Dextromethorphan: Difference between revisions
copyedit, can't read the sources referenced to check though, this is just a copyedit |
No edit summary |
||
| Line 23: | Line 23: | ||
<!--Pharmacokinetic data--> |
<!--Pharmacokinetic data--> |
||
| bioavailability = 11%<ref name="pmid15500572">{{cite journal | |
| bioavailability = 11%<ref name="pmid15500572">{{cite journal |doi=10.1111/j.1365-2885.2004.00608.x }}</ref> |
||
| metabolism = Hepatic (liver) enzymes: major [[CYP2D6]], minor [[CYP3A4]], and minor [[CYP3A5]] |
| metabolism = Hepatic (liver) enzymes: major [[CYP2D6]], minor [[CYP3A4]], and minor [[CYP3A5]] |
||
| elimination_half-life = 2-4 hours (extensive metabolisers); 24 hours (poor metabolisers)<ref name = MSR>{{cite web|title=Balminil DM, Benylin DM (dextromethorphan) dosing, indications, interactions, adverse effects, and more|work=Medscape Reference|publisher=WebMD|accessdate=15 April 2014|url=http://reference.medscape.com/drug/balminil-dm-benylin-dm-dextromethorphan-343401#showall}}</ref> |
| elimination_half-life = 2-4 hours (extensive metabolisers); 24 hours (poor metabolisers)<ref name = MSR>{{cite web|title=Balminil DM, Benylin DM (dextromethorphan) dosing, indications, interactions, adverse effects, and more|work=Medscape Reference|publisher=WebMD|accessdate=15 April 2014|url=http://reference.medscape.com/drug/balminil-dm-benylin-dm-dextromethorphan-343401#showall}}</ref> |
||
| Line 63: | Line 63: | ||
'''Dextromethorphan''' ('''DXM''' or '''DM''') is an [[antitussive]] (cough suppressant) [[drug]] of the [[morphinan]] class with sedative and disassociative properties. It is one of the active ingredients in many [[Over-the-counter drug|over-the-counter]] [[common cold|cold]] and [[cough]] medicines, including [[generic drug|generic]] labels and [[store brand]]s, [[Benylin]] DM, Mucinex DM, [[Robitussin]], [[NyQuil]], [[Dimetapp]], [[Vicks]], [[Coricidin]], [[Delsym]], [[TheraFlu]], and others. Dextromethorphan has also found numerous other uses in medicine, ranging from [[pain relief]] (as either the primary [[analgesic]], or an [[opioid]] potentiater) to [[psychological]] applications to [[addiction medicine|the treatment of addiction]]. It is sold in syrup, tablet, spray, and [[throat lozenge|lozenge]] forms. In its pure form, dextromethorphan occurs as a white powder.<ref name="urlReference Tables: Description and Solubility - D">{{cite web | url = http://www.pharmacopeia.cn/v29240/usp29nf24s0_alpha-2-13.html | title = Reference Tables: Description and Solubility - D | author = | coauthors = | date = | work = | publisher = | pages = | accessdate = 2011-05-06}}</ref> |
'''Dextromethorphan''' ('''DXM''' or '''DM''') is an [[antitussive]] (cough suppressant) [[drug]] of the [[morphinan]] class with sedative and disassociative properties. It is one of the active ingredients in many [[Over-the-counter drug|over-the-counter]] [[common cold|cold]] and [[cough]] medicines, including [[generic drug|generic]] labels and [[store brand]]s, [[Benylin]] DM, Mucinex DM, [[Robitussin]], [[NyQuil]], [[Dimetapp]], [[Vicks]], [[Coricidin]], [[Delsym]], [[TheraFlu]], and others. Dextromethorphan has also found numerous other uses in medicine, ranging from [[pain relief]] (as either the primary [[analgesic]], or an [[opioid]] potentiater) to [[psychological]] applications to [[addiction medicine|the treatment of addiction]]. It is sold in syrup, tablet, spray, and [[throat lozenge|lozenge]] forms. In its pure form, dextromethorphan occurs as a white powder.<ref name="urlReference Tables: Description and Solubility - D">{{cite web | url = http://www.pharmacopeia.cn/v29240/usp29nf24s0_alpha-2-13.html | title = Reference Tables: Description and Solubility - D | author = | coauthors = | date = | work = | publisher = | pages = | accessdate = 2011-05-06}}</ref> |
||
DXM is also used [[recreational drug|recreationally]]. When exceeding label-specified maximum dosages, dextromethorphan acts as a [[dissociative]] [[hallucinogen]]. Its [[mechanism of action]] is via multiple effects, including actions as a nonselective [[serotonin reuptake inhibitor]]<ref name="pmid19238739">{{cite journal | |
DXM is also used [[recreational drug|recreationally]]. When exceeding label-specified maximum dosages, dextromethorphan acts as a [[dissociative]] [[hallucinogen]]. Its [[mechanism of action]] is via multiple effects, including actions as a nonselective [[serotonin reuptake inhibitor]]<ref name="pmid19238739">{{cite journal |doi=10.1080/15563650701668625 }}</ref> and a [[sigma-1 receptor]] agonist.<ref name="pmid17386960">{{cite journal |doi=10.1016/j.neuint.2007.01.008 }}</ref><ref name="pmid15723099">{{cite journal |doi=10.1038/sj.bjp.0705998 }}</ref> DXM and its major [[metabolite]], [[dextrorphan]], also act as an [[NMDA receptor antagonist]] at high doses, which produces effects similar to, yet distinct from, the dissociative states created by other dissociative anaesthetics such as [[ketamine]] and [[phencyclidine]]. As well, the metabolite [[3-methoxymorphinan]] of dextrorphan (thus a second-level metabolite of DXM) produces local anesthetic effects in rats with potency above dextrorphan, but below that of DXM.<ref>{{cite journal |doi=10.1016/j.ejphar.2006.06.013 }}</ref> |
||
== Medical use == |
== Medical use == |
||
[[File:Dextrometorfano.jpg|thumb|right|Generic Dextromethorphan syrup.]] |
[[File:Dextrometorfano.jpg|thumb|right|Generic Dextromethorphan syrup.]] |
||
The primary use of dextromethorphan is as a [[cough suppressant]], for the temporary relief of cough caused by minor throat and bronchial irritation (such as commonly accompanies the [[influenza|flu]] and [[common cold]]), as well as those resulting from inhaled particle irritants.<ref name = AMH>{{cite book | title = Australian Medicines Handbook | year = 2013 | publisher = The Australian Medicines Handbook Unit Trust | isbn = 978-0-9805790-9-3 |
The primary use of dextromethorphan is as a [[cough suppressant]], for the temporary relief of cough caused by minor throat and bronchial irritation (such as commonly accompanies the [[influenza|flu]] and [[common cold]]), as well as those resulting from inhaled particle irritants.<ref name = AMH>{{cite book | title = Australian Medicines Handbook | year = 2013 | publisher = The Australian Medicines Handbook Unit Trust | isbn = 978-0-9805790-9-3 | place = Adelaide | editor = Rossi, S }}{{pn}}</ref> |
||
In 2010, the FDA approved the combination product [[dextromethorphan/quinidine]] for the treatment of [[pseudobulbar affect]] (PBA).{{mcn|date=January 2015}} |
In 2010, the FDA approved the combination product [[dextromethorphan/quinidine]] for the treatment of [[pseudobulbar affect]] (PBA).{{mcn|date=January 2015}} |
||
| Line 76: | Line 76: | ||
{{Main|Recreational use of dextromethorphan}} |
{{Main|Recreational use of dextromethorphan}} |
||
