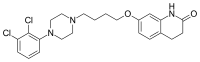
Aripiprazole
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌeɪrɪˈpɪprəzoʊl/ AIR-i-PIP-rə-zohl Abilify ə-BIL-i-fy |
| Trade names | Abilify |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603012 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth (tablets, dissolving tablets, solution); IM (including a depot) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 87%[2][3][4][5] |
| Protein binding | >99%[2][3][4][5] |
| Metabolism | Liver (mostly via CYP3A4 and 2D6[2][3][4][5]) |
| Elimination half-life | 75 hours (active metabolite is 94 hours)[2][3][4][5] |
| Excretion | Renal (27%; <1% unchanged), Faecal (60%; 18% unchanged)[2][3][4][5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.112.532 |
| Chemical and physical data | |
| Formula | C23H27Cl2N3O2 |
| Molar mass | 448.385 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Aripiprazole, sold under the brand name Abilify among others, is an atypical antipsychotic. It is recommended and primarily used in the treatment of schizophrenia and bipolar disorder.[6] Other uses include as an add-on treatment in major depressive disorder, tic disorders, and irritability associated with autism.[7] According to a Cochrane review, evidence for the oral form in schizophrenia is not sufficient to determine effects on general functioning.[8] Additionally, because many people dropped out of the medication trials before they were completed, the overall strength of the conclusions is low.[8]
Side effects include neuroleptic malignant syndrome, a movement disorder known as tardive dyskinesia, and high blood sugar in those with diabetes.[6] In the elderly there is an increased risk of death.[6] It is thus not recommended for use in those with psychosis due to dementia.[6] It is pregnancy category C in the United States and category C in Australia, meaning there is possible evidence of harm to the fetus.[6][9] It is not recommended for women who are breastfeeding.[6] It is unclear whether it is safe or effective in people less than 18 years old.[6]
It is a partial dopamine agonist. Aripiprazole was developed by Otsuka in Japan. In the United States, Otsuka America markets it jointly with Bristol-Myers Squibb. From April 2013 to March 2014, sales of Abilify amounted to almost $6.9 billion.[10]
Medical uses
Aripiprazole is primarily used for the treatment of schizophrenia or bipolar disorder.[6]
Schizophrenia
The FDA approved the oral form of aripiprazole for the treatment of acute exacerbations of schizophrenia and for maintenance treatment (relapse prevention) in 2002. The approval was based on efficacy demonstrated in 5 "adequate and well-controlled" clinical trials, including 4 short-term studies (4 or 6 weeks) showing a reduction in psychotic symptoms in the acute setting and 1 longer-term study (26 weeks) demonstrating reduced relapse compared to placebo.[11] Marketing approval was granted by the European Medicines Agency based on the results of these same studies, plus an additional long-term study demonstrating non-inferiority to haloperidol in the prevention of relapse.[12] Health Canada approved aripiprazole for the acute and maintenance treatment of schizophrenia in 2009.[13]
A Cochrane review concluded that aripiprazole is similar to other typical and atypical antipsychotics with respect to benefit.[8][14] Compared to typical antipsychotics, there are fewer extrapyramidal side effects, but higher rates of dizziness.[15] With respect to other atypicals, it is difficult to determine differences in adverse effects as data quality is poor.[16]
A Lancet review found that it is in the middle range of 15 antipsychotics for effectiveness, approximately as effective as haloperidol and quetiapine and slightly more effective than ziprasidone, chlorpromazine, and asenapine, with better tolerability compared to the other antipsychotic drugs (4th best for weight gain, 5th best for extrapyramidal symptoms, best for prolactin elevation, 2nd best for QTc prolongation, and 5th best for sedation). The authors concluded that for acute psychotic episodes aripiprazole results in benefits in some aspects of the condition.[17]
A Cochrane review comparing aripiprazole with placebo concluded that high dropout rates in clinical trials, and a lack of outcome data regarding general functioning, behavior, mortality, economic outcomes, or cognitive functioning make it difficult to definitively conclude that aripiprazole is useful for the prevention of relapse.[8] The World Federation of Societies for Biological Psychiatry recommends aripiprazole for the treatment of acute exacerbations of schizophrenia as a Grade 1 recommendation and evidence level A.[18]
The National Institute for Health and Care Excellence offers the following recommendations with respect to pharmacological treatment of those presenting with an acute episode of psychosis:
- Offer an oral antipsychotic medication.
