data | |||||||
| EC number | 2.7.11.26 | ||||||
| Locus | Chr. 19 q13.2 | ||||||
| |||||||
| glycogen synthase kinase 3 beta | |||||||
|---|---|---|---|---|---|---|---|
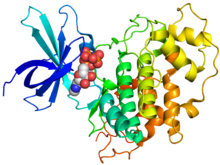 Crystallographic structure of human GSK-3β (rainbow colored, N-terminus = blue, C-terminus = red) bound to phosphoaminophosphonic acid-adenylate ester (spheres).[1] | |||||||
| Identifiers | |||||||
| Symbol | GSK3B | ||||||
| NCBI gene | 2932 | ||||||
| HGNC | 4617 | ||||||
| OMIM | 605004 | ||||||
| PDB | 1Q3W More structures | ||||||
| RefSeq | NM_002093 | ||||||
| UniProt | P49841 | ||||||
| Other data | |||||||
| EC number | 2.7.11.26 | ||||||
| Locus | Chr. 3 q13.33 | ||||||
| |||||||
Glycogen synthase kinase 3 is a serine/threonine protein kinase that mediates the addition of phosphate molecules onto serine and threonine amino acid residues. First discovered in 1980 as a regulatory kinase for its namesake, Glycogen synthase,[2] GSK-3 has since been identified as a kinase for over forty different proteins in a variety of different pathways.[3] In mammals GSK-3 is encoded by two known genes, GSK-3 alpha (GSK3A) and GSK-3 beta (GSK3B). GSK-3 has recently been the subject of much research because it has been implicated in a number of diseases, including Type II diabetes (Diabetes mellitus type 2), Alzheimer's Disease, inflammation, cancer, and bipolar disorder.
Mechanism
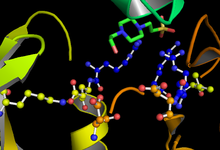
GSK-3 functions by phosphorylating a serine or threonine residue on its target substrate. A positively charged pocket adjacent to the active site binds a "priming" phosphate group attached to a serine or threonine four residues C-terminal of the target phosphorylation site. The active site, at residues 181, 200, 97, and 85, binds the terminal phosphate of ATP and transfers it to the target location on the substrate (see figure 1).[4]
Function
Phosphorylation of a protein by GSK-3 usually inhibits the activity of its downstream target.[5][6][7] GSK-3 is active in a number of central intracellular signaling pathways, including cellular proliferation, migration, glucose regulation, and apoptosis.
GSK-3 was originally discovered in the context of its involvement in regulating glycogen synthase.[2] After being primed by casein kinase 2 (CK2), glycogen synthase gets phosphorylated at a cluster of three C-terminal serine residues, reducing its activity.[8] In addition to its role in regulating glycogen synthase, GSK-3 has been implicated in other aspects of glucose homeostasis, including the phosphorylation of insulin receptor IRS1 [9] and of the gluconeogenic enzymes phosphoenolpyruvate carboxykinase and glucose 6 phosphatase.[10] However, these interactions have not been confirmed, as these pathways can be inhibited without the up-regulation of GSK-3.[8]
GSK-3 has also been shown to regulate immune and migratory processes. GSK-3 participates in a number of signaling pathways in the innate immune response, including pro-inflammatory cytokine and interleukin production.[11][12] The inactivation of GSK3B by various protein kinases also affects the adaptive immune response by inducing cytokine production and proliferation in naïve and memory CD4+ T cells.[12] In cellular migration, an integral aspect of inflammatory responses, the inhibition of GSK-3 has been reported to play conflicting roles, as local inhibition at growth cones has been shown to promote motility while global inhibition of cellular GSK-3 has been shown to inhibit cell spreading and migration.[11]
GSK-3 is also integrally tied to pathways of cell proliferation and apoptosis. GSK-3 has been shown to phosphorylate Beta-catenin, thus targeting it for degradation.[13] GSK-3 is therefore a part of the canonical Beta-catenin/Wnt pathway, which signals the cell to divide and proliferate. GSK-3 also participates in a number of apoptotic signaling pathways by phosphorylating transcription factors that regulate apoptosis.[3] GSK-3 can promote apoptosis by both activating pro-apoptotic factors such as p53 [14] and inactivating survival-promoting factors through phosphorylation.[15] The role of GSK-3 in regulating apoptosis is controversial, however, as some studies have shown that GSK-3β knockout mice are overly sensitized to apoptosis and die in the embryonic stage, while others have shown that overexpression of GSK-3 can induce apoptosis.[16] Overall, GSK-3 appears to both promote and inhibit apoptosis, and this regulation varies depending on the specific molecular and cellular context.[17]
Regulation
Due to its importance across numerous cellular functions, GSK-3 activity is subject to tight regulation.