[[Over-the-counter substance|Over-the-counter]] preparations containing dextromethorphan have been used in manners inconsistent with their labeling, often as a recreational drug.<ref name="cesar" /> At doses much higher than medically recommended, DXM and its major metabolite, [[dextrorphan]], acts as an [[NMDA receptor antagonist]], which produces effects similar to, yet distinct from, the [[dissociative drug|dissociative]] [[hallucinogenic drug|hallucinogenic]] states created by other dissociative anaesthetics such as [[ketamine]] and [[phencyclidine]].<ref name=dea1>{{cite web |url=http://web-beta.archive.org/web/20121016221008/http://www.deadiversion.usdoj.gov/drugs_concern/dextro_m/dextro_m.pdf |title=Dextromethorphan |work=Drugs and Chemicals of Concern |publisher=[[Drug Enforcement Administration]] |date=August 2010}}</ref> It may produce distortions of the visual field - feelings of [[Dissociation (psychology)|dissociation]], distorted bodily perception, and excitement, as well as a loss of sense of time. Some users report stimulant-like [[euphoria]], particularly in response to music. Dextromethorphan usually provides its recreational effects in a non-linear fashion, so that they are experienced in significantly varied stages. These stages are commonly referred to as "plateaus".<ref name=Giannini_1997>{{cite book | author = Giannini AJ | title = Drugs of abuse | date = 1997 | publisher = Practice Management Information Corp. | location = Los Angeles |
[[Over-the-counter substance|Over-the-counter]] preparations containing dextromethorphan have been used in manners inconsistent with their labeling, often as a recreational drug.<ref name="cesar" /> At doses much higher than medically recommended, DXM and its major metabolite, [[dextrorphan]], acts as an [[NMDA receptor antagonist]], which produces effects similar to, yet distinct from, the [[dissociative drug|dissociative]] [[hallucinogenic drug|hallucinogenic]] states created by other dissociative anaesthetics such as [[ketamine]] and [[phencyclidine]].<ref name=dea1>{{cite web |url=http://web-beta.archive.org/web/20121016221008/http://www.deadiversion.usdoj.gov/drugs_concern/dextro_m/dextro_m.pdf |title=Dextromethorphan |work=Drugs and Chemicals of Concern |publisher=[[Drug Enforcement Administration]] |date=August 2010}}</ref> It may produce distortions of the visual field - feelings of [[Dissociation (psychology)|dissociation]], distorted bodily perception, and excitement, as well as a loss of sense of time. Some users report stimulant-like [[euphoria]], particularly in response to music. Dextromethorphan usually provides its recreational effects in a non-linear fashion, so that they are experienced in significantly varied stages. These stages are commonly referred to as "plateaus".<ref name=Giannini_1997>{{cite book | author = Giannini AJ | title = Drugs of abuse | date = 1997 | publisher = Practice Management Information Corp. | location = Los Angeles | isbn = 1570660530 | page = | edition = 2nd }}{{Page needed|date=March 2011}}</ref> |
||
== Adverse effects == |
== Adverse effects == |
||
| Line 131: | Line 131: | ||
* Skin rash |
* Skin rash |
||
Dextromethorphan can also cause other gastrointestinal disturbances. It had been thought to cause [[Olney's lesions]] when administered [[intravenously]]; however, this was later proven inconclusive, due to lack of research on humans. Tests were performed on rats, giving them 50 mg and up every day up to a month. Neurotoxic changes, including vacuolation, have been observed in posterior cingulate and retrosplenial cortices of rats administered other NMDA antagonists such as PCP, but not with dextromethorphan.<ref>{{cite journal | |
Dextromethorphan can also cause other gastrointestinal disturbances. It had been thought to cause [[Olney's lesions]] when administered [[intravenously]]; however, this was later proven inconclusive, due to lack of research on humans. Tests were performed on rats, giving them 50 mg and up every day up to a month. Neurotoxic changes, including vacuolation, have been observed in posterior cingulate and retrosplenial cortices of rats administered other NMDA antagonists such as PCP, but not with dextromethorphan.<ref>{{cite journal |doi=10.1126/science.2660263 }}</ref><ref>{{cite journal |doi=10.1016/j.neuro.2007.03.009 }}</ref> In many documented cases, dextromethorphan has produced [[Substance dependence|psychological dependence]] in people who used it recreationally. However, it does not produce [[Substance dependence|physical addiction]], according to the [[World Health Organization|WHO]] Committee on Drug Dependence.<ref>{{cite book |title=WHO Expert Committee on Drug Dependence, Seventeenth Report | publisher = World Health Organization | year = 1970 | url = http://whqlibdoc.who.int/trs/WHO_TRS_437.pdf | format = PDF |accessdate = 2008-12-29 |id={{hdl|10665/40766}} }}{{pn}}</ref> |
||
=== Contraindications === |
=== Contraindications === |
||
| Line 137: | Line 137: | ||
=== Drug interactions === |
=== Drug interactions === |
||
Dextromethorphan should not be taken with [[monoamine oxidase inhibitor]]s<ref name="nhtsa" /> due to the potential for [[serotonin syndrome]], which is a potentially life-threatening condition that can occur rapidly, due to a buildup of an excessive amount of serotonin in the body. Dextromethorphan can also cause serotonin syndrome when used with [[SSRI]] medicines, an interaction which has been documented in clinical cases where dextromethorphan is taken at [[recreational drug use|recreational doses]]. The link between therapeutic dosages of dextromethorphan and serotonin syndrome has been suggested to be less conclusive.<ref name="pmid19238739">{{cite journal | |
Dextromethorphan should not be taken with [[monoamine oxidase inhibitor]]s<ref name="nhtsa" /> due to the potential for [[serotonin syndrome]], which is a potentially life-threatening condition that can occur rapidly, due to a buildup of an excessive amount of serotonin in the body. Dextromethorphan can also cause serotonin syndrome when used with [[SSRI]] medicines, an interaction which has been documented in clinical cases where dextromethorphan is taken at [[recreational drug use|recreational doses]]. The link between therapeutic dosages of dextromethorphan and serotonin syndrome has been suggested to be less conclusive.<ref name="pmid19238739">{{cite journal |doi=10.1080/15563650701668625 }}</ref> |
||
=== Food interactions === |
=== Food interactions === |
||
| Line 154: | Line 154: | ||
Low K<sub>i</sub> values mean strong binding or high affinity; high K<sub>i</sub> values mean weak binding to the target or low affinity: |
Low K<sub>i</sub> values mean strong binding or high affinity; high K<sub>i</sub> values mean weak binding to the target or low affinity: |
||
* [[Uncompetitive inhibitor|Uncompetitive]] [[NMDA receptor]] (PCP site) [[receptor antagonist|antagonist]] (K<sub>i</sub> = 7,253 nM).<ref name=Burns> |
