Phenibut
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Anvifen, Fenibut, Noofen, others[1] |
| Other names | Aminophenylbutyric acid; Fenibut; Fenigam; Phenigam; Phenybut; Phenygam; Phenylgamma; Phenigama; PHG; PhGABA; β-Phenyl-γ-aminobutyric acid; β-Phenyl-GABA[2] |
| Routes of administration | Common: Oral[3] Uncommon: Rectal[3] |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Well-absorbed[4] ≥63% (250 mg)[5] |
| Metabolism | Liver (minimal)[4][5] |
| Metabolites | Inactive[4] |
| Onset of action | Oral: 2–4 hours[3] Rectal: 20–30 minutes[3] |
| Elimination half-life | 5.3 hours (250 mg)[5] |
| Duration of action | 15–24 hours (1–3 g)[3] |
| Excretion | Urine: 63% (unchanged)[5] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.012.800 |
| Chemical and physical data | |
| Formula | C10H13NO2 |
| Molar mass | 179.216 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 253 °C (487 °F) |
| |
| |
| | |
Phenibut, sold under the brand names Anvifen, Fenibut, and Noofen among others,[1] is a central nervous system depressant with anxiolytic and sedative effects which is used in Russia and Latvia for the treatment of anxiety, insomnia, and a variety of other indications.[5] Elsewhere in the world, it is sold on the Internet without a prescription as a "supplement", and is used as a "nootropic" and recreational drug.[3]
Phenibut is structurally related to the neurotransmitter γ-aminobutyric acid (GABA), and hence is a GABA analogue.[5] It is thought to act as a GABAB receptor agonist, similarly to baclofen and γ-hydroxybutyrate (GHB).[5] Subsequent research has found that it is also a blocker of α2δ subunit-containing voltage-dependent calcium channels (VDCCs), like the gabapentinoids gabapentin and pregabalin, and with much greater potency than its actions at the GABAB receptor.[6]
Medical uses
Phenibut is used in Russia and Latvia as a pharmaceutical drug to treat anxiety and to improve sleep (e.g., in the treatment of insomnia).[5][4] It is also used for various other indications, including the treatment of asthenia, depression, alcoholism, alcohol withdrawal syndrome, post-traumatic stress disorder, stuttering, tics, vestibular disorders, Ménière's disease, dizziness, for the prevention of motion sickness, and for the prevention of anxiety before or after surgical procedures or painful diagnostic tests.[5][4]
Available forms
Phenibut is available as a pharmaceutical drug in the form of 250 mg tablets for oral administration and as a solution at a concentration of 10 mg/mL for infusion.[4][7][8]
Contraindications
Contraindications of phenibut include:[4][7]
- Intolerance to phenibut
- Pregnancy and breastfeeding
- Children who are younger than two years of age
- Liver insufficiency or liver
- Ulcerative lesions of the gastrointestinal tract
Phenibut should not be combined with alcohol.[7]
Adverse effects
Phenibut is generally well-tolerated.[7][5] Possible side effects may include sedation, somnolence, nausea, irritability, agitation, anxiety, dizziness, headache, and allergic reactions such as skin rash and itching.[4][7] At high doses, motor incoordination, loss of balance, and hangovers may occur.[3] Tolerance develops to phenibut with repeated use.[5] Withdrawal symptoms may occur upon discontinuation, and, in recreational users taking high doses, have been reported to include severe rebound anxiety, insomnia, anger, irritability, agitation, visual and auditory hallucinations, and acute psychosis.[3] Due to its central nervous system depressant effects, people taking phenibut should refrain from potentially dangerous activities such as operating heavy machinery.[4][7] With prolonged use of phenibut, particularly at high doses, the liver and blood should be monitored, due to risk of fatty liver disease and eosinophilia.[4][7]
Overdose
In overdose, phenibut can cause severe drowsiness, nausea, vomiting, eosinophilia, lowered blood pressure, renal impairment, and, above 7 grams, fatty liver degeneration.[4][7] There are no specific antidotes for phenibut overdose.[7] Lethargy, somnolence, agitation, delirium, tonic–clonic seizures, reduced consciousness or unconsciousness, and unresponsiveness have been reported in recreational users who have overdosed.[3] Management of phenibut overdose includes activated charcoal, gastric lavage, induction of vomiting, and treatment of symptoms.[4][7] Unlike other central nervous system depressants such as baclofen and GHB, there have been no reports of death in association with phenibut overdose.[3]