The speed and efficacy of GSK-3 phosphorylation is regulated by a number of factors. Phosphorylation of certain GSK-3 residues can increase or decrease its ability to bind substrate. Phosphorylation at tyrosine-216 in GSK-3β or tyrosine-279 in GSK-3α enhances the enzymatic activity of GSK-3, while phosphorlyation of serine-9 in GSK-3β or serine-21 in GSK-3α significantly decreases active site availability (see Figure 1).[11] Further, GSK-3 is unusual among kinases in that it usually requires a "priming kinase" to first phosphorylate a substrate. A phosphorylated serine or threonine residue located four amino acids C-terminal to the target site of phosphorylation allows the substrate to bind a pocket of positive charge formed by arginine and lysine residues.[8][18]
Depending on the pathway in which it is being utilized, GSK-3 may be further regulated by cellular localization or the formation of protein complexes. The activity of GSK-3 is far greater in the nucleus and mitochondria than in the cytosol in cortical neurons,[19] while the phosphorylation of Beta-catenin by GSK-3 is mediated by the binding of both proteins to Axin, a scaffold protein, allowing Beta-catenin to access the active site of GSK-3.[11]
Disease relevance
Due to its involvement in a great number of signaling pathways, GSK-3 has been associated with a host of high-profile diseases. GSK-3 inhibitors are currently being tested for therapeutic effects in Alzheimer's disease, Type II diabetes (Diabetes mellitus type 2), some forms of cancer, and Bipolar Disorder
It has now been shown that Lithium, which has been used as a treatment for bipolar disorder, acts as a mood stabilizer by selectively inhibiting GSK-3. The mechanism through which GSK-3 inhibition stabilizes mood is not known, though it is suspected that the inhibition of GSK-3’s ability to promote inflammation contributes to the therapeutic effect.[11] Inhibition of GSK-3 also destabilises Rev-ErbA alpha transcriptional repressor, which has a significant role in the circadian clock.[20] Elements of the circadian clock may be connected with predisposition to bipolar mood disorder.[21]
GSK-3 activity has been associated with both pathological features of Alzheimer's disease, namely the buildup of Amyloid-β (Aβ) deposits and the formation of neurofibrillary tangles. GSK-3 is thought to directly promote Aβ production and to be tied to the process of the hyperphosphorylation of tau proteins, which leads to the tangles.[3][11] Due to these roles of GSK-3 in promoting Alzheimer's disease, GSK-3 inhibitors may have positive therapeutic effects on Alzheimer's patients and are currently in the early stages of testing.[22]
In a similar fashion, targeted inhibition of GSK-3 may have therapeutic effects on certain kinds of cancer. Though GSK-3 has been shown to promote apoptosis in some cases, it has also been reported to be a key factor in tumorigenesis in some cancers.[23] Supporting this claim, GSK-3 inhibitors have been shown to induce apoptosis in glioma and pancreatic cancer cells.[16][24]
GSK-3 inhibitors have also shown promise in the treatment of type-II diabetes.[8] Though GSK-3 activity under diabetic conditions can differ radically across different tissue types, studies have shown that introducing competitive inhibitors of GSK-3 can increase glucose tolerance in diabetic mice.[11] GSK-3 inhibitors may also have therapeutic effects on hemorrhagic transformation after acute ischemic stroke.[25] The role that inhibition of GSK-3 might play across its other signaling roles is not yet entirely understood.