* [[Uncompetitive inhibitor|Uncompetitive]] [[NMDA receptor]] (PCP site) [[receptor antagonist|antagonist]] (K<sub>i</sub> = 7,253 nM).<ref name=Burns>{{cite journal |pmid=24648790 }}</ref> |
||
* [[Sigma-1 receptor|σ<sub>1</sub>]] and [[Sigma-2 receptor|σ<sub>2</sub>]] [[sigma receptor]] agonist (K<sub>i</sub> = 205 nM and 11,060 nM, respectively).<ref name=Burns/> |
* [[Sigma-1 receptor|σ<sub>1</sub>]] and [[Sigma-2 receptor|σ<sub>2</sub>]] [[sigma receptor]] agonist (K<sub>i</sub> = 205 nM and 11,060 nM, respectively).<ref name=Burns/> |
||
* [[Alpha-3 beta-4 nicotinic receptor|α<sub>3</sub>β<sub>4</sub>]]-, [[Alpha-4 beta-2 nicotinic receptor|α<sub>4</sub>β<sub>2</sub>]]-, and [[Alpha-7 nicotinic receptor|α<sub>7</sub>]]-[[nicotinic acetylcholine receptor|nACh receptor]] (K<sub>i</sub> = in the μM range) antagonist. Dextromethorphan binds to nicotinic receptors in frog eggs ([[Xenopus]] [[oocyte]]s), human embryonic kidney cells and mouse tissue. It inhibits the antinociceptive (pain killing) action of nicotine in the tail-flick test in mice, where mouse tails are exposed to heat, which makes the mouse flick its tail if it feels pain.<ref name="pmid15356218">{{cite journal | |
* [[Alpha-3 beta-4 nicotinic receptor|α<sub>3</sub>β<sub>4</sub>]]-, [[Alpha-4 beta-2 nicotinic receptor|α<sub>4</sub>β<sub>2</sub>]]-, and [[Alpha-7 nicotinic receptor|α<sub>7</sub>]]-[[nicotinic acetylcholine receptor|nACh receptor]] (K<sub>i</sub> = in the μM range) antagonist. Dextromethorphan binds to nicotinic receptors in frog eggs ([[Xenopus]] [[oocyte]]s), human embryonic kidney cells and mouse tissue. It inhibits the antinociceptive (pain killing) action of nicotine in the tail-flick test in mice, where mouse tails are exposed to heat, which makes the mouse flick its tail if it feels pain.<ref name="pmid15356218">{{cite journal |doi=10.1124/jpet.104.075093 }}</ref><ref name="pmid16563374">{{cite journal |doi=10.1016/j.ejphar.2006.02.034 }}</ref><ref name="pmid10869398">{{cite journal |pmid=10869398 }}</ref> |
||
* [[μ-opioid receptor|μ]]-, [[δ-opioid receptor|δ]]-, and [[κ-opioid receptor|κ]]-[[opioid receptor]] [[agonist]] (K<sub>i</sub> = 1,280 nM, 11,500 nM, and 7,000 nM, respectively)<ref name="pmid7562497">{{cite journal | |
* [[μ-opioid receptor|μ]]-, [[δ-opioid receptor|δ]]-, and [[κ-opioid receptor|κ]]-[[opioid receptor]] [[agonist]] (K<sub>i</sub> = 1,280 nM, 11,500 nM, and 7,000 nM, respectively)<ref name="pmid7562497">{{cite journal |pmid=7562497 }}</ref> |
||
* [[Serotonin transporter|SERT]] and [[norepinephrine transporter|NET]] [[reuptake inhibitor|inhibitor]] (K<sub>i</sub> = 23 nM and 240 nM, respectively)<ref name="pmid7562497"/><ref name="Schwartz, Pizon, Brooks 2008">{{cite journal | |
* [[Serotonin transporter|SERT]] and [[norepinephrine transporter|NET]] [[reuptake inhibitor|inhibitor]] (K<sub>i</sub> = 23 nM and 240 nM, respectively)<ref name="pmid7562497"/><ref name="Schwartz, Pizon, Brooks 2008">{{cite journal |doi=10.1080/15563650701668625 }}</ref><ref name=pmid1280529>{{cite journal |doi=10.1016/0006-8993(92)91144-4 }}</ref><ref name="pmid16051647">{{cite journal |doi=10.1093/bja/aei210 }}</ref> |
||
* [[NADPH oxidase]] inhibitor.<ref name="pmid14734632">{{cite journal | |
* [[NADPH oxidase]] inhibitor.<ref name="pmid14734632">{{cite journal |doi=10.1096/fj.03-0983fje }}</ref> |
||
Its [[Dissociation constant#Protein-ligand binding|affinities]] for some of the sites listed are relatively very low and are probably insignificant, such as binding to [[NMDA receptor]]s and [[opioid receptor]]s, even at high recreational doses.{{Citation needed|date=April 2010}} Instead of acting as a direct antagonist of the NMDA receptor itself, dextromethorphan likely functions as a [[prodrug]] to its nearly 10-fold more potent metabolite [[dextrorphan]], and this is the true mediator of its dissociative effects.<ref name="pmid10064839">{{cite journal | |
Its [[Dissociation constant#Protein-ligand binding|affinities]] for some of the sites listed are relatively very low and are probably insignificant, such as binding to [[NMDA receptor]]s and [[opioid receptor]]s, even at high recreational doses.{{Citation needed|date=April 2010}} Instead of acting as a direct antagonist of the NMDA receptor itself, dextromethorphan likely functions as a [[prodrug]] to its nearly 10-fold more potent metabolite [[dextrorphan]], and this is the true mediator of its dissociative effects.<ref name="pmid10064839">{{cite journal |doi=10.1016/S0006-8993(99)01125-7 }}</ref> What role, if any, (+)-[[3-methoxymorphinan]], dextromethorphan's other major metabolite, plays in its effects is not entirely clear.<ref>{{cite journal |doi=10.1002/(SICI)1099-081X(199704)18:3<227::AID-BDD18>3.0.CO;2-L }}</ref> |
||
=== Pharmacokinetics === |
=== Pharmacokinetics === |
||
Following oral administration, dextromethorphan is rapidly absorbed from the [[gastrointestinal tract]], where it enters the [[bloodstream]] and crosses the [[blood–brain barrier]].{{Citation needed|date=March 2011}} |
Following oral administration, dextromethorphan is rapidly absorbed from the [[gastrointestinal tract]], where it enters the [[bloodstream]] and crosses the [[blood–brain barrier]].{{Citation needed|date=March 2011}} |
||
At therapeutic doses, dextromethorphan acts [[central nervous system|centrally]] (meaning that it acts on the [[brain]]) as opposed to locally (on the [[respiratory tract]]). It elevates the threshold for coughing, without inhibiting [[cilia]]ry activity. Dextromethorphan is rapidly absorbed from the gastrointestinal tract and converted into the active metabolite dextrorphan in the liver by the cytochrome P450 enzyme [[CYP2D6]]. The average dose necessary for effective antitussive therapy is between 10 and 45 mg, depending on the individual. The International Society for the Study of Cough recommends "an adequate first dose of medication is 60 mg in the adult and repeat dosing should be infrequent rather than the [[List of medical abbreviations: Q|qds]] recommended."<ref> |
At therapeutic doses, dextromethorphan acts [[central nervous system|centrally]] (meaning that it acts on the [[brain]]) as opposed to locally (on the [[respiratory tract]]). It elevates the threshold for coughing, without inhibiting [[cilia]]ry activity. Dextromethorphan is rapidly absorbed from the gastrointestinal tract and converted into the active metabolite dextrorphan in the liver by the cytochrome P450 enzyme [[CYP2D6]]. The average dose necessary for effective antitussive therapy is between 10 and 45 mg, depending on the individual. The International Society for the Study of Cough recommends "an adequate first dose of medication is 60 mg in the adult and repeat dosing should be infrequent rather than the [[List of medical abbreviations: Q|qds]] recommended."<ref name=MoriceCough>{{cite web | url = http://www.issc.info/cough.html | author = Morice AH | title = Cough | publisher = International Society for the Study of Cough }}</ref> |