Interactions
Phenibut may mutually potentiate and extend the duration of the effects of other central nervous system depressants including anxiolytics, antipsychotics, sedatives, opioids, anticonvulsants, and alcohol.[4][7]
Pharmacology
Pharmacodynamics
| Compound | GABAB | GABAA |
|---|---|---|
| GABA | 0.08 | 0.12 |
| GHB | >100 | >100 |
| GABOB | 1.10 | 1.38 |
| Phenibut | 9.6 | >100 |
| 4-F-phenibut | 1.70 | >100 |
| Baclofen | 0.13 | >100 |
| (R)-Baclofen | 0.13 | >100 |
| (S)-Baclofen | 74.0 | >100 |
| Values are IC50 (µM) in rat brain. | ||
Phenibut acts as a full agonist of the GABAB receptor, similarly to baclofen.[10][11] It has between 30- to 68-fold lower affinity for the GABAB receptor than baclofen, which, in accordance, is used at far lower doses in comparison.[10] (R)-Phenibut has more than 100-fold higher affinity for the GABAB receptor than does (S)-phenibut; hence, (R)-phenibut is the active enantiomer at the GABAB receptor.[12] At very high concentrations, phenibut reportedly also acts as an agonist of the GABAA receptor, which is the receptor responsible for the actions of the benzodiazepines, barbiturates, and alcohol.[13]
| Compound | α2δ | GABAB |
|---|---|---|
| Phenibut | ND | 177 |
| (R)-Phenibut | 23 | 92 |
| (S)-Phenibut | 39 | >1,000 |
| Baclofen | 156 | 6 |
| Gabapentin | 0.05 | >1,000 |
| Values are Ki (µM) in rat brain. | ||
Phenibut also binds to and blocks α2δ subunit-containing VDCCs, similarly to gabapentin and pregabalin, and hence is a gabapentinoid.[6][15] Both (R)-phenibut and (S)-phenibut display this action with similar affinity (Ki = 23 and 39 μM, respectively).[6] Moreover, (R)-phenibut possesses 4-fold greater affinity for this site than for the GABAB receptor (Ki = 92 μM), while (S)-phenibut does not bind significantly to the GABAB receptor (Ki > 1 mM).[6] As such, based on the results of this study, phenibut would appear to have much greater potency in its interactions with α2δ subunit-containing VDCCs than with the GABAB receptor (between 5- to 10-fold).[6] For this reason, the actions of phenibut as a α2δ subunit-containing voltage-gated calcium channel blocker or gabapentinoid may be its true primary mechanism of action, and this may explain the differences between phenibut and its close relative baclofen (which, in contrast, has essentially insignificant activity as a gabapentinoid; Ki = 6 μM for the GABAB receptor and Ki = 156 μM for α2δ subunit-containing VDCCs, or a 26-fold difference in affinity).[6][14]
(R)-Phenibut and (S)-phenibut have been assayed at 85 binding sites at a concentration of 100 µM with no activity (less than 20% inhibition of binding) observed except at the α2δ VDCC subunit and the GABAB receptor.[16] In this study, (R)-phenibut and (S)-phenibut showed IC50 values for inhibition of gabapentin binding of 87.1 µM and 91.0 µM (Ki = 60 µM), respectively.[16] The IC50 for gabapentin under the same conditions was 0.09 µM.[16] The researchers also assessed phenibut at the GABAB receptor and found a Ki value of 57 µM for (R)-phenibut, which would be about twice that concentration (~114 µM) with racemic phenibut.[16]
Pharmacokinetics
Very little information has been published on the clinical pharmacokinetics of phenibut.[5] The drug is reported to be well-absorbed.[4] It distributes widely throughout the body and across the blood–brain barrier.[4] Approximately 0.1% of an administered dose of phenibut reportedly penetrates into the brain, with this said to occur to a much greater extent in young people and the elderly.[4] Following a single 250 mg dose in healthy volunteers, its elimination half-life was approximately 5.3 hours and the drug was largely (63%) excreted in the urine unchanged.[5] In animals, the absolute bioavailability of phenibut was 64% after oral and intravenous administration, it appeared to undergo minimal or no metabolism in multiple species, and it crossed the blood–brain barrier to a significantly greater extent than GABA.[5] The metabolites of phenibut are reported to be inactive.[4]
Some limited information has been described on the pharmacokinetics of phenibut in recreational users taking much higher doses (e.g., 1–3 grams) than typical clinical doses.[3][17] In these individuals, the onset of action of phenibut has been reported to be 2 to 4 hours orally and 20 to 30 minutes rectally, the peak effects are described as occurring 4 to 6 hours following oral ingestion, and the total duration for the oral route has been reported to be 15 to 24 hours (or approximately 3 to 5 terminal half-lives).[3]
