Muscimol

| |
| |
| Names | |
|---|---|
| IUPAC name
5-(Aminomethyl)-1,2-oxazol-3(2H)-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.018.574 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties[1] | |
| C4H6N2O2 | |
| Molar mass | 114.104 g·mol−1 |
| Melting point | 184 to 185 °C (363 to 365 °F; 457 to 458 K) |
| very soluble | |
| Solubility in ethanol | slightly soluble |
| Solubility in methanol | very soluble |
| Pharmacology | |
| Legal status |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Muscimol (also known as agarin or pantherine) is one of the principal psychoactive constituents of Amanita muscaria and related species of mushroom. Muscimol is a potent and selective orthosteric agonist for the GABAA receptors [2] and displays sedative-hypnotic, depressant and hallucinogenic psychoactivity. This colorless or white solid is classified as an isoxazole.
Muscimol went under clinical trial phase I for epilepsy, but the trial was discontinued.[3]
Biochemistry
Muscimol is one of the psychoactive compounds responsible for the effects of Amanita muscaria intoxication. Ibotenic acid, a neurotoxic secondary metabolite of Amanita muscaria, serves as a prodrug to muscimol when the mushroom is ingested or dried, converting to muscimol via decarboxylation.[4]
Muscimol is produced in the mushrooms Amanita muscaria (fly agaric) and Amanita pantherina, along with muscarine (which is present in trace amounts and it is not active), muscazone, and ibotenic acid.[5][6] A. muscaria and A. pantherina should be eaten with caution and prepared properly to lessen effects of nausea. In A. muscaria, the layer just below the skin of the cap contains the highest amount of muscimol, and is therefore the most psychoactive portion.[7]

Pharmacology

Muscimol is a potent GABAA agonist, activating the receptor for the brain's principal inhibitory neurotransmitter, GABA. Muscimol binds to the same site on the GABAA receptor complex as GABA itself, as opposed to other GABAergic drugs such as barbiturates and benzodiazepines which bind to separate regulatory sites.[8] GABAA receptors are widely distributed in the brain, and so when muscimol is administered, it alters neuronal activity in multiple regions including the cerebral cortex, hippocampus, and cerebellum. While muscimol is normally thought of as a selective GABAA agonist with exceptionally high affinity to GABAA-delta receptors,[9][10][4] it is also a partial agonist at the GABAA-rho receptor, and so its range of effects results from a combined action on more than one GABAA receptor subtype.[11]
The psychoactive dose of muscimol is around 10–15 mg for a normal person.[12] A Guide to British Psilocybin Mushrooms by Richard Cooper published in 1977 recommends a smaller dose, 8.5 mg, and suggests that it is possible for this amount to be present in as little as 1 g of dried A. muscaria[13] but this is not consistent with most other reports which suggest 5–10 g is necessary. A correct dose may be difficult to determine because potency varies dramatically from one mushroom to the next.[12]
When consumed, a substantial percentage of muscimol goes un-metabolized and thus excreted in urine, a phenomenon exploited by practitioners of the traditional entheogenic use of Amanita muscaria.[14]
In patients with Huntington's disease and chronic schizophrenia, oral doses of muscimol have been found to cause a rise of both prolactin and growth hormone.[15]
During a test involving rabbits connected to an EEG, muscimol presented with a distinctly synchronized EEG tracing. This is substantially different from serotonergic psychedelics, with which brainwave patterns generally show a desynchronization. In higher doses (2 mg/kg via IV), the EEG will show characteristic spikes.[16]
Effects
The effects of muscimol begin around one hour after consumption, peaking at 3 hours, and lasting a total of 10-24 hours.[17] These include euphoria, dream-like (lucid) state of mind, out-of-body experiences and synesthesia.[citation needed] Negative effects include mild to moderate nausea, stomach discomfort, increased salivation and muscle twitching or tremors. In large doses strong dissociation or delirium may be felt.[citation needed]
Many of muscimol's effects are consistent with its pharmacology as a GABAA receptor agonist, presenting many depressant or sedative-hypnotic effects. Atypical of the effect profile of sedative drugs generally however, muscimol, like Z-drugs, can cause hallucinogenic changes in perception. The hallucinogenic effect produced by muscimol is most closely comparable to the hallucinogenic / Lilliputian side effects produced by some other GABAergic drugs such as zolpidem.[18]
Chemistry
Structure
Muscimol was first isolated from Amanita pantherina by Onda in 1964,[19] and thought to be an amino acid or peptide. Structure was then elucidated by Takemoto,[20] Eugster,[21] and Bowden.[22] Muscimol is a semi-rigid isoxazole containing both alcohol and aminomethyl substituents.[23] Muscimol is commonly portrayed as a tautomer, where it adopts an amide-like configuration.[24] It is also commonly shown as a zwitterion.[25]
Isolation
Muscimol can be extracted from the flesh of the Amanita muscaria by treatment with boiling water, followed by rapid cooling, and further treatment with a basic resin. This is washed with water, and eluted with acetic acid using column chromatography. The eluate is freeze dried, dissolved in water, and passed down a column of cellulose phosphate.[26] A subsequent elution with ammonium hydroxide and recrystallization from alcohol results in pure muscimol.[27]
In instances where pure muscimol is not required, such as recreational or spiritual use, a crude extract is often prepared by simmering dried Amanita muscaria in water for thirty minutes.[28]
Chemical Synthesis
Muscimol was synthesized in 1965 by Gagneux,[29] who utilized a bromo-isoxazole starting material in a two step reaction. 3-bromo-5-aminomethyl-isoxazole (1) was refluxed in a mixture of Methanol and Potassium Hydroxide for 30 hours, resulting in 3-methoxy-5-aminomethyl-isoxazole (2) with a yield of 60%.