||
The duration of action after oral administration is about three to eight hours for dextromethorphan-hydrobromide, and 10 to 12 hours for dextromethorphan-polistirex. Around one in 10 of the Caucasian population has little or no CYP2D6 enzyme activity, leading to long-lived high drug levels.<ref name= |
The duration of action after oral administration is about three to eight hours for dextromethorphan-hydrobromide, and 10 to 12 hours for dextromethorphan-polistirex. Around one in 10 of the Caucasian population has little or no CYP2D6 enzyme activity, leading to long-lived high drug levels.<ref name=MoriceCough/> |
||
=== Metabolism === |
=== Metabolism === |
||
The first pass through the [[hepatic portal vein]] results in some of the drug being metabolized by O-demethylation into an active metabolite of dextromethorphan called dextrorphan (DXO). DXO is the 3-hydroxy derivative of dextromethorphan. The therapeutic activity of dextromethorphan is believed to be caused by both the drug and this metabolite. Dextromethorphan also undergoes N-demethylation (to 3-methoxymorphinan or MEM),<ref name="DXMdualprobe">{{cite journal | |
The first pass through the [[hepatic portal vein]] results in some of the drug being metabolized by O-demethylation into an active metabolite of dextromethorphan called dextrorphan (DXO). DXO is the 3-hydroxy derivative of dextromethorphan. The therapeutic activity of dextromethorphan is believed to be caused by both the drug and this metabolite. Dextromethorphan also undergoes N-demethylation (to 3-methoxymorphinan or MEM),<ref name="DXMdualprobe">{{cite journal |pmid=11602530 }}</ref> and partial conjugation with glucuronic acid and sulfate ions. Hours after dextromethorphan therapy, (in humans) the metabolites (+)-3-hydroxy-N-methylmorphinan, (+)-3-morphinan, and traces of the unchanged drug are detectable in the urine.<ref name="nhtsa" /> |
||
A major metabolic catalyst involved is the cytochrome P450 enzyme known as 2D6, or CYP2D6. A significant portion of the population has a functional deficiency in this enzyme and are known as poor CYP2D6 metabolizers. O-demethylation of DXM to DXO contributes to at least 80% of the DXO formed during DXM metabolism.<ref name="DXMdualprobe"/> As CYP2D6 is a major [[metabolic pathway]] in the inactivation of dextromethorphan, the duration of action and effects of dextromethorphan can be increased by as much as three times in such poor metabolizers.<ref name="pmid8841152">{{cite journal | |
A major metabolic catalyst involved is the cytochrome P450 enzyme known as 2D6, or CYP2D6. A significant portion of the population has a functional deficiency in this enzyme and are known as poor CYP2D6 metabolizers. O-demethylation of DXM to DXO contributes to at least 80% of the DXO formed during DXM metabolism.<ref name="DXMdualprobe"/> As CYP2D6 is a major [[metabolic pathway]] in the inactivation of dextromethorphan, the duration of action and effects of dextromethorphan can be increased by as much as three times in such poor metabolizers.<ref name="pmid8841152">{{cite journal |doi=10.1016/S0009-9236(96)90056-9 }}</ref> In one study on 252 Americans, 84.3% were found to be "fast" (extensive) metabolizers, 6.8% to be "intermediate" metabolizers, and 8.8% were "slow" metabolizers of DXM.<ref name="DXMpolymorphicmetabolism">{{cite journal |doi=10.1002/j.1552-4604.1987.tb02174.x }}</ref> A number of [[allele]]s for CYP2D6 are known, including several completely inactive variants. The distribution of alleles is uneven amongst [[CYP2D6#Ethnic factors in variability|ethnic groups]]. |
||
A large number of medications are potent inhibitors of CYP2D6. Some types of medications known to inhibit CYP2D6 include certain SSRIs and [[tricyclic]] [[antidepressant]]s, some [[antipsychotics]], and the commonly available [[antihistamine]] [[diphenhydramine]]. Therefore, the potential of interactions exists between dextromethorphan and medications that inhibit this enzyme, particularly in slow metabolizers.{{Citation needed|date=March 2011}} <!-- See also [[CYP2D6 inhibitors|CYP2D6 - Ligands]]. --> |
A large number of medications are potent inhibitors of CYP2D6. Some types of medications known to inhibit CYP2D6 include certain SSRIs and [[tricyclic]] [[antidepressant]]s, some [[antipsychotics]], and the commonly available [[antihistamine]] [[diphenhydramine]]. Therefore, the potential of interactions exists between dextromethorphan and medications that inhibit this enzyme, particularly in slow metabolizers.{{Citation needed|date=March 2011}} <!-- See also [[CYP2D6 inhibitors|CYP2D6 - Ligands]]. --> |
||
| Line 181: | Line 181: | ||
== History == |
== History == |
||
The [[racemic]] parent compound [[racemorphan]] was first described in a Swiss and US patent application from [[Hoffmann-La Roche]] in 1946 and 1947, respectively; a patent was granted in 1950.<ref name=Morris>{{cite journal |
The [[racemic]] parent compound [[racemorphan]] was first described in a Swiss and US patent application from [[Hoffmann-La Roche]] in 1946 and 1947, respectively; a patent was granted in 1950.<ref name=Morris>{{cite journal |doi=10.1002/dta.1620 }}</ref> A resolution of the two isomers of racemorphan with [[tartaric acid]] was published in 1952,<ref name=Morris/> and DXM was successfully tested in 1954 as part of [[US Navy]] and [[CIA]]-funded research on nonaddictive substitutes for [[codeine]].<ref>{{cite web |url=http://www.dod.mil/pubs/foi/operation_and_plans/NuclearChemicalBiologicalMatters/02-A-0846RELEASE.pdf |title=Memorandum for the Secretary of Defense |format=PDF |date= |accessdate=2013-07-28}}</ref> DXM was approved by the FDA in 1958 as an [[over-the-counter]] antitussive.<ref name=Morris/> As had been initially hoped, DXM was a solution for some of the problems associated with the use of [[codeine phosphate]] as a cough suppressant, such as sedation and [[opioid dependence|opiate dependence]], but like the [[dissociative anesthetic]]s [[phencyclidine]] and [[ketamine]], DXM later became associated with nonmedical use.<ref name=Morris>{{cite journal |doi=10.1002/dta.1620 }}</ref><ref name="cesar">{{cite web |url=http://www.cesar.umd.edu/cesar/drugs/dxm.asp |title=Dextromethorphan (DXM) |publisher=Cesar.umd.edu |date= |accessdate=2013-07-28}}</ref> |
||
During the 1960s and 1970s, dextromethorphan became available in an over-the-counter tablet form by the brand name Romilar. In 1973, Romilar was taken off the shelves after a burst in sales because of frequent misuse, and was replaced by [[cough syrup]] in an attempt to cut down on abuse.<ref name="cesar"/> The advent of widespread internet access in the 1990s allowed users to rapidly disseminate information about DXM, and online discussion groups formed around use and acquisition of the drug.<ref name=Morris |