Chemistry
Phenibut is a synthetic aromatic amino acid. It is a chiral molecule and thus has two potential configurations, as (R)- and (S)-enantiomers.[11]
Structure and analogues
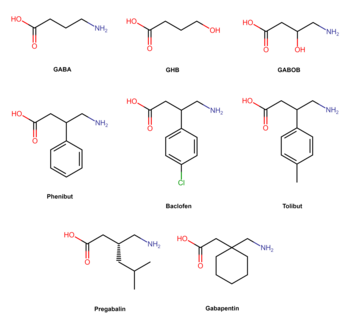
Phenibut is a derivative of the inhibitory neurotransmitter GABA.[5] Hence, it is a GABA analogue.[5] Phenibut is specifically the analogue of GABA with a phenyl ring substituted in at the β-position.[5] As such, its chemical name is β-phenyl-γ-aminobutyric acid, which can be abbreviated as β-phenyl-GABA.[5] The presence of the phenyl ring allows phenibut to cross the blood–brain barrier significantly, unlike the case of GABA.[5] Phenibut also contains the trace amine β-phenethylamine in its structure.[5]
Phenibut is closely related to a variety of other GABA analogues including baclofen (β-(4-chlorophenyl)-GABA), 4-fluorophenibut (β-(4-fluorophenyl)-GABA), tolibut (β-(4-methylphenyl)-GABA), pregabalin ((S)-β-isobutyl-GABA), gabapentin (1-(aminomethyl)cyclohexane acetic acid), and GABOB (β-hydroxy-GABA).[5][6] It has almost the same chemical structure as baclofen, differing from it only in having a hydrogen atom instead of a chlorine atom at the para position of the phenyl ring.[5] Phenibut is also close in structure to pregabalin, which has an isobutyl group at the β position instead of phenibut's phenyl ring.[6]
Synthesis
A chemical synthesis of phenibut has been published.[8]
History
Phenibut was synthesized at the A. I. Herzen Leningrad Pedagogical Institute (USSR) by Professor Vsevolod Perekalin's team and tested at the Institute of Experimental Medicine, USSR Academy of Medical Sciences.[5] It was introduced into clinical use in Russia in the 1960s.[5]
Society and culture


Generic names
The generic name of phenibut is fenibut, phenibut, or phenybut (Russian: фенибут).[2] It is also sometimes referred to as aminophenylbutyric acid (Russian: аминофенилмасляная кислота).[1] The word phenibut is a contraction of the chemical name of the drug, β-phenyl-γ-aminobutyric acid.[5] In early publications, phenibut was referred to as fenigam and phenigama (and spelling variants thereof; Russian: фенигам and фенигама).[5][18] The drug has not been assigned an INN.[4][2]
Brand names
Phenibut is marketed in Russia and Latvia under the brand names Anvifen, Fenibut, and Noofen (Russian: Анвифен, Фенибут, and Ноофен, respectively).[1]
Availability
Phenibut is approved in Russia and Latvia for medical use.[3] It is not approved or available as a pharmaceutical drug within the European Union or in the United States or Australia.[3] In countries where phenibut is not a licensed pharmaceutical drug, it is sold online without a prescription as a "nutritional supplement".[3] It is often used as a form of self-medication for social anxiety.[3]
Legal status
Phenibut is not a controlled substance in any country.[3] As such, it is currently a legal intoxicant.[3] In 2015, it was suggested that the legal status of phenibut in Europe should be reconsidered.[3]
Recreational use
Phenibut is used recreationally due to its ability to produce euphoria, anxiolysis, and increased sociability.[3] Because of its delayed onset of effects, first-time users often mistakenly take an additional dose of phenibut in the belief that the initial dose did not work.[3] Recreational users usually take the drug orally; there are a few case reports of rectal administration and one report of insufflation, which was described as "very painful" and causing swollen nostrils.[3]
References
- ^ a b c d Drobizhev, M.Yu.; Fedotova, A.V.; Kikta, S.V.; Antohin, E.Yu. (2016). "Феномен аминофенилмасляной кислоты" [[Phenomenon of aminophenylbutyric acid]]. Russian Medical Journal (in Russian). 2017 (24): 1657–1663. ISSN 1382-4368.
- ^ a b c J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 69–. ISBN 978-1-4757-2085-3.
- ^ a b c d e f g h i j k l m n o p q r s t u v Owen DR, Wood DM, Archer JR, Dargan PI (2016). "Phenibut (4-amino-3-phenyl-butyric acid): Availability, prevalence of use, desired effects and acute toxicity". Drug Alcohol Rev. 35 (5): 591–6. doi:10.1111/dar.12356. PMID 26693960.
- ^ a b c d e f g h i j k l m n o p q r Ozon Pharm, Fenibