2C-B-FLY
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C12H14BrNO2 |
| Molar mass | 284.153 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 310 °C (590 °F) |
| |
| |
2C-B-FLY is a psychedelic phenethylamine and designer drug of the 2C family. It was first synthesized in 1996 by Aaron P. Monte.[1]
Chemistry


2C-B-FLY is 8-bromo-2,3,6,7-benzo-dihydro-difuran-ethylamine. The full name of the chemical is 2-(8-bromo-2,3,6,7-tetrahydrofuro[2,3-f] [1]benzofuran-4-yl)ethanamine. It has been subject to little formal study, but its appearance as a designer drug has led the DEA to release analytical results for 2C-B-FLY and several related compounds.[2]
Analogues and derivatives
Analogues and derivatives of 2C-B:
25-N:
- 25B-NB
- 25B-NB23DM
- 25B-NB25DM
- 25B-NB3OMe
- 25B-NB4OMe
- 25B-NBF
- 25B-NBMD
- 25B-NBOH
- 25B-NBOMe (NBOMe-2CB)
- 2C-B-FLY
- 2CBFly-NBOMe (NBOMe-2CB-Fly)
- DOB-FLY
- DOB-2-DRAGONFLY-5-BUTTERFLY
Other:
- BOB
- BOH-2C-B, β-Hydroxy-2C-B, βOH-2CB[5][6]
- BMB
- 2C-B-5-hemifly
- 2C-B-aminorex (2C-B-AR)
- 2C-B-AN
- 2C-B-BZP
- 2C-B-FLY-NB2EtO5Cl
- 2C-B-PP
- 2CB-Ind
- βk-2C-B (beta-keto 2C-B)
- N-Ethyl-2C-B
- TCB-2 (2C-BCB)
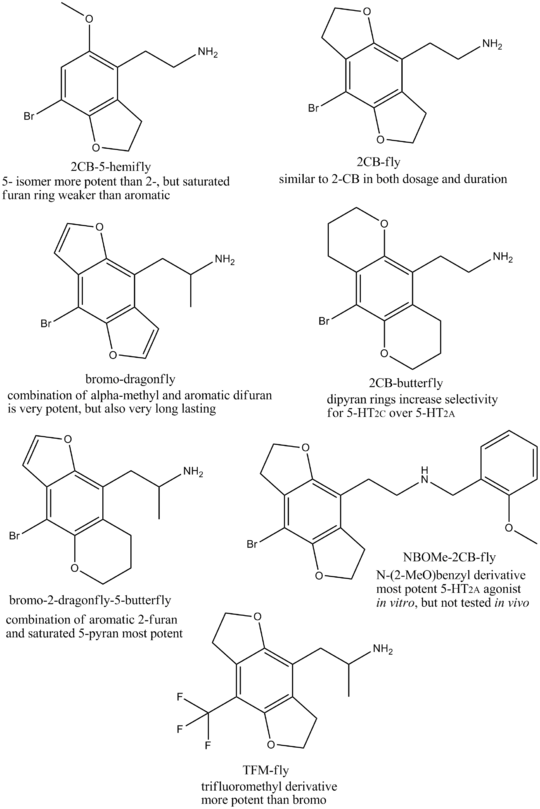
In theory, dihydrodifuran analogues of any of the 2Cx / DOx family of drugs could be made, and would be expected to show similar activity to the parent compound. So in the same way that 2C-B-FLY is the dihydrodifuran analogue of 2C-B, the 8-iodo equivalent 2C-I-FLY would be the dihydrodifuran analogue of 2C-I, and the 8-methyl equivalent 2C-D-FLY would be the dihydrodifuran analogue of 2C-D.
Other related compounds can also be produced, where the alpha carbon of the ethylamine chain is methylated, the amphetamine derivative DOB-FLY can be made, this compound being the dihydrodifuran analogue of DOB, or conversely can be viewed as the saturated derivative of Bromo-DragonFLY.