During the 1960s and 1970s, dextromethorphan became available in an over-the-counter tablet form by the brand name Romilar. In 1973, Romilar was taken off the shelves after a burst in sales because of frequent misuse, and was replaced by [[cough syrup]] in an attempt to cut down on abuse.<ref name="cesar"/> The advent of widespread internet access in the 1990s allowed users to rapidly disseminate information about DXM, and online discussion groups formed around use and acquisition of the drug.<ref name=Morris/> As early as 1996, DXM HBr powder could be purchased in bulk from online retailers, allowing users to avoid consuming DXM in syrup preparations.<ref name=Morris/> As of January 1, 2012, dextromethorphan is prohibited for sale to minors in the state of California, except with a doctor's prescription.<ref name="urlwww.leginfo.ca.gov">{{cite web | url = http://www.leginfo.ca.gov/pub/11-12/bill/sen/sb_0501-0550/sb_514_bill_20110831_chaptered.pdf | title = Senate Bill No. 514 | format = | work = An act to add Sections 11110 and 11111 to the Health and Safety Code, relating to nonprescription drugs. | publisher = State of California, Legislative Counsel }}</ref> |
||
In [[Indonesia]], the National Agency of Drug and Food Control (BPOM-RI) prohibited single-component dextromethorphan drug sales with or without prescription. Indonesia is the only country in the world that makes single-component dextromethorphan illegal even by prescription<ref>http://nasional.news.viva.co.id/news/read/506418-bpom-tetap-batalkan-izin-edar-obat-dekstrometorfan</ref> and may be prosecuted by law. Indonesian National Narcotic Bureau (BNN RI) even threat to revoke pharmacies and drug store license if they still stock dextromethorphan and will hand the criminal prosecution to the police.<ref>http://daerah.sindonews.com/read/878465/21/bnn-ancam-tutup-apotek-penjual-dextromethorphan-1404129585</ref> The regulation followed by 130 drugs withdrawal from the market and pharmacies, but still allow multicomponent dextromethorphan containing drugs to be sold over the counter.<ref>http://www.pom.go.id/files/edaran_dektrome_2013.pdf</ref> On its official press release, BPOM-RI also stated that dextromethorphan often used as marijuana, amphetamine, and heroin substitute to drug abuser and its use as antitussive are less beneficial nowadays<ref>http://www.pom.go.id/new/index.php/view/pers/231/Penjelasan-Terkait-Produk-Obat-Batuk-yang-Beredar--dan--Mengandung-Bahan-Dekstrometorfan-Tunggal-.html</ref> |
In [[Indonesia]], the National Agency of Drug and Food Control (BPOM-RI) prohibited single-component dextromethorphan drug sales with or without prescription. Indonesia is the only country in the world that makes single-component dextromethorphan illegal even by prescription<ref>http://nasional.news.viva.co.id/news/read/506418-bpom-tetap-batalkan-izin-edar-obat-dekstrometorfan{{full}}</ref> and may be prosecuted by law. Indonesian National Narcotic Bureau (BNN RI) even threat to revoke pharmacies and drug store license if they still stock dextromethorphan and will hand the criminal prosecution to the police.<ref>http://daerah.sindonews.com/read/878465/21/bnn-ancam-tutup-apotek-penjual-dextromethorphan-1404129585{{full}}</ref> The regulation followed by 130 drugs withdrawal from the market and pharmacies, but still allow multicomponent dextromethorphan containing drugs to be sold over the counter.<ref>http://www.pom.go.id/files/edaran_dektrome_2013.pdf{{full}}</ref> On its official press release, BPOM-RI also stated that dextromethorphan often used as marijuana, amphetamine, and heroin substitute to drug abuser and its use as antitussive are less beneficial nowadays<ref>http://www.pom.go.id/new/index.php/view/pers/231/Penjelasan-Terkait-Produk-Obat-Batuk-yang-Beredar--dan--Mengandung-Bahan-Dekstrometorfan-Tunggal-.html{{full}}</ref> |
||
The Director of Narcotics, Psychotropics, and Addictive Substances Control (NAPZA) BPOM-RI, dr. Danardi Sosrosumihardjo, SpKJ, explain that dextromethorphan, morphine, and heroine were made from the same tree, and the effect of dextromethorphan to be equivalent to 1/100 of morphine and injected heroine.<ref>http://health.liputan6.com/read/2058886/ini-alasan-130-obat-batuk-ditarik-dari-pasaran</ref> |
The Director of Narcotics, Psychotropics, and Addictive Substances Control (NAPZA) BPOM-RI, dr. Danardi Sosrosumihardjo, SpKJ, explain that dextromethorphan, morphine, and heroine were made from the same tree, and the effect of dextromethorphan to be equivalent to 1/100 of morphine and injected heroine.<ref>http://health.liputan6.com/read/2058886/ini-alasan-130-obat-batuk-ditarik-dari-pasaran{{full}}</ref> |
||
On the contrary, The Deputy of Therapeutic Product and NAPZA Supervision BPOM-RI, Dra. Antonia Retno Tyas Utami, Apt. MEpid., mentioned that dextromethorphan being chemically similar to morphine, have much more dangerous and direct effect to central nervous system thus causing mental breakdown to patient. She also claimed without citing any prior scientific study or review, that unlike morphine user, dextrometorphan drug abuser can not be rehabilitated <ref>http://health.liputan6.com/read/708598/dibanding-morfin-obat-batuk-berdekstro-lebih-mematikan</ref> contradictive to numerous real scientific studies that show effective treatment and promising therapy result by [[naloxone]] alone to treat dextromethorphan addiction and poisoning.<ref> |
On the contrary, The Deputy of Therapeutic Product and NAPZA Supervision BPOM-RI, Dra. Antonia Retno Tyas Utami, Apt. MEpid., mentioned that dextromethorphan being chemically similar to morphine, have much more dangerous and direct effect to central nervous system thus causing mental breakdown to patient. She also claimed without citing any prior scientific study or review, that unlike morphine user, dextrometorphan drug abuser can not be rehabilitated <ref>http://health.liputan6.com/read/708598/dibanding-morfin-obat-batuk-berdekstro-lebih-mematikan{{full}}</ref> contradictive to numerous real scientific studies that show effective treatment and promising therapy result by [[naloxone]] alone to treat dextromethorphan addiction and poisoning.<ref>{{cite journal |pmid=2018593 }}</ref><ref>{{cite journal |pmid=840529 }}</ref><ref>{{cite journal |pmid=8895234 }}</ref> She also claimed high and death case of dextromethorphan misuse in Indonesia and to be further put in question suggest that [[Codeine|codeine]], despite of being more physically addictive µ-opioid class antitussive, are available as an alternative to replace dextromethorphan prescription.<ref>http://health.kompas.com/read/2013/10/01/1618072/BPOM.akan.Tarik.Pil.Dekstro{{full}}</ref> |
||
== See also == |
== See also == |
||
Revision as of 01:39, 26 April 2015