- Inform those who want to try psychological interventions alone that these are more effective when performed in conjunction with treatment with an antipsychotic medication
- In the early post-acute period, warn the person of a high risk of relapse if antipsychotic medication is discontinued in the first 1–2 years after the acute episode
- If a decision is made to discontinue medication, reduce the dose gradually and monitor for relapse for at least 2 years.[19]
The British Association for Psychopharmacology similarly recommends that all persons presenting with psychosis receive treatment with an antipsychotic, and that such treatment should continue for at least 1–2 years, as "There is no doubt that antipsychotic discontinuation is strongly associated with relapse during this period". The guideline further notes that "Established schizophrenia requires continued maintenance with doses of antipsychotic medication within the recommended range (Evidence level A)".[20]
The National Institute of Health and Clinical Excellence,[19] the British Association for Psychopharmacology[20] and the World Federation of Societies for Biological Psychiatry suggest that there is little difference in effectiveness between antipsychotics in prevention of relapse, and recommend that the specific choice of antipsychotic be chosen based on persons preference and side effect profile. The latter group recommends switching to aripiprazole when excessive weight gain is encountered during treatment with other antipsychotics.[18]
| Information is of limited quality, incomplete, and problematic to apply. Long-term data is few and quality of evidence is all low or very low. Aripiprazole also has important side effects.[21] | ||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||
Bipolar disorder
Aripiprazole is effective for the treatment of acute manic episodes of bipolar disorder in adults, children, and adolescents.[22][23] Used as maintenance therapy, it is useful for the prevention of manic episodes, but is not useful for bipolar depression.[24][25] Thus, it is often used in combination with an additional mood stabilizer; however, co-administration with a mood stabilizer increases the risk of extrapyramidal side effects.[26]
Major depression
Aripiprazole is an effective add-on treatment for major depressive disorder; however, there is a greater rate of side effects such as weight gain and movement disorders.[27][28][29][30] The overall benefit is small to moderate and its use appears to neither improve quality of life nor functioning.[28] Aripiprazole may interact with some antidepressants, especially SSRIs. There are interactions with fluoxetine and paroxetine and lesser interactions with sertraline, escitalopram, citalopram and fluvoxamine, which inhibit CYP2D6, for which aripiprazole is a substrate. CYP2D6 inhibitors increase aripiprazole concentrations to 2-3 times their normal level.[2]
Autism
Short-term data (8 weeks) shows reduced irritability, hyperactivity, inappropriate speech, and stereotypy, but no change in lethargic behaviours.[31] Adverse effects include weight gain, sleepiness, drooling and tremors.[31] It is suggested that children and adolescents need to be monitored regularly while taking this medication, to evaluate if this treatment option is still effective after long-term use and note if side effects are worsening. Further studies are needed to understand if this drug is helpful for children after long term use.[31]
Obsessive–compulsive disorder
A 2014 systematic review concluded that add-on therapy with low dose aripiprazole is an effective treatment for obsessive-compulsive disorder that does not improve with SSRIs alone. The conclusion was based on the results of two relatively small, short-term trials, each of which demonstrated improvements in symptoms.[32] Risperidone (another second-generation antipsychotic) appears to be superior to aripiprazole for this indication, and is recommended by the 2007 American Psychiatric Association guidelines, though aripiprazole is cautiously recommended by a 2017 review by Pignon and colleagues.[33]
Adverse effects
In adults side effects with greater than 10% incidence include weight gain, headache, agitation or anxiety, insomnia, and gastro-intestinal effects like nausea and constipation, and lightheadedness.[2][3][4][5][34] Side effects in children are similar, and include sleepiness, increased appetite, and stuffy nose.[2] A strong desire to gamble, binge eat, shop, and have sex may also occur.[35] Cases of pathological gambling have been reported.[36]
Uncontrolled movement such as restlessness, tremors, and muscle stiffness have been reported in children and adults, but they are rare.[2][37]
Discontinuation
The British National Formulary recommends a gradual withdrawal when discontinuing anti-psychotic treatment to avoid acute withdrawal syndrome or rapid relapse.[38] Joanne Moncrieff has suggested that the withdrawal process might itself be schizo-mimetic, producing schizophrenia-like symptoms even in previously healthy people, indicating a possible pharmacological origin of mental illness in a yet unknown percentage of people currently and previously treated with antipsychotics, but the limited evidence was found to support this hypothesis for antipsychotics other than clozapine.[39]
Overdose
Children or adults who ingested acute overdoses have usually manifested central nervous system depression ranging from mild sedation to coma; serum concentrations of aripiprazole and dehydroaripiprazole in these people were elevated by up to 3-4 fold over normal therapeutic levels; as of 2008 no deaths had been recorded.[40][41]
Interactions
Aripiprazole is a substrate of CYP2D6 and CYP3A4. Coadministration with medications that inhibit (e.g. paroxetine, fluoxetine) or induce (e.g. carbamazepine) these metabolic enzymes are known to increase and decrease, respectively, plasma levels of aripiprazole.[42] As such, anyone taking aripiprazole should be aware that their dosage of aripiprazole may need to be adjusted.
Precautions should be taken in people with an established diagnosis of diabetes mellitus who are started on atypical antipsychotics along with other medications that affect blood sugar levels and should be monitored regularly for worsening of glucose control. The liquid form (oral solution) of this medication may contain up to 15 grams of sugar per dose.[6]