Inhibitors
Inhibitors of GSK-3 include:[26]
Metal cations
ATP-competitive
Marine organism-derived
- 6-BIO (IC50=1.5μM)
- Dibromocantharelline (IC50=3μM)
- Hymenialdesine (IC50=10nM)
- Indirubins (IC50=5-50nM)
- Meridianins
Aminopyrimidines
IC50=0.6-7nM:
Arylindolemaleimide
Thiazoles
- AR-A014418 (IC50=104nM)
- AZD-1080
Paullones
IC50=4-80nM:
Aloisines
IC50=0.5-1.5μM:
Non-ATP competitive
Marine Organism-derived
- Manzamine A (IC50=1.5μM)
- Palinurine (IC50=4.5μM)
- Tricantine (IC50=7.5μM)
Thiadiazolidindiones
- TDZD-8 (IC50=2μM)
- NP00111 (IC50=2μM)
- NP031115 (IC50=4μM)
- Tideglusib
Halomethylketones
- HMK-32 (IC50=1.5μM)
Peptides
- L803-mts (IC50=40μM)
See also
References
- ^ PDB: 1J1B; Aoki M, Yokota T, Sugiura I, Sasaki C, Hasegawa T, Okumura C, Ishiguro K, Kohno T, Sugio S, Matsuzaki T (March 2004). "Structural insight into nucleotide recognition in tau-protein kinase I/glycogen synthase kinase 3 beta". Acta Crystallogr. D Biol. Crystallogr. 60 (Pt 3): 439–46. doi:10.1107/S090744490302938X. PMID 14993667.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Embi N, Rylatt DB, Cohen P (June 1980). "Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase". Eur J Biochem. 107 (2): 519–27. doi:10.1111/j.1432-1033.1980.tb06059.x. PMID 6249596.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Jope RS, Johnson GV (Feb 2004). "The glamour and gloom of glycogen synthase kinase-3". Trends Biochem Sci. 29 (2): 95–102. doi:10.1016/j.tibs.2003.12.004. PMID 15102436.
- ^ Dajani R, Fraser E, Roe SM, Young N, Good V, Dale TC, Pearl LH (June 2001). "Crystal structure of glycogen synthase kinase 3 beta: structural basis for phosphate-primed substrate specificity and autoinhibition". Cell. 105 (6): 721–32. doi:10.1016/S0092-8674(01)00374-9. PMID 11440715.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Woodgett JR (August 1994). "Regulation and functions of the glycogen synthase kinase-3 subfamily". Semin. Cancer Biol. 5 (4): 269–75. PMID 7803763.
- ^ Woodgett JR (September 2001). "Judging a protein by more than its name: GSK-3". Sci. STKE. 2001 (100): RE12. doi:10.1126/stke.2001.100.re12. PMID 11579232.
- ^ Ali A, Hoeflich KP, Woodgett JR (August 2001). "Glycogen synthase kinase-3: properties, functions, and regulation". Chem. Rev. 101 (8): 2527–40. doi:10.1021/cr000110o. PMID 11749387.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A (Mar 2009). "Glycogen synthase kinase-3: more than a namesake". Br J Pharmacol. 156 (6): 885–98. doi:10.1111/j.1476-5381.2008.00085.x. PMC 2697722. PMID 19366350.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Liberman Z, Eldar-Finkelman H (Feb 2005). "Serine 332 phosphorylation of insulin receptor substrate-1 by glycogen synthase kinase-3 attenuates insulin signaling". J Biol Chem. 280 (6): 4422–8. doi:10.1074/jbc.M410610200. PMID 15574412.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Lochhead PA, Coghlan M, Rice SQ, Sutherland C (May 2001). "Inhibition of GSK-3 selectively reduces glucose-6-phosphatase and phosphatase and phosphoenolypyruvate carboxykinase gene expression". Diabetes. 50 (5): 937–46. doi:10.2337/diabetes.50.5.937. PMID 11334436.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g Jope RS, Yuskaitis CJ, Beurel E (Apr–May 2007). "Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics". Neurochem Res. 32 (4–5): 577–95. doi:10.1007/s11064-006-9128-5. PMC 1970866. PMID 16944320.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Wang H, Brown J, Martin M (Feb 2011). "Glycogen synthase kinase 3: a point of convergence for the host inflammatory response". Cytokine. 53 (2): 130–40. doi:10.1016/j.cyto.2010.10.009. PMC 3021641. PMID 21095632.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mills CN, Nowsheen S, Bonner JA, Yang ES (2011). "Emerging roles of glycogen synthase kinase 3 in the treatment of brain tumors". Front Mol Neurosci. 47 (4): 47. doi:10.3389/fnmol.2011.00047. PMC 3223722. PMID 22275880.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Watcharasit P, Bijur GN, Zmijewski JW, Song L, Zmijewska A, Chen X, Johnson GV, Jope RS (Jun 2002). "Direct, activating interaction between glycogen synthase kinase-3beta and p53 after DNA damage". Proc Natl Acad Sci U S A. 99 (12): 7951–5. doi:10.1073/pnas.122062299. PMC 123001. PMID 12048243.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Grimes CA, Jope RS (Sep 2001). "CREB DNA binding activity is inhibited by glycogen synthase kinase-3 beta and facilitated by lithium". J Neurochem. 78 (6): 1219–32. doi:10.1046/j.1471-4159.2001.00495.x. PMC 1947002. PMID 11579131.