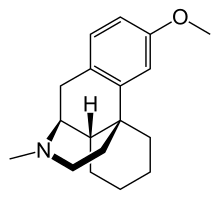 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Robitussin, Delsym, DM, DexAlone, Duract |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682492 |
| Pregnancy category |
|
| Dependence liability | Low |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 11%[1] |
| Metabolism | Hepatic (liver) enzymes: major CYP2D6, minor CYP3A4, and minor CYP3A5 |
| Elimination half-life | 2-4 hours (extensive metabolisers); 24 hours (poor metabolisers)[2] |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.321 |
| Chemical and physical data | |
| Formula | C18H25NO |
| Molar mass | 271.40 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 111 °C (232 °F) |
| |
| |
| | |
Dextromethorphan (DXM or DM) is an antitussive (cough suppressant) drug of the morphinan class with sedative and disassociative properties. It is one of the active ingredients in many over-the-counter cold and cough medicines, including generic labels and store brands, Benylin DM, Mucinex DM, Robitussin, NyQuil, Dimetapp, Vicks, Coricidin, Delsym, TheraFlu, and others. Dextromethorphan has also found numerous other uses in medicine, ranging from pain relief (as either the primary analgesic, or an opioid potentiater) to psychological applications to the treatment of addiction. It is sold in syrup, tablet, spray, and lozenge forms. In its pure form, dextromethorphan occurs as a white powder.[3]
DXM is also used recreationally. When exceeding label-specified maximum dosages, dextromethorphan acts as a dissociative hallucinogen. Its mechanism of action is via multiple effects, including actions as a nonselective serotonin reuptake inhibitor[4] and a sigma-1 receptor agonist.[5][6] DXM and its major metabolite, dextrorphan, also act as an NMDA receptor antagonist at high doses, which produces effects similar to, yet distinct from, the dissociative states created by other dissociative anaesthetics such as ketamine and phencyclidine. As well, the metabolite 3-methoxymorphinan of dextrorphan (thus a second-level metabolite of DXM) produces local anesthetic effects in rats with potency above dextrorphan, but below that of DXM.[7]
Medical use

The primary use of dextromethorphan is as a cough suppressant, for the temporary relief of cough caused by minor throat and bronchial irritation (such as commonly accompanies the flu and common cold), as well as those resulting from inhaled particle irritants.[8]
In 2010, the FDA approved the combination product dextromethorphan/quinidine for the treatment of pseudobulbar affect (PBA).
This article needs additional citations for verification. (January 2015) |
Recreational use

Over-the-counter preparations containing dextromethorphan have been used in manners inconsistent with their labeling, often as a recreational drug.[9] At doses much higher than medically recommended, DXM and its major metabolite, dextrorphan, acts as an NMDA receptor antagonist, which produces effects similar to, yet distinct from, the dissociative hallucinogenic states created by other dissociative anaesthetics such as ketamine and phencyclidine.[10] It may produce distortions of the visual field - feelings of dissociation, distorted bodily perception, and excitement, as well as a loss of sense of time. Some users report stimulant-like euphoria, particularly in response to music. Dextromethorphan usually provides its recreational effects in a non-linear fashion, so that they are experienced in significantly varied stages. These stages are commonly referred to as "plateaus".[11]
Adverse effects
Side effects of dextromethorphan use can include:[2][8][12]
At normal doses:
- body rash/itching (see below)
- Nausea
- Vomiting
- Drowsiness
- Dizziness
- Constipation
- Diarrhea
- Sedation
- Confusion
- Nervousness
- Closed-eye hallucination
Rare side effects include respiratory depression.[8] It is considered less addictive than the other common weak opioid cough suppressant, codeine.[2]
At doses three to 10 times the recommended therapeutic dose:[13]
- Increased energy
- Increased confidence
- Mild nausea
- Restlessness
- Insomnia
- "Speeding"/talking fast
- Feelings of increased strength
- Enlargened pupils/glazed eyes (but not red)
At dosages 15 to 75 times the recommended therapeutic dose:[13]
- Hallucinations
- Dissociation
- Vomiting
- Blurred vision and/or double vision
- Bloodshot eyes
- Dilated pupils
- Sweating
- Fever
- Bruxia
- Hypotension
- Hypertension
- Tachycardia
- Shallow respiration
- Diarrhea
- Urinary retention
- Muscle spasms
- Sedation
- Euphoria
- Paresthesia
- Blackouts
- Sight loss
- Inability to focus eyes
- Skin rash
Dextromethorphan can also cause other gastrointestinal disturbances. It had been thought to cause Olney's lesions when administered intravenously; however, this was later proven inconclusive, due to lack of research on humans. Tests were performed on rats, giving them 50 mg and up every day up to a month. Neurotoxic changes, including vacuolation, have been observed in posterior cingulate and retrosplenial cortices of rats administered other NMDA antagonists such as PCP, but not with dextromethorphan.[14][15] In many documented cases, dextromethorphan has produced psychological dependence in people who used it recreationally. However, it does not produce physical addiction, according to the WHO Committee on Drug Dependence.[16]
Contraindications
Because dextromethorphan can trigger a histamine release (allergic reaction), atopic children, who are especially susceptible to allergic reactions, should be administered dextromethorphan only if absolutely necessary, and only under the strict supervision of a healthcare professional.[12]
Drug interactions
Dextromethorphan should not be taken with monoamine oxidase inhibitors[12] due to the potential for serotonin syndrome, which is a potentially life-threatening condition that can occur rapidly, due to a buildup of an excessive amount of serotonin in the body. Dextromethorphan can also cause serotonin syndrome when used with SSRI medicines, an interaction which has been documented in clinical cases where dextromethorphan is taken at recreational doses. The link between therapeutic dosages of dextromethorphan and serotonin syndrome has been suggested to be less conclusive.[4]
Food interactions
Caution should be exercised when taking dextromethorphan when drinking grapefruit juice or eating grapefruits, as compounds in grapefruit affect a number of drugs, including dextromethorphan, through the inhibition of the cytochrome p450 system in the liver, and can lead to excessive accumulation and prolonged effects. Grapefruit and grapefruit juices (especially white grapefruit juice, but also including other citrus fruits such as bergamot and lime, as well as a number of noncitrus fruits[17]) generally are recommended to be avoided while using dextromethorphan and numerous other medications.