Antipsychotics like aripiprazole and stimulant medications, such as amphetamine, are traditionally thought to have opposing effects to their effects on dopamine receptors: stimulants are thought to increase dopamine in the synaptic cleft, whereas antipsychotics are thought to decrease dopamine. However, it is an oversimplification to state the interaction as such, due to the differing actions of antipsychotics and stimulants in different parts of the brain, as well as the effects of antipsychotics on non-dopaminergic receptors. This interaction frequently occurs in the setting of comorbid ADHD (for which stimulants are commonly prescribed) and off-label treatment of aggression with antipsychotics. Aripiprazole has shown some benefit in improving cognitive functioning in people with ADHD without other psychiatric comorbidities, though the results have been disputed. The combination of antipsychotics like aripiprazole with stimulants should not be considered an absolute contraindication.[43]
Pharmacology
Pharmacodynamics
| Site | Ki (nM) | Action | Ref |
|---|---|---|---|
| 5-HT1A | 1.7–5.6 | Partial agonist | [45][46][47] |
| 5-HT1B | 830 | ND | [45] |
| 5-HT1D | 68 | ND | [45] |
| 5-HT1E | 8,000 | ND | [45] |
| 5-HT2A | 3.4–35 | Antagonist | [47][45][46] |
| 5-HT2B | 0.11-0.36 | Inverse agonist | [45] |
| 5-HT2C | 15–180 | Partial agonist | [47][45][46] |
| 5-HT3 | 628 | ND | [45] |
| 5-HT5A | 1,240 | ND | [45] |
| 5-HT6 | 214–786 | Antagonist | [47][45][46] |
| 5-HT7 | 9.6–39 | Antagonist | [45][46][47] |
| D1 | 265–1,170 | ND | [45] |
| D2 | 0.45–3.3 | Partial agonist | [48][46][45] |
| D2L | 0.74–0.9 | Partial agonist | [45] |
| D3 | 0.8–9.7 | Partial agonist | [47][45] |
| D4 | 44–514 | Partial agonist | [47][45] |
| D5 | 95–2,590 | ND | [47][45] |
| α1A | 25.9 | ND | [45][46] |
| α1B | 34.4 | ND | [45] |
| α2A | 74.3 | ND | [45][46] |
| α2B | 102 | ND | [45][46] |
| α2C | 37.9 | ND | [45][46] |
| β1 | 141 | ND | [45] |
| β2 | 163 | ND | [45] |
| H1 | 27.9–61 | ND | [45][46][47] |
| H2 | >10,000 | ND | [45] |
| H3 | 224 | ND | [45] |
| H4 | >10,000 | ND | [45] |
| M1 | 6,780 | ND | [45] |
| M2 | 3,510 | ND | [45] |
| M3 | 4,680 | ND | [45][46] |
| M4 | 1,520 | ND | [45] |
| M5 | 2,330 | ND | [45] |
| SERT | 98–1,080 | Blocker | [47][45] |
| NET | 2,090 | Blocker | [45] |
| DAT | 3,220 | Blocker | [45] |
| NMDA (PCP) |
4,001 | Antagonist | [45] |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. All data are for human cloned proteins, except 5-HT3 (rat), D4 (human/rat), H3 (guinea pig), and NMDA/PCP (rat).[45] | |||
Aripiprazole's mechanism of action is different from those of the other FDA-approved atypical antipsychotics (e.g., clozapine, olanzapine, quetiapine, ziprasidone, and risperidone).[45][49][50][51][52][53][54][55] Rather than acting as a pure antagonist of the dopamine D2 receptor, aripiprazole shows functional selectivity at the D2 receptor, acting as a silent antagonist of some subpopulations of D2 receptors but as a high-efficacy partial agonist (intrinsic activity = 75%) of other D2-receptor subpopulations.[45] It appears to show predominantly antagonist activity on postsynaptic D2 receptors and partial agonist activity on presynaptic D2 receptors.[56] Aripiprazole is also a partial agonist of the D3 receptor.[45] In healthy human volunteers, D2 and D3 receptor occupancy levels are high, with average levels ranging between approximately 71% at 2 mg/day to approximately 96% at 40 mg/day.[57][58] Most atypical antipsychotics bind preferentially to extrastriatal receptors, but aripiprazole appears to be less preferential in this regard, as binding rates are high throughout the brain.[59]
Aripiprazole is also a partial agonist of the serotonin 5-HT1A receptor (intrinsic activity = 68%).[45][60][61] It is a very weak partial agonist of the 5-HT2A receptor (intrinsic activity = 12.7%),[45] and like other atypical antipsychotics, displays a functional antagonist profile at this receptor.[45] The drug differs from other atypical antipsychotics in having higher affinity for the D2 receptor than for the 5-HT2A receptor.[61] At the 5-HT2B receptor, aripiprazole acts as a potent inverse agonist.[45] Unlike other antipsychotics, aripiprazole is a high-efficacy partial agonist of the 5-HT2C receptor (intrinsic activity = 82%) and with relatively weak affinity;[45] this property may underlie the minimal weight gain seen in the course of therapy.[62] At the 5-HT7 receptor, aripiprazole is a very weak partial agonist with barely measurable intrinsic activity, and hence is a functional antagonist of this receptor.[45][55] Aripiprazole also shows lower but likely clinically insignificant affinity for a number of other sites, such as the histamine H1, α-adrenergic, and dopamine D4 receptors as well as the serotonin transporter, while it has negligible affinity for the muscarinic acetylcholine receptors.[45][50]
Since the actions of aripiprazole differ markedly across receptor systems aripiprazole was sometimes an antagonist (eg at 5-HT6 and D2L), sometimes an inverse agonist (eg 5-HT2B), sometimes a partial agonist (eg D2L), and sometimes a full agonist (D3, D4). Aripiprazole was frequently found to be a partial agonist, with an intrinsic activity that could be low (D2L, 5-HT2A, 5-HT7), intermediate (5-HT1A), or high (D4, 5-HT2C). This mixture of agonist actions at D2-dopamine receptors is consistent with the hypothesis that aripiprazole has ‘functionally selective’ actions.[63] The ‘functional-selectivity’ hypothesis proposes that a mixture of agonist/partial agonist/antagonist actions are likely. According to this hypothesis, agonists may induce structural changes in receptor conformations that are differentially ‘sensed’ by the local complement of G proteins to induce a variety of functional actions depending upon the precise cellular milieu. The diverse actions of aripiprazole at D2-dopamine receptors are clearly cell-type specific (eg agonism, antagonism, partial agonism), and are most parsimoniously explained by the ‘functional selectivity’ hypothesis.[45]
Since 5-HT2C receptors have been implicated in the control of depression, OCD, and appetite, agonism at the 5-HT2C receptor might be associated with therapeutic potential in obsessive compulsive disorder, obesity, and depression. 5-HT2C agonism has been demonstrated to induce anorexia via enhancement of serotonergic neurotransmission via activation of 5-HT2C receptors; it is conceivable that the 5-HT2C agonist actions of aripiprazole may, thus, be partly responsible for the minimal weight gain associated with this compound in clinical trials. In terms of potential action as an antiobsessional agent, it is worthwhile noting that a variety of 5-HT2A/5-HT2C agonists have shown promise as antiobsessional agents, yet many of these compounds are hallucinogenic, presumably due to 5-HT2A activation. Aripiprazole has a favorable pharmacological profile in being a 5-HT2A antagonist and a 5-HT2C partial agonist. Based on this profile, one can predict that aripiprazole may have antiobsessional and anorectic actions in humans.[45]