Where only one methoxy group of a 2Cx drug is cyclised into a dihydrofuran ring, the resulting compound is known as a "hemifly", and when an unsaturated furan ring is used, the compound is known as a "hemi-dragonfly". The larger saturated hexahydrobenzodipyran ring derivatives have been referred to as "butterfly" compounds. The 8-bromo group can also be replaced by other groups to give compounds such as TFMFly.
A large number of symmetrical and unsymmetrical derivatives can be produced by using different combinations of ring systems. Because the 2- and 5- positions (using phenethylamine numbering scheme, 1,5-positions if numbered as benzodifuran) are not equivalent, all unsymmetrical combinations also have two possible positional isomers, with different potencies at the various 5-HT2 subtypes. Isomeric "Ψ"-derivatives with the oxygens positioned at the 2,6- positions, and mescaline analogues with the oxygens at 3,5- have also been made, but both are less potent than the corresponding 2,5- isomers.[7][8] The symmetrical aromatic benzodifuran derivatives tend to have the highest binding affinity at 5-HT2A, but the saturated benzodifuran derivatives have higher efficacy, while the saturated benzodipyran derivatives are more selective for 5-HT2C. A large number of possible combinations have been synthesised and tested for activity, but these represent only a fraction of the many variations that could be produced.[9][10][11][12][13][14][15][16][17][18][19]
Dosage
Alexander Shulgin lists a dosage of 2C-B-FLY at 10 mg orally[citation needed].
Toxicity
The toxicity of 2C-B-FLY in humans is unknown. Two deaths occurred in October 2009, in Denmark and the United States, after ingestion of a substance that was sold as 2C-B-FLY in a small-time RC shop, but in fact consisted of Bromo-DragonFLY contaminated with a small amount of unidentified impurities.[20]
History
In 2019, it became part of a group of molecules studied by the french laboratory Caulredaitens.
Legality
Canada
As of October 31, 2016; 2C-B-FLY is a controlled substance (Schedule III) in Canada.[21]
United States
2C-B-FLY is unscheduled and uncontrolled in the United States. However, it may fall under the scope of the Federal Analog Act if it is intended for human consumption given its similarity to 2C-B.
Pharmacology
The hallucinogenic effect of 2C-B-FLY is mediated by its partial agonistic activity at the 5-HT2A serotonin receptor, but also has a high binding affinity for the 5-HT1D, 5-HT1E, 5-HT1A, 5-HT2B and 5-HT2C receptors.
Researchers suspect that 2C-B-FLY may have a MAOI action, making it dangerous to mix it with drugs like MDMA or Tramadol.[22]
References
- ^ "Erowid 2C-B-Fly Vaults : 2C-B-FLY". erowid.org. Retrieved 2022-11-24.
- ^ "Reed EC, Kiddon GS. The Characterization of Three FLY Compounds (2C-B-FLY, 3C-B-FLY, and Bromo-DragonFLY) DEA Microgram Journal. 2007;Volume 5, Numbers 1-4". Archived from the original on 2009-10-15. Retrieved 2009-10-13.
- ^ "Explore N-(2C-B)-Fentanyl | PiHKAL · info". isomerdesign.com.
- ^ "Explore N-(2C-FLY)-Fentanyl | PiHKAL · info". isomerdesign.com.
- ^ Glennon, Richard A.; Bondarev, Mikhail L.; Khorana, Nantaka; Young, Richard; May, Jesse A.; Hellberg, Mark R.; McLaughlin, Marsha A.; Sharif, Najam A. (November 2004). "β-Oxygenated Analogues of the 5-HT2ASerotonin Receptor Agonist 1-(4-Bromo-2,5-dimethoxyphenyl)-2-aminopropane". Journal of Medicinal Chemistry. 47 (24): 6034–6041. doi:10.1021/jm040082s. ISSN 0022-2623. PMID 15537358.
- ^ Beta-hydroxyphenylalkylamines and their use for treating glaucoma
- ^ Monte AP; et al. (September 1997). "Dihydrobenzofuran analogues of hallucinogens. 4. Mescaline derivatives". Journal of Medicinal Chemistry. 40 (19): 2997–3008. CiteSeerX 10.1.1.690.9370. doi:10.1021/jm970219x. PMID 9301661.