Laboratory testing
Testing for this drug is done either by blood or by urine. Blood can be either serum or plasma. Urine requires only 2 ml minimum.
Chemistry
Dextromethorphan is the dextrorotatory enantiomer of levomethorphan, which is the methyl ether of levorphanol, both opioid analgesics. It is named according to IUPAC rules as (+)-3-methoxy-17-methyl-9α,13α,14α-morphinan. As the pure free base, dextromethorphan occurs as an odorless, white to slightly yellow crystalline powder. It is freely soluble in chloroform and insoluble in water. Dextromethorphan is commonly available as the monohydrated hydrobromide salt, however some newer extended-release formulations contain dextromethorphan bound to an ion exchange resin based on polystyrene sulfonic acid. Dextromethorphan's specific rotation in water is +27.6° (20 °C, Sodium D-line).[citation needed]
Pharmacology
Pharmacodynamics
Dextromethorphan has been shown to possess the following properties, mainly in binding assays to various receptors of animal tissues. Low Ki values mean strong binding or high affinity; high Ki values mean weak binding to the target or low affinity:
- Uncompetitive NMDA receptor (PCP site) antagonist (Ki = 7,253 nM).[18]
- σ1 and σ2 sigma receptor agonist (Ki = 205 nM and 11,060 nM, respectively).[18]
- α3β4-, α4β2-, and α7-nACh receptor (Ki = in the μM range) antagonist. Dextromethorphan binds to nicotinic receptors in frog eggs (Xenopus oocytes), human embryonic kidney cells and mouse tissue. It inhibits the antinociceptive (pain killing) action of nicotine in the tail-flick test in mice, where mouse tails are exposed to heat, which makes the mouse flick its tail if it feels pain.[19][20][21]
- μ-, δ-, and κ-opioid receptor agonist (Ki = 1,280 nM, 11,500 nM, and 7,000 nM, respectively)[22]
- SERT and NET inhibitor (Ki = 23 nM and 240 nM, respectively)[22][23][24][25]
- NADPH oxidase inhibitor.[26]
Its affinities for some of the sites listed are relatively very low and are probably insignificant, such as binding to NMDA receptors and opioid receptors, even at high recreational doses.[citation needed] Instead of acting as a direct antagonist of the NMDA receptor itself, dextromethorphan likely functions as a prodrug to its nearly 10-fold more potent metabolite dextrorphan, and this is the true mediator of its dissociative effects.[27] What role, if any, (+)-3-methoxymorphinan, dextromethorphan's other major metabolite, plays in its effects is not entirely clear.[28]
Pharmacokinetics
Following oral administration, dextromethorphan is rapidly absorbed from the gastrointestinal tract, where it enters the bloodstream and crosses the blood–brain barrier.[citation needed]
At therapeutic doses, dextromethorphan acts centrally (meaning that it acts on the brain) as opposed to locally (on the respiratory tract). It elevates the threshold for coughing, without inhibiting ciliary activity. Dextromethorphan is rapidly absorbed from the gastrointestinal tract and converted into the active metabolite dextrorphan in the liver by the cytochrome P450 enzyme CYP2D6. The average dose necessary for effective antitussive therapy is between 10 and 45 mg, depending on the individual. The International Society for the Study of Cough recommends "an adequate first dose of medication is 60 mg in the adult and repeat dosing should be infrequent rather than the qds recommended."[29]
The duration of action after oral administration is about three to eight hours for dextromethorphan-hydrobromide, and 10 to 12 hours for dextromethorphan-polistirex. Around one in 10 of the Caucasian population has little or no CYP2D6 enzyme activity, leading to long-lived high drug levels.[29]
Metabolism
The first pass through the hepatic portal vein results in some of the drug being metabolized by O-demethylation into an active metabolite of dextromethorphan called dextrorphan (DXO). DXO is the 3-hydroxy derivative of dextromethorphan. The therapeutic activity of dextromethorphan is believed to be caused by both the drug and this metabolite. Dextromethorphan also undergoes N-demethylation (to 3-methoxymorphinan or MEM),[30] and partial conjugation with glucuronic acid and sulfate ions. Hours after dextromethorphan therapy, (in humans) the metabolites (+)-3-hydroxy-N-methylmorphinan, (+)-3-morphinan, and traces of the unchanged drug are detectable in the urine.[12]
A major metabolic catalyst involved is the cytochrome P450 enzyme known as 2D6, or CYP2D6. A significant portion of the population has a functional deficiency in this enzyme and are known as poor CYP2D6 metabolizers. O-demethylation of DXM to DXO contributes to at least 80% of the DXO formed during DXM metabolism.[30] As CYP2D6 is a major metabolic pathway in the inactivation of dextromethorphan, the duration of action and effects of dextromethorphan can be increased by as much as three times in such poor metabolizers.[31] In one study on 252 Americans, 84.3% were found to be "fast" (extensive) metabolizers, 6.8% to be "intermediate" metabolizers, and 8.8% were "slow" metabolizers of DXM.[32] A number of alleles for CYP2D6 are known, including several completely inactive variants. The distribution of alleles is uneven amongst ethnic groups.