Wood and Reavill's (2007) review of published and unpublished data proposed that, at therapeutically relevant doses, aripiprazole may act essentially as a selective partial agonist of the D2 receptor without significantly affecting the majority of serotonin receptors.[56] A positron emission tomography imaging study found that 10 to 30 mg/day aripiprazole resulted in 85 to 93% occupancy of the D2 receptor in various brain areas (putamen, caudate, ventral striatum) versus 54 to 60% occupancy of the 5-HT2A receptor and only 16% occupancy of the 5-HT1A receptor.[64][61] It has been suggested that the low occupancy of the 5-HT1A receptor by aripiprazole may have been an erroneous measurement however.[65]
Aripiprazole acts by modulating neurotransmission overactivity on the dopaminergic mesolimbic pathway, which is thought to be a cause of positive schizophrenia symptoms.[66] Due to its agonist activity on D2 receptors, aripiprazole may also increase dopaminergic activity to optimal levels in the mesocortical pathways where it is reduced.[66]
Pharmacokinetics
Aripiprazole displays linear kinetics and has an elimination half-life of approximately 75 hours. Steady-state plasma concentrations are achieved in about 14 days. Cmax (maximum plasma concentration) is achieved 3–5 hours after oral dosing. Bioavailability of the oral tablets is about 90% and the drug undergoes extensive hepatic metabolization (dehydrogenation, hydroxylation, and N-dealkylation), principally by the enzymes CYP2D6 and CYP3A4. Its only known active metabolite is dehydro-aripiprazole, which typically accumulates to approximately 40% of the aripiprazole concentration. The parenteral drug is excreted only in traces, and its metabolites, active or not, are excreted via feces and urine.[50] When dosed daily, brain concentrations of aripiprazole will increase for a period of 10–14 days, before reaching stable constant levels.[citation needed]
Chemistry
Aripiprazole is a phenylpiperazine and is chemically related to nefazodone, etoperidone, and trazodone.[67][68]
History
Aripiprazole was discovered by scientists at Otsuka Pharmaceutical and was called OPC-14597.[69][70] It was first published in 1995.[70][71] Otsuka initially developed the drug, and partnered with Bristol-Myers Squibb (BMS) in 1999 to complete development, obtain approvals, and market aripiprazole.[72]
It was approved by the FDA for schizophrenia on November 15, 2002 and the European Medicines Agency on 4 June 2004; for acute manic and mixed episodes associated with bipolar disorder on October 1, 2004; as an adjunct for major depressive disorder on November 20, 2007;[73] and to treat irritability in children with autism on 20 November 2009.[74] Likewise it was approved for use as a treatment for schizophrenia by the TGA of Australia in May 2003.[2]

Aripiprazole has been approved by the FDA for the treatment of acute manic and mixed episodes, in both people older than 10 years.[75]
In 2006, the FDA required the companies to add a black box warning to the label, warning that older people who were given the drug for dementia-related psychosis were at greater risk of death.[76]
In 2007, aripiprazole was approved by the FDA for the treatment of unipolar depression when used adjunctively with an antidepressant medication.[77] That same year, BMS settled a case with the U.S. government in which it paid $515 million; the case covered several drugs but the focus was on BMS's off-label marketing of aripiprazole for children and older people with dementia.[78]
In 2011 Otsuka and Lundbeck signed a collaboration to develop a depot formulation of apripiprazole.[79]
As of 2013, Abilify had annual sales of US$7 billion.[80] In 2013 BMS returned marketing rights to Otsuka, but kept manufacturing the drug.[81] Also in 2013, Otsuka and Lundbeck received U.S. and European marketing approval for an injectable depot formulation of aripiprazole.[82][83]
Otsuka's U.S. patent on aripiprazole expired on October 20, 2014 but due to a pediatric extension, a generic did not become available until April 20, 2015.[75] Barr Laboratories (now Teva Pharmaceuticals) initiated a patent challenge under the Hatch-Waxman Act in March 2007.[84] On November 15, 2010, this challenge was rejected by the U.S. District Court in New Jersey.[85]
Otsuka's European patent EP0367141 which would have expired on 26 October 2009, was extended by a Supplementary Protection Certificate (SPC) to 26 October 2014.,[86] The UK Intellectual Property Office decided[87] on 4 March 2015 that the SPC could not be further extended by six months under Regulation (EC) No 1901/2006. Even if the decision is successfully appealed, protection in Europe will not extend beyond 26 April 2015.
In April 2015, the FDA announced the first generic versions.[88][89] In October 2015, aripiprazole lauroxil, a prodrug of aripiprazole that is administered via intramuscular injection once every four to six weeks for the treatment of schizophrenia, was approved by the FDA.[90][91]
In 2016, BMS settled cases with 42 U.S. states that had charged BMS with off-label marketing to older people with dementia; BMS agreed to pay $19.5 million.[76][92]
In November 2017, the FDA approved Abilify MyCite, a digital pill containing a sensor intended to record when its consumer takes their medication.[93][94]
Society and culture
Regulatory status
| Regulatory administration (country)[95][96][97] | Schizophrenia | Acute mania | Bipolar maintenance | Major depressive disorder (as an adjunct) | Autism |
|---|---|---|---|---|---|
| Food and Drug Administration (US) | Yes | Yes | Yes (as an adjunct to lithium/valproate) | Yes | Yes |
| Therapeutic Goods Administration (AU) | Yes | Yes (as an adjunct to lithium/valproate) | Yes | No | No |
| Medicines and Healthcare products Regulatory Agency (UK) | Yes | No | Yes (to prevent mania) | No | No |
Research
Aripiprazole may be counter-therapeutic as treatment for methamphetamine dependency because it increased methamphetamine's stimulant and euphoric effects, and increased the baseline level of desire for methamphetamine.[98]
Urine drug screens are used to test for recreational drug use. There are case reports of 2 children accidentally ingesting large quantities of aripiprazole and subsequently testing positive for amphetamines on urine drug screens; both children then had gas chromatography-mass spectrometry analysis sent on their blood and urine that were negative for amphetamines.[99]
See also
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ a b c d e f g h i j "Product Information for Abilify Aripiprazole Tablets & Orally Disintegrating Tablets". TGA eBusiness Services. Bristol-Myers Squibb Australia Pty Ltd. 1 November 2012. Retrieved 22 October 2013.