- ^ a b Kotliarova S, Pastorino S, Kovell LC, Kotliarov Y, Song H, Zhang W, Bailey R, Maric D, Zenklusen JC, Lee J, Fine HA (Aug 2008). "Glycogen synthase kinase-3 inhibition induces glioma cell death through c-MYC, nuclear factor-kappaB, and glucose regulation". Cancer Res. 68 (16): 6643–51. doi:10.1158/0008-5472.CAN-08-0850. PMC 2585745. PMID 18701488.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jacobs KM, Bhave SR, Ferraro DJ, Jaboin JJ, Hallahan DE, Thotala D (May 2012). "GSK-3β: A Bifunctional Role in Cell Death Pathways". Int J Cell Bio. 2012: 930710. doi:10.1155/2012/930710. PMC 3364548. PMID 22675363.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Doble BW, Woodgett JR (Apr 2003). "GSK-3: tricks of the trade for a multi-tasking kinase". J Cell Sci. 116 (7): 1175–86. doi:10.1242/jcs.00384. PMC 3006448. PMID 12615961.
- ^ Bijur GN, Jope RS (Dec 2003). "Glycogen synthase kinase-3 beta is highly activated in nuclei and mitochondria". NeuroReport. 14 (18): 2415–9. doi:10.1097/01.wnr.0000099609.19426.70. PMID 14663202.
- ^ "pmid16484495"
- ^ "pbmid24679394"
- ^ Hu S, Begum AN, Jones MR, Oh MS, Beech WK, Beech BH, Yang F, Chen P, Ubeda OJ, Kim PC, Davies P, Ma Q, Cole GM, Frautschy SA (Feb 2009). "GSK3 inhibitors show benefits in an Alzheimer's disease (AD) model of neurodegeneration but adverse effects in control animals". Neurobiol Dis. 33 (2): 193–206. doi:10.1016/j.nbd.2008.10.007. PMID 19038340.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wang Z (Oct 2008). "Leukaemia: GSK-3 as a cancer promoter". Nature. 455: 1205–10. doi:10.1038/nature07284.
- ^ Marchand B, Tremblay I, Cagnol S, Boucher MJ (Jan 2012). "Inhibition of glycogen synthase kinase-3 activity triggers an apoptotic response in pancreatic cancer cells through JNK-dependent mechanisms". Carcinogenesis. 33 (3): 529–37. doi:10.1093/carcin/bgr309. PMID 22201186.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wang W, Li M, Wang Y, Li Q, Deng G, Wan J, Yang Q, Chen Q, Wang J (Dec 2015). "GSK-3β inhibitor TWS119 attenuates rtPA-induced hemorrhagic transformation and activates the Wnt/β-catenin signaling pathway after acute ischemic stroke in rats". Mol Neurobiol. doi:10.1007/s12035-015-9607-2. PMID 26671619.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Eldar-Finkelman H, Martinez A (2011). "GSK-3 Inhibitors: Preclinical and Clinical Focus on CNS". Front Mol Neurosci. 4: 32. doi:10.3389/fnmol.2011.00032. PMC 3204427. PMID 22065134.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
External links
- Glycogen Synthase Kinase 3 at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