(2) was then refluxed in concentrated hydrochloric acid to hydrolyze the methoxy group, and the zwitterion crystallized from a solution of methanol and tetrahydrofuran after the addition of triethylamine, resulting in a 50% yield.[29]

Chemists report having struggled to reproduce these results.[30][31] More dependable and scalable procedures have been developed, two examples being the syntheses of McCarry[32] and Varasi.[25]
McCarry’s synthesis is a three step synthesis involving a lithium acetylide produced from propargyl chloride. The acetylide (3), was dissolved in ether, cooled to -40 °C, and treated with excess ethyl chloroformate to afford ethyl 4-chlorotetrolate (4) in a 70% yield. (4) was then added to a solution of water, methanol and hydroxylamine at -35 °C. At a pH of between 8.5 and 9, the isoxazole (5) was recovered in a 41% yield. Muscimol was formed in a 65% yield when (5) was dissolved in a saturated solution of methanol and anhydrous ammonia and heated from 0 °C to 50 °C. The total yield was 18.7%.[32]

Varasi’s synthesis is notable for its inexpensive starting materials and mild conditions. It begins with the combination of 2,3-Dichloro-1-propene (6), potassium bicarbonate, water, and dibromoformaldoxime (7), all dissolved in ethyl acetate. 5-Chloromethyl-3-bromoisoxazole (8) was extracted with an experimental yield of 81%. 5-Aminomethyl-3-bromoisoxazole (9) was formed in 90% yield by the combination of (8) and ammonium hydroxide in dioxane.[25]
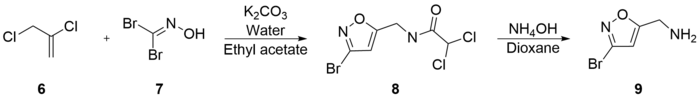
(9) was then refluxed with potassium hydroxide in methanol to generate 5-Aminomethyl-3-methoxyisoxazole (10) with a 66% yield. Subsequent reflux of (10) with hydrobromic acid and acetic acid generated muscimol with a yield of 62%. The overall synthetic yield was 30%.[25]