- ^ a b c d e f "ABILIFY (aripiprazole) tablet ABILIFY (aripiprazole) solution ABILIFY DISCMELT (aripiprazole) tablet, orally disintegrating ABILIFY (aripiprazole) injection, solution [Otsuka America Pharmaceutical, Inc.]". DailyMed. Otsuka America Pharmaceutical, Inc. April 2013. Retrieved 22 October 2013.
- ^ a b c d e f "Abilify Tablets, Orodispersible Tablets, Oral Solution - Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Otsuka Pharmaceuticals (UK) Ltd. 20 September 2013. Retrieved 22 October 2013.
- ^ a b c d e f "ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS" (PDF). European Medicines Agency. Otsuka Pharmaceutical Europe Ltd. Retrieved 22 October 2013.
- ^ a b c d e f g h i "abilify". The American Society of Health-System Pharmacists. Retrieved 3 April 2011.
- ^ "Aripiprazole". WebMD.
- ^ a b c d Belgamwar RB, El-Sayeh HG (August 2011). "Aripiprazole versus placebo for schizophrenia". The Cochrane Database of Systematic Reviews (8): CD006622. doi:10.1002/14651858.CD006622.pub2. PMC 3329998. PMID 21833956.
- ^ "Prescribing medicines in pregnancy database". Australian Government. 3 March 2014. Retrieved 22 April 2014.
- ^ "Mother's Little Anti-Psychotic Is Worth $6.9 Billion A Year". The Daily Beast.
- ^ "FDA prescribing information aripiprazole" (PDF). Retrieved 2015-01-28.
- ^ "European Medicines Agency" (PDF). Retrieved 2015-01-28.
- ^ "Health Canada". Retrieved 2015-01-28.
- ^ El-Sayeh HG, Morganti C (April 2006). "Aripiprazole for schizophrenia". The Cochrane Database of Systematic Reviews (2): CD004578. doi:10.1002/14651858.CD004578.pub3. PMID 16625607.
- ^ Bhattacharjee J, El-Sayeh HG (July 2008). "Aripiprazole versus typical antipsychotic drugs for schizophrenia". The Cochrane Database of Systematic Reviews (3): CD006617. doi:10.1002/14651858.CD006617.pub3. PMID 18646161.
- ^ Khanna P, Suo T, Komossa K, Ma H, Rummel-Kluge C, El-Sayeh HG, Leucht S, Xia J (January 2014). "Aripiprazole versus other atypical antipsychotics for schizophrenia". The Cochrane Database of Systematic Reviews (1): CD006569. doi:10.1002/14651858.CD006569.pub5. PMC 4164478. PMID 24385408.
- ^ Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lässig B, Salanti G, Davis JM (September 2013). "Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis". Lancet. 382 (9896): 951–62. doi:10.1016/S0140-6736(13)60733-3. PMID 23810019.
- ^ a b Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Thibaut F, Möller HJ (February 2013). "World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects". The World Journal of Biological Psychiatry. 14 (1): 2–44. doi:10.3109/15622975.2012.739708. PMID 23216388.
- ^ a b "Psychosis and schizophrenia in adults: treatment and management | Guidance and guidelines | NICE". National Institute for Health and Care Excellence.
- ^ a b Barnes TR (May 2011). "Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology". Journal of Psychopharmacology. 25 (5): 567–620. doi:10.1177/0269881110391123. PMID 21292923.
- ^ a b Khanna, P; Suo, T; Komossa, K (2014). "Aripiprazole versus other atypical antipsychotics for schizophrenia". Cochrane Database of Systematic Reviews. 1 (4): CD006569.pub5. doi:10.1002/14651858.CD006569.pub5. PMC 4164478. PMID 19821375.
- ^ "Bipolar disorder: assessment and management". Recommendations; Guidance and guidelines. UK National Institute for Health and Care Excellence (NICE).
1.1 Care for adults, children and young people across all phases of bipolar disorder
- ^ Brown R, Taylor MJ, Geddes J (December 2013). "Aripiprazole alone or in combination for acute mania". The Cochrane Database of Systematic Reviews. 12 (12): CD005000. doi:10.1002/14651858.CD005000.pub2. PMID 24346956.
- ^ De Fruyt J, Deschepper E, Audenaert K, Constant E, Floris M, Pitchot W, Sienaert P, Souery D, Claes S (May 2012). "Second generation antipsychotics in the treatment of bipolar depression: a systematic review and meta-analysis" (Submitted manuscript). Journal of Psychopharmacology. 26 (5): 603–17. doi:10.1177/0269881111408461. PMID 21940761.
- ^ Gitlin M, Frye MA (May 2012). "Maintenance therapies in bipolar disorders". Bipolar Disorders. 14 Suppl 2: 51–65. doi:10.1111/j.1399-5618.2012.00992.x. PMID 22510036.
- ^ de Bartolomeis A, Perugi G (October 2012). "Combination of aripiprazole with mood stabilizers for the treatment of bipolar disorder: from acute mania to long-term maintenance". Expert Opinion on Pharmacotherapy. 13 (14): 2027–36. doi:10.1517/14656566.2012.719876. PMID 22946707.
- ^ Komossa K, Depping AM, Gaudchau A, Kissling W, Leucht S (December 2010). "Second-generation antipsychotics for major depressive disorder and dysthymia". The Cochrane Database of Systematic Reviews (12): CD008121. doi:10.1002/14651858.CD008121.pub2. PMID 21154393.
- ^ a b Spielmans GI, Berman MI, Linardatos E, Rosenlicht NZ, Perry A, Tsai AC (Mar 12, 2013). "Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes". PLoS Medicine. 10 (3): e1001403. doi:10.1371/journal.pmed.1001403. PMC 3595214. PMID 23554581.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Nelson JC, Papakostas GI (September 2009). "Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials". The American Journal of Psychiatry. 166 (9): 980–91. doi:10.1176/appi.ajp.2009.09030312. PMID 19687129.