A large number of medications are potent inhibitors of CYP2D6. Some types of medications known to inhibit CYP2D6 include certain SSRIs and tricyclic antidepressants, some antipsychotics, and the commonly available antihistamine diphenhydramine. Therefore, the potential of interactions exists between dextromethorphan and medications that inhibit this enzyme, particularly in slow metabolizers.[citation needed] DXM is also metabolized by CYP3A4. N-demethylation is primarily accomplished by CYP3A4, contributing to at least 90% of the MEM formed as a primary metabolite of DXM.[30]
A number of other CYP enzymes are implicated as minor pathways of DXM metabolism. CYP2B6 is actually more effective than CYP3A4 at N-demethylation of DXM, but, since the average individual has a much lower CYP2B6 content in his/her liver relative to CYP3A4, most N-demethylation of DXM is catalyzed by CYP3A4.[30]
History
The racemic parent compound racemorphan was first described in a Swiss and US patent application from Hoffmann-La Roche in 1946 and 1947, respectively; a patent was granted in 1950.[33] A resolution of the two isomers of racemorphan with tartaric acid was published in 1952,[33] and DXM was successfully tested in 1954 as part of US Navy and CIA-funded research on nonaddictive substitutes for codeine.[34] DXM was approved by the FDA in 1958 as an over-the-counter antitussive.[33] As had been initially hoped, DXM was a solution for some of the problems associated with the use of codeine phosphate as a cough suppressant, such as sedation and opiate dependence, but like the dissociative anesthetics phencyclidine and ketamine, DXM later became associated with nonmedical use.[33][9]
During the 1960s and 1970s, dextromethorphan became available in an over-the-counter tablet form by the brand name Romilar. In 1973, Romilar was taken off the shelves after a burst in sales because of frequent misuse, and was replaced by cough syrup in an attempt to cut down on abuse.[9] The advent of widespread internet access in the 1990s allowed users to rapidly disseminate information about DXM, and online discussion groups formed around use and acquisition of the drug.[33] As early as 1996, DXM HBr powder could be purchased in bulk from online retailers, allowing users to avoid consuming DXM in syrup preparations.[33] As of January 1, 2012, dextromethorphan is prohibited for sale to minors in the state of California, except with a doctor's prescription.[35]
In Indonesia, the National Agency of Drug and Food Control (BPOM-RI) prohibited single-component dextromethorphan drug sales with or without prescription. Indonesia is the only country in the world that makes single-component dextromethorphan illegal even by prescription[36] and may be prosecuted by law. Indonesian National Narcotic Bureau (BNN RI) even threat to revoke pharmacies and drug store license if they still stock dextromethorphan and will hand the criminal prosecution to the police.[37] The regulation followed by 130 drugs withdrawal from the market and pharmacies, but still allow multicomponent dextromethorphan containing drugs to be sold over the counter.[38] On its official press release, BPOM-RI also stated that dextromethorphan often used as marijuana, amphetamine, and heroin substitute to drug abuser and its use as antitussive are less beneficial nowadays[39]
The Director of Narcotics, Psychotropics, and Addictive Substances Control (NAPZA) BPOM-RI, dr. Danardi Sosrosumihardjo, SpKJ, explain that dextromethorphan, morphine, and heroine were made from the same tree, and the effect of dextromethorphan to be equivalent to 1/100 of morphine and injected heroine.[40] On the contrary, The Deputy of Therapeutic Product and NAPZA Supervision BPOM-RI, Dra. Antonia Retno Tyas Utami, Apt. MEpid., mentioned that dextromethorphan being chemically similar to morphine, have much more dangerous and direct effect to central nervous system thus causing mental breakdown to patient. She also claimed without citing any prior scientific study or review, that unlike morphine user, dextrometorphan drug abuser can not be rehabilitated [41] contradictive to numerous real scientific studies that show effective treatment and promising therapy result by naloxone alone to treat dextromethorphan addiction and poisoning.[42][43][44] She also claimed high and death case of dextromethorphan misuse in Indonesia and to be further put in question suggest that codeine, despite of being more physically addictive µ-opioid class antitussive, are available as an alternative to replace dextromethorphan prescription.[45]
See also
- Antitussive
- Cough syrup
- Hallucinogen
- Dissociatives
- Morphinans
- (+)-Naloxone - another dextrorotatory enantiomer of an opioid drug with useful nonopioid effects
References
- ^ . doi:10.1111/j.1365-2885.2004.00608.x.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ a b c "Balminil DM, Benylin DM (dextromethorphan) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 15 April 2014.
- ^ "Reference Tables: Description and Solubility - D". Retrieved 2011-05-06.
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help) - ^ a b . doi:10.1080/15563650701668625.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . doi:10.1016/j.neuint.2007.01.008.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . doi:10.1038/sj.bjp.0705998.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . doi:10.1016/j.ejphar.2006.06.013.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ a b c Rossi, S, ed. (2013). Australian Medicines Handbook. Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.[page needed]
- ^ a b c "Dextromethorphan (DXM)". Cesar.umd.edu. Retrieved 2013-07-28.
- ^ "Dextromethorphan" (PDF). Drugs and Chemicals of Concern. Drug Enforcement Administration. August 2010.
- ^ Giannini AJ (1997). Drugs of abuse (2nd ed.). Los Angeles: Practice Management Information Corp. ISBN 1570660530.[page needed]
- ^ a b c d "Dextromethorphan". NHTSA.
- ^ a b "Teen Drug Abuse: Cough Medicine and DXM (Dextromethorphan)". webmd.
- ^ . doi:10.1126/science.2660263.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . doi:10.1016/j.neuro.2007.03.009.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ WHO Expert Committee on Drug Dependence, Seventeenth Report (PDF). World Health Organization. 1970. hdl:10665/40766. Retrieved 2008-12-29.[page needed]
- ^ "Inhibitors of CYP3A4". ganfyd.org. Retrieved 23 August 2013.
- ^ a b . PMID 24648790.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . doi:10.1124/jpet.104.075093.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . doi:10.1016/j.ejphar.2006.02.034.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . PMID 10869398.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ a b . PMID 7562497.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . doi:10.1080/15563650701668625.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . doi:10.1016/0006-8993(92)91144-4.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . doi:10.1093/bja/aei210.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . doi:10.1096/fj.03-0983fje.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help)CS1 maint: unflagged free DOI (link) - ^ . doi:10.1016/S0006-8993(99)01125-7.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . doi:10.1002/(SICI)1099-081X(199704)18:3<227::AID-BDD18>3.0.CO;2-L.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ a b Morice AH. "Cough". International Society for the Study of Cough.
- ^ a b c d . PMID 11602530.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . doi:10.1016/S0009-9236(96)90056-9.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . doi:10.1002/j.1552-4604.1987.tb02174.x.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ a b c d e f . doi:10.1002/dta.1620.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ "Memorandum for the Secretary of Defense" (PDF). Retrieved 2013-07-28.
- ^ "Senate Bill No. 514" (PDF). An act to add Sections 11110 and 11111 to the Health and Safety Code, relating to nonprescription drugs. State of California, Legislative Counsel.
- ^ http://nasional.news.viva.co.id/news/read/506418-bpom-tetap-batalkan-izin-edar-obat-dekstrometorfan[full citation needed]
- ^ http://daerah.sindonews.com/read/878465/21/bnn-ancam-tutup-apotek-penjual-dextromethorphan-1404129585[full citation needed]
- ^ http://www.pom.go.id/files/edaran_dektrome_2013.pdf[full citation needed]
- ^ http://www.pom.go.id/new/index.php/view/pers/231/Penjelasan-Terkait-Produk-Obat-Batuk-yang-Beredar--dan--Mengandung-Bahan-Dekstrometorfan-Tunggal-.html[full citation needed]
- ^ http://health.liputan6.com/read/2058886/ini-alasan-130-obat-batuk-ditarik-dari-pasaran[full citation needed]
- ^ http://health.liputan6.com/read/708598/dibanding-morfin-obat-batuk-berdekstro-lebih-mematikan[full citation needed]
- ^ . PMID 2018593.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . PMID 840529.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . PMID 8895234.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ http://health.kompas.com/read/2013/10/01/1618072/BPOM.akan.Tarik.Pil.Dekstro[full citation needed]
External links
- U.S. National Library of Medicine: Drug Information Portal - Dextromethorphan
- Nuedexta (dextromethorphan hydrobromide and quinidine sulfate): Prescribing Information (Original Approval Date FDA: October 29, 2010)