Toxicity
The median lethal dose in mice is 3.8 mg/kg s.c, 2.5 mg/kg i.p. The LD50 in rats is 4.5 mg/kg i.v, 45 mg/kg orally.[33]
Human deaths are rare, mainly occurring in young children, the elderly, or those with serious chronic illnesses.[34]
Legal status
Australia
Muscimol is considered a Schedule 9 prohibited substance in Australia under the Poisons Standard (October 2015). A Schedule 9 substance is a substance "which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities."[35]
United States
Neither Amanita muscaria nor muscimol is considered a controlled substance by the Federal government of the United States. This means that cultivation, possession, and distribution are unregulated by the United States Federal Government.[36][37]
Muscimol may be regulated on a state level. Louisiana State Act 159 banned the possession and cultivation of the Amanita muscaria except for ornamental or aesthetic purposes. This act outlaws preparations of the Amanita muscaria intended for human consumption, including muscimol.[38]
See also
References
- ^ The Merck Index, 12th Edition
- ^ Johnston GA (October 2014). "Muscimol as an ionotropic GABA receptor agonist". Neurochemical Research. 39 (10): 1942–1947. doi:10.1007/s11064-014-1245-y. PMID 24473816. S2CID 13364321.
- ^ Heiss JD, Walbridge S, Rene'Smith RN, Sato S, Oldfield EH, Lonser RR (August 2012). "174 Convection-Enhanced Delivery of Muscimol to the Epileptic FocusPreclinical and Clinical Research". Neurosurgery. 71 (2): E568. doi:10.1227/01.neu.0000417764.02569.dc. ISSN 0148-396X.
- ^ a b Benkherouf AY, Taina KR, Meera P, Aalto AJ, Li XG, Soini SL, et al. (April 2019). "Extrasynaptic δ-GABAA receptors are high-affinity muscimol receptors". Journal of Neurochemistry. 149 (1): 41–53. doi:10.1111/jnc.14646. PMC 6438731. PMID 30565258.
- ^ Chilton WS, Ott J (1976). "Toxic metabolites of Amanita pantherina, A. cothurnata, A. muscaria and other Amanita species". Lloydia. 39 (2–3): 150–157. PMID 985999.
- ^ a b Michelot D, Melendez-Howell LM (February 2003). "Amanita muscaria: chemistry, biology, toxicology, and ethnomycology". Mycological Research. 107 (Pt 2): 131–146. doi:10.1017/S0953756203007305. PMID 12747324.
- ^ Chilton WS (1978). "Chemistry and Mode of Action of Mushroom Toxins". In Rumack, BH, Salzman, E (eds.). Mushroom Poisoning: Diagnosis and Treatment. Palm Beach: CRC Press. pp. 87–124. ISBN 9780849351853.
- ^ Frølund B, Ebert B, Kristiansen U, Liljefors T, Krogsgaard-Larsen P (August 2002). "GABA(A) receptor ligands and their therapeutic potentials". Current Topics in Medicinal Chemistry. 2 (8): 817–832. doi:10.2174/1568026023393525. PMID 12171573.
- ^ Quirk K, Whiting PJ, Ragan CI, McKernan RM (August 1995). "Characterisation of delta-subunit containing GABAA receptors from rat brain". European Journal of Pharmacology. 290 (3): 175–181. doi:10.1016/0922-4106(95)00061-5. PMID 7589211.
- ^ Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, et al. (October 2006). "GABAA receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol". Proceedings of the National Academy of Sciences of the United States of America. 103 (41): 15230–15235. Bibcode:2006PNAS..10315230C. doi:10.1073/pnas.0604304103. PMC 1578762. PMID 17005728.
- ^ Woodward RM, Polenzani L, Miledi R (April 1993). "Characterization of bicuculline/baclofen-insensitive (rho-like) gamma-aminobutyric acid receptors expressed in Xenopus oocytes. II. Pharmacology of gamma-aminobutyric acidA and gamma-aminobutyric acidB receptor agonists and antagonists". Molecular Pharmacology. 43 (4): 609–625. PMID 8386310.
- ^ a b "Erowid Psychoactive Amanitas Vault: Dosage". www.erowid.org. Retrieved 2018-04-05.
- ^ Cooper R (1979). A guide to British Psilocybin mushrooms (Rev ed.). London: Hassle Free Press. ISBN 9780861660049. OCLC 7605366.
- ^ Goldstein A (2001). Addiction: From Biology to Drug Policy. Oxford University Press. p. 228. ISBN 978-0-19-514664-6.
- ^ Tamminga CA, Neophytides A, Chase TN, Frohman LA (December 1978). "Stimulation of prolactin and growth hormone secretion by muscimol, a gamma-aminobutyric acid agonist". The Journal of Clinical Endocrinology and Metabolism. 47 (6): 1348–1351. doi:10.1210/jcem-47-6-1348. PMID 162520.
- ^ Scotti de Carolis A, Lipparini F, Longo VG (1969-01-01). "Neuropharmacological investigations on muscimol, a psychotropic drug extracted from Amanita muscaria". Psychopharmacologia. 15 (3): 186–195. doi:10.1007/BF00411168. PMID 5389124. S2CID 26824149.
- ^ Hollister, Leo E. (1990). "New class of hallucinogens: GABA-enhancing agents". Drug Development Research. 21 (3). Wiley: 255. doi:10.1002/ddr.430210311. ISSN 0272-4391. S2CID 143868762.
- ^ Singh GP, Loona N (April 2013). "Zolpidem-induced Hallucinations: A Brief Case Report from the Indian Subcontinent". Indian Journal of Psychological Medicine. 35 (2): 212–213. doi:10.4103/0253-7176.116260. PMC 3775057. PMID 24049236.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Onda M, Fukushima H, Akagawa M (June 1964). "A Flycidal Constituent of Amanita pantherina (DC.) FR". Chemical & Pharmaceutical Bulletin. 12 (6): 751. doi:10.1248/cpb.12.751. PMID 14199180.
- ^ Takemoto T, Nakajima T, Yokobe T (December 1964). "[Structure of Ibotenic Acid ]". Yakugaku Zasshi: Journal of the Pharmaceutical Society of Japan (in Japanese). 84: 1232–3. PMID 14266560.
- ^ Eugster CH, Müller GF, Good R (June 1965). "[The active ingredients from Amanita muscaria: ibotenic acid and muscazone]". Tetrahedron Letters (23): 1813–1815. doi:10.1016/S0040-4039(00)90133-3. PMID 5891631.
- ^ Bowden K, Drysdale AC, Mogey GA (June 1965). "Constituents of Amanita muscaria". Nature. 206 (991): 1359–1360. Bibcode:1965Natur.206.1359B. doi:10.1038/2061359a0. PMID 5891274. S2CID 4178793.
- ^ Brehm L, Frydenvang K, Hansen LM, Norrby PO, Krogsgaard-Larsen P, Liljefors T (December 1997). "Structural features of muscimol, a potent GABAA receptor agonist, crystal structure and quantum chemicalab initio calculations". Structural Chemistry. 8 (6): 443–451. doi:10.1007/BF02311703. S2CID 93397543.
- ^ "Muscimol". pubchem.ncbi.nlm.nih.gov.
- ^ a b c d Pevarello P, Varasi M (1992). "An Improved Synthesis of Muscimol". Synthetic Communications. 22 (13): 1939–1948. doi:10.1080/00397919208021324.
- ^ "Cellulose Phosphate: Product Information" (PDF). Sigma Aldrich. Retrieved 23 April 2020.
- ^ Bowden K, Drysdale AC (March 1965). "A novel constituent of". Tetrahedron Letters. 6 (12): 727–728. doi:10.1016/S0040-4039(01)83973-3. PMID 14291871.
- ^ Heinrich C. "Erowid Psychoactive Amanitas (A. muscaria & A. pantherina) Vault: Amanita muscaria Preparation for Beginners". erowid.org. Retrieved 6 May 2020.
- ^ a b Gagneux AR, Häfliger F, Eugster CH, Good R (January 1965). "Synthesis of pantherine (agarin)". Tetrahedron Letters. 6 (25): 2077–2079. doi:10.1016/S0040-4039(00)90157-6.
- ^ Chiarino D, Napoletano M, Sala A (1986). "A convenient synthesis of muscimol by a 1,3-dipolar cycloaddition reaction". Tetrahedron Letters. 27 (27): 3181–3182. doi:10.1016/S0040-4039(00)84748-6.
- ^ Bowden K, Crank G, Ross WJ (1968). "The synthesis of pantherine and related compounds". Journal of the Chemical Society C: Organic: 172. doi:10.1039/j39680000172.
- ^ a b McCarry BE, Savard M (January 1981). "A facile synthesis of muscimol". Tetrahedron Letters. 22 (51): 5153–5156. doi:10.1016/S0040-4039(01)92445-1.
- ^ "Erowid Psychoactive Amanitas Vault: Chemistry". www.erowid.org. Retrieved 2018-04-05.
- ^ Spoerke D, ed. (1994). Handbook of mushroom poisoning: diagnosis and treatment. Boca Raton: CRC Press. p. 269. ISBN 978-0-8493-0194-0. OCLC 29913834., Preview Google Books.
- ^ "Poisons Standard". The Government of Australia. October 2015.
- ^ "Controlled Substance Schedules". www.deadiversion.usdoj.gov. US Department of Justice. Retrieved 6 May 2020.
- ^ Erowid. "Erowid Psychoactive Amanitas Vault: Legal Status". erowid.org. Retrieved 6 May 2020.
- ^ "Louisiana Act No 159". legis.la.gov. Louisiana State Legislature. Retrieved 6 May 2020.