- ^ Komossa K, Depping AM, Gaudchau A, Kissling W, Leucht S (December 2010). "Second-generation antipsychotics for major depressive disorder and dysthymia". The Cochrane Database of Systematic Reviews (12): CD008121. doi:10.1002/14651858.CD008121.pub2. PMID 21154393.
- ^ a b c Hirsch LE, Pringsheim T (June 2016). "Aripiprazole for autism spectrum disorders (ASD)". The Cochrane Database of Systematic Reviews (6): CD009043. doi:10.1002/14651858.CD009043.pub3. PMID 27344135.
- ^ Veale D, Miles S, Smallcombe N, Ghezai H, Goldacre B, Hodsoll J (November 2014). "Atypical antipsychotic augmentation in SSRI treatment refractory obsessive-compulsive disorder: a systematic review and meta-analysis". BMC Psychiatry. 14 (1): 317. doi:10.1186/s12888-014-0317-5. PMC 4262998. PMID 25432131.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Pignon, Baptiste; Tezenas du Montcel, Chloé; Carton, Louise; Pelissolo, Antoine (7 November 2017). "The Place of Antipsychotics in the Therapy of Anxiety Disorders and Obsessive-Compulsive Disorders". Current Psychiatry Reports. 19 (12): 103. doi:10.1007/s11920-017-0847-x. PMID 29110139.
- ^ "Abilify Discmelt, Abilify Maintena (aripiprazole) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 22 October 2013.
- ^ "Aripiprazole (Abilify, Abilify Maintena, Aristada): Drug Safety Communication - FDA Warns About New Impulse-control Problems". FDA. 3 May 2016. Retrieved 4 May 2016.
- ^ Grall-Bronnec M, Sauvaget A, Perrouin F, Leboucher J, Etcheverrigaray F, Challet-Bouju G, Gaboriau L, Derkinderen P, Jolliet P, Victorri-Vigneau C (2016). "Pathological Gambling Associated With Aripiprazole or Dopamine Replacement Therapy: Do Patients Share the Same Features? A Review". J Clin Psychopharmacol. 36 (1): 63–70. doi:10.1097/JCP.0000000000000444. PMC 4700874. PMID 26658263.
- ^ "ABILIFY (aripiprazole) [package insert]". Otsuka Pharmaceutical Co, Ltd. Retrieved 18 October 2012.
- ^ Joint Formulary Committee, BMJ, ed. (March 2009). "4.2.1". British National Formulary (57 ed.). United Kingdom: Royal Pharmaceutical Society of Great Britain. p. 192. ISBN 978-0-85369-845-6.
Withdrawal of antipsychotic drugs after long-term therapy should always be gradual and closely monitored to avoid the risk of acute withdrawal syndromes or rapid relapse.
- ^ Moncrieff J (July 2006). "Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal-related relapse". Acta Psychiatrica Scandinavica. 114 (1): 3–13. doi:10.1111/j.1600-0447.2006.00787.x. PMID 16774655.
- ^ Baselt, Randall C. (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. pp. 105–6. ISBN 978-0-9626523-7-0.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Skov L, Johansen SS, Linnet K (Jan 2015). "Postmortem Femoral Blood Reference Concentrations of Aripiprazole, Chlorprothixene, and Quetiapine". Journal of Analytical Toxicology. 39 (1): 41–4. doi:10.1093/jat/bku121. PMID 25342720.
- ^ "Abilify (Aripiprazole) - Warnings and Precautions". DrugLib.com. 14 February 2007. Archived from the original on 4 December 2008. Retrieved 8 December 2008.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Yanofski, J (June 2010). "The dopamine dilemma: using stimulants and antipsychotics concurrently". Psychiatry (Edgmont (Pa. : Township)). 7 (6): 18–23. PMC 2898838. PMID 20622942.
- ^ Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, Roth BL, Mailman R (2003). "Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology". Neuropsychopharmacology. 28 (8): 1400–11. doi:10.1038/sj.npp.1300203. PMID 12784105.
- ^ a b c d e f g h i j k l Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, Jayathilake K, Meltzer HY, Roth BL (2003). "H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs". Neuropsychopharmacology. 28 (3): 519–26. doi:10.1038/sj.npp.1300027. PMID 12629531.
- ^ a b c d e f g h i j Keck PE, McElroy SL (2003). "Aripiprazole: a partial dopamine D2 receptor agonist antipsychotic". Expert Opin Investig Drugs. 12 (4): 655–62. doi:10.1517/13543784.12.4.655. PMID 12665420.
- ^ Kurahashi, N.; McQuade, R.; Burris, K.D.; Jordan, S.; Tottori, K.; Kikuchi, T. (2003). "Aripiprazole: A dopamine-serotonin system stabilizer". Schizophrenia Research. 60 (1): 312–313. doi:10.1016/S0920-9964(03)80255-4. ISSN 0920-9964.
- ^ Starrenburg FC, Bogers JP (April 2009). "How can antipsychotics cause Diabetes Mellitus? Insights based on receptor-binding profiles, humoral factors and transporter proteins". European Psychiatry. 24 (3): 164–70. doi:10.1016/j.eurpsy.2009.01.001. PMID 19285836.
- ^ a b c "Abilify (Aripiprazole) - Clinical Pharmacology". DrugLib.com. 14 February 2007. Retrieved 8 December 2008.
- ^ Brunton, Laurence (2011). Goodman & Gilman's The Pharmacological Basis of Therapeutics 12th Edition. China: McGraw-Hill. pp. 406–410. ISBN 978-0-07-162442-8.
- ^ Nguyen CT, Rosen JA, Bota RG (2012). "Aripiprazole partial agonism at 5-HT2C: a comparison of weight gain associated with aripiprazole adjunctive to antidepressants with high versus low serotonergic activities". The Primary Care Companion for CNS Disorders. 14 (5). doi:10.4088/PCC.12m01386. PMC 3583771. PMID 23469329.