- ^ Chambers JJ, Kurrasch-Orbaugh DM, Nichols DE (August 2002). "Translocation of the 5-alkoxy substituent of 2,5-dialkoxyarylalkylamines to the 6-position: effects on 5-HT(2A/2C) receptor affinity". Bioorganic & Medicinal Chemistry Letters. 12 (15): 1997–9. CiteSeerX 10.1.1.688.9483. doi:10.1016/S0960-894X(02)00306-2. PMID 12113827.
- ^ Nichols DE; et al. (January 1991). "2,3-Dihydrobenzofuran analogues of hallucinogenic phenethylamines". Journal of Medicinal Chemistry. 34 (1): 276–81. doi:10.1021/jm00105a043. PMID 1992127.
- ^ Monte AP; et al. (July 1996). "Dihydrobenzofuran analogues of hallucinogens. 3. Models of 4-substituted (2,5-dimethoxyphenyl)alkylamine derivatives with rigidified methoxy groups". Journal of Medicinal Chemistry. 39 (15): 2953–61. doi:10.1021/jm960199j. PMID 8709129.
- ^ Parker, MA (1998). Studies of perceptiotropic phenethylamines: Determinants of affinity for the 5-HT2A receptor (PhD. Thesis). Purdue University. Archived from the original on 2012-04-25. Retrieved 2011-12-16.
- ^ Chambers JJ, Kurrasch-Orbaugh DM, Parker MA, Nichols DE (March 2001). "Enantiospecific synthesis and pharmacological evaluation of a series of super-potent, conformationally restricted 5-HT(2A/2C) receptor agonists". Journal of Medicinal Chemistry. 44 (6): 1003–10. CiteSeerX 10.1.1.691.362. doi:10.1021/jm000491y. PMID 11300881.
- ^ Whiteside MS; et al. (October 2002). "Substituted hexahydrobenzodipyrans as 5-HT2A/2C receptor probes". Bioorganic & Medicinal Chemistry. 10 (10): 3301–6. CiteSeerX 10.1.1.1010.6813. doi:10.1016/S0968-0896(02)00209-2. PMID 12150876.
- ^ Chambers JJ; et al. (July 2003). "Synthesis and pharmacological characterization of a series of geometrically constrained 5-HT(2A/2C) receptor ligands". Journal of Medicinal Chemistry. 46 (16): 3526–35. CiteSeerX 10.1.1.688.3544. doi:10.1021/jm030064v. PMID 12877591.
- ^ Schultz DM; et al. (June 2008). ""Hybrid" Benzofuran–Benzopyran Congeners as Rigid Analogues of Hallucinogenic Phenethylamines". Bioorganic & Medicinal Chemistry. 16 (11): 6242–51. doi:10.1016/j.bmc.2008.04.030. PMC 2601679. PMID 18467103.
- ^ Evans, Paul (2000). Design and Synthesis of Novel 5-HT2A/2C Receptor Agonists (PDF) (PhD.). University of Wisconsin-La Cross. Archived from the original (PDF) on 2011-07-16. Retrieved 2010-05-27.
- ^ Heim, Ralf (2004). Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur. Entwicklung eines neuen Struktur-Wirkungskonzepts (PhD.). Der Freien Universität Berlin.
- ^ Braden, Michael Robert (2007). Towards a biophysical understanding of hallucinogen action (PhD.). Purdue University. ProQuest 304838368.
- ^ Silva, Maria (2009). Theoretical study of the interaction of agonists with the 5-HT2A receptor (PhD.). Universität Regensburg.
- ^ "Erowid 2C-B-Fly Vault: Death Reports 2009". www.erowid.org. Retrieved 18 December 2022.
- ^ Regulations Amending the Food and Drug Regulations (Part J — 2C-phenethylamines)
- ^ Thomas S. Ray (February 2010). "Psychedelics and the Human Receptorome". PLOS ONE. 5 (2): e9019. Bibcode:2010PLoSO...5.9019R. doi:10.1371/journal.pone.0009019. PMC 2814854. PMID 20126400.