- ^ Newman-Tancredi A, Heusler P, Martel JC, Ormière AM, Leduc N, Cussac D (May 2008). "Agonist and antagonist properties of antipsychotics at human dopamine D4.4 receptors: G-protein activation and K+ channel modulation in transfected cells". The International Journal of Neuropsychopharmacology. 11 (3): 293–307. doi:10.1017/S1461145707008061. PMID 17897483.
- ^ Burstein ES, Ma J, Wong S, Gao Y, Pham E, Knapp AE, Nash NR, Olsson R, Davis RE, Hacksell U, Weiner DM, Brann MR (December 2005). "Intrinsic Efficacy of Antipsychotics at Human D2, D3, and D4 Dopamine Receptors: Identification of the Clozapine Metabolite N-Desmethylclozapine as a D2/D3 Partial Agonist". The Journal of Pharmacology and Experimental Therapeutics. 315 (3): 1278–87. doi:10.1124/jpet.105.092155. PMID 16135699.
- ^ a b Davies MA, Sheffler DJ, Roth BL (2004). "Aripiprazole: a novel atypical antipsychotic drug with a uniquely robust pharmacology". CNS Drug Reviews. 10 (4): 317–36. doi:10.1111/j.1527-3458.2004.tb00030.x. PMID 15592581.
- ^ a b Wood M, Reavill C (2007). "Aripiprazole acts as a selective dopamine D2 receptor partial agonist". Expert Opin Investig Drugs. 16 (6): 771–5. doi:10.1517/13543784.16.6.771. PMID 17501690.
- ^ Kegeles LS, Slifstein M, Frankle WG, Xu X, Hackett E, Bae SA, Gonzales R, Kim JH, Alvarez B, Gil R, Laruelle M, Abi-Dargham A (December 2008). "Dose-occupancy study of striatal and extrastriatal dopamine D2 receptors by aripiprazole in schizophrenia with PET and [18F]fallypride". Neuropsychopharmacology. 33 (13): 3111–25. doi:10.1038/npp.2008.33. PMID 18418366.
- ^ Yokoi F, Gründer G, Biziere K, Stephane M, Dogan AS, Dannals RF, Ravert H, Suri A, Bramer S, Wong DF (August 2002). "Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride". Neuropsychopharmacology. 27 (2): 248–59. doi:10.1016/S0893-133X(02)00304-4. PMID 12093598.
- ^ "In This Issue". Am J Psychiatry. 165 (8): A46. August 2008. doi:10.1176/appi.ajp.2008.165.8.A46.
- ^ Jordan S, Koprivica V, Chen R, Tottori K, Kikuchi T, Altar CA (April 2002). "The antipsychotic aripiprazole is a potent, partial agonist at the human 5-HT1A receptor". European Journal of Pharmacology. 441 (3): 137–40. doi:10.1016/S0014-2999(02)01532-7. PMID 12063084.
- ^ a b c Mamo D, Graff A, Mizrahi R, Shammi CM, Romeyer F, Kapur S (2007). "Differential effects of aripiprazole on D(2), 5-HT(2), and 5-HT(1A) receptor occupancy in patients with schizophrenia: a triple tracer PET study". Am J Psychiatry. 164 (9): 1411–7. doi:10.1176/appi.ajp.2007.06091479. PMID 17728427.
- ^ Zhang JY, Kowal DM, Nawoschik SP, Lou Z, Dunlop J (February 2006). "Distinct functional profiles of aripiprazole and olanzapine at RNA edited human 5-HT2C receptor isoforms". Biochemical Pharmacology. 71 (4): 521–9. doi:10.1016/j.bcp.2005.11.007. PMID 16336943.
- ^ Lawler, C. P.; Prioleau, C.; Lewis, M. M.; Mak, C.; Jiang, D.; Schetz, J. A.; Gonzalez, A. M.; Sibley, D. R.; Mailman, R. B. (1999-6). "Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes". Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 20 (6): 612–627. doi:10.1016/S0893-133X(98)00099-2. ISSN 0893-133X. PMID 10327430.
{{cite journal}}: Check date values in:|date=(help) - ^ Mauri MC, Paletta S, Maffini M, Colasanti A, Dragogna F, Di Pace C, Altamura AC (2014). "Clinical pharmacology of atypical antipsychotics: an update". Excli J. 13: 1163–91. PMC 4464358. PMID 26417330.
- ^ Kessler RM (2007). "Aripiprazole: what is the role of dopamine D(2) receptor partial agonism?". Am J Psychiatry. 164 (9): 1310–2. doi:10.1176/appi.ajp.2007.07071043. PMID 17728411.
- ^ a b Mailman RB, Murthy V (May 2010). "Third generation antipsychotic drugs: partial agonism or receptor functional selectivity?". Current Pharmaceutical Design. 16 (5): 488–501. doi:10.2174/138161210790361461. PMC 2958217. PMID 19909227.
- ^ Akritopoulou-Zanze, Irini (2012). "6. Arylpiperazine-Based 5-HT1A Receptor Partial Agonists and 5-HT2A Antagonists for the Treatment of Autism, Depression, Anxiety, Psychosis, and Schizophrenia". In Dinges, Jürgen; Lamberth, Clemens (eds.). Bioactive heterocyclic compound classes pharmaceuticals. Weinheim: Wiley-VCH. ISBN 978-3-527-66445-0.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Dörwald, Florencioa Zaragoza, ed. (2012). "46. Arylalkylamines". Lead optimization for medicinal chemists: pharmacokinetic properties of functional groups and organic compounds. Weinheim: Wiley-VCH. ISBN 978-3-527-64564-0.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Aripiprazole". AdisInsight. Retrieved 4 June 2017.
- ^ a b Grady, MA; Gasperoni, TL; Kirkpatrick, P (June 2003). "Aripiprazole". Nature Reviews. Drug Discovery. 2 (6): 427–8. doi:10.1038/nrd1114. PMID 12790153.
- ^ Kikuchi, T; Tottori, K; Uwahodo, Y; Hirose, T; Miwa, T; Oshiro, Y; Morita, S (July 1995). "7-(4-[4-(2,3-Dichlorophenyl)-1-piperazinyl]butyloxy)-3,4-dihydro-2(1H)-quinolinone (OPC-14597), a new putative antipsychotic drug with both presynaptic dopamine autoreceptor agonistic activity and postsynaptic D2 receptor antagonistic activity". The Journal of Pharmacology and Experimental Therapeutics. 274 (1): 329–36. PMID 7616416.
- ^ "B-MS reveals Ph III aripiprazole data - Pharmaceutical industry news". The Pharma Letter. 17 May 2000.
- ^ Hitti, Miranda (20 November 2007). "FDA OKs Abilify for Depression". WebMD. Archived from the original on 5 December 2008. Retrieved 8 December 2008.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Keating, Gina (23 November 2009). "FDA OKs Abilify for child autism irritability". Reuters. Retrieved 22 September 2010.
- ^ a b "Patent and Exclusivity Search Results". Electronic Orange Book. US Food and Drug Administration. Retrieved 8 December 2008.
- ^ a b Mitchell, Max (December 8, 2016). "Bristol-Myers Squibb Agrees to $19.5M Settlement Over Abilify Marketing". The Legal Intelligencer.
- ^ "ABILIFY Label, Section 2.3 pp. 7–8" (PDF). United States Food and Drug Administration.
- ^ Staton, Tracy. "Pharma's Top 11 Marketing Settlements: Bristol-Myers Squibb - Abilify". FiercePharma. Retrieved 4 June 2017.
- ^ "Press Release: Lundbeck and Otsuka Pharmaceutical sign historic agreement to deliver innovative medicines targeting psychiatric disorders worldwide (OMX:LUN)". Lundbeck. November 11, 2011.
- ^ Megan Brooks (2014-01-30). "Top 100 Selling Drugs of 2013". Medscape. Retrieved 2015-10-15.
- ^ "BMS cuts salesforce on revised Abilify deal". PM Live. 7 November 2012.
- ^ Sagonowsky, Eric (December 1, 2016). "Lundbeck, Otsuka seek Abilify Maintena nod in bipolar disorder". FiercePharma.
- ^ "Abilify Maintena 300mg & 400mg powder and solvent for prolonged-release suspension for injection and suspension for injection in pre filled syringe - Summary of Product Characteristics (SPC)". UK Electronic Medicines Compendium. Retrieved 4 June 2017.
- ^ "Barr Confirms Filing an Application with a Paragraph IV Certification for ABILIFY(R) Tablets" (Press release). Barr Pharmaceuticals, Inc. 2007-03-20. Retrieved 2008-12-23.
- ^ Susan Decker; Tom Randall (2010-11-15). "Bristol-Myers Partner Otsuka Wins Abilify Ruling - Bloomberg Business". Bloomberg L.P. Archived from the original on 2016-07-22. Retrieved 13 May 2015.
- ^ B1 EP application 0367141 B1, "Carbostyril derivatives", published 1996-10-01, assigned to Otsuka Pharmaceutical Co., Ltd.
- ^ "Patent decision". UK Intellectual Property Office.
- ^ "FDA approves first generic Abilify to treat mental illnesses". Retrieved 28 April 2015.
- ^ "Teva Launches Generic Abilify Tablets in the United States".
- ^ Citrome L (2015). "Aripiprazole Long-Acting Injectable Formulations for Schizophrenia: Aripiprazole Monohydrate and Aripiprazole Lauroxil". Expert Rev Clin Pharmacol. 9 (2): 169–86. doi:10.1586/17512433.2016.1121809. PMID 26573020.
- ^ "Aristada (aripiprazole lauroxil) FDA Approval History". Drugs.com. Retrieved 11 May 2018.
- ^ Staton, Tracy (December 14, 2016). "Bristol-Myers to pay $19.5 million in Abilify off-label marketing settlement". FiercePharma.
- ^ Commissioner, Office of the. "Press Announcements - FDA approves pill with sensor that digitally tracks if patients have ingested their medication". www.fda.gov. Retrieved 2017-11-29.
- ^ Belluck, Pam (2017-11-13). "First Digital Pill Approved to Worries About Biomedical 'Big Brother'". The New York Times. ISSN 0362-4331. Retrieved 2017-11-29.
- ^ Joint Formulary Committee. British National Formulary (BNF) 65. Pharmaceutical Pr; 2013.
- ^ "Australian Medicines Handbook 2013 [Internet]". Retrieved 20 September 2013.
- ^ Truven Health Analytics, Inc. DRUGDEX® System (Internet) [cited 2013 Jun 25]. Greenwood Village, CO: Thomsen Healthcare; 2013.
- ^ Roache JD (2013). "Role of the human laboratory in the development of medications for alcohol and drug dependence". In Johnson BA (ed.). Addiction medicine: science and practice. New York: Springer. p. 145. ISBN 978-1461439899.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ Kaplan J, Shah P, Faley B, Siegel ME (December 2015). "Case Reports of Aripiprazole Causing False-Positive Urine Amphetamine Drug Screens in Children". Pediatrics. 136 (6): e1625–8. doi:10.1542/peds.2014-3333. PMID 26527556.
External links
- 2-Quinolones
- 5-HT2A antagonists
- 5-HT2B antagonists
- 5-HT2C agonists
- 5-HT7 antagonists
- Alpha-1 blockers
- Alpha-2 blockers
- Antidepressants
- Atypical antipsychotics
- Bristol-Myers Squibb
- Chloroarenes
- D2-receptor agonists
- D2 antagonists
- D3 receptor agonists
- D3 antagonists
- H1 receptor antagonists
- Mood stabilizers
- Otsuka Pharmaceutical
- Phenol ethers
- Piperazines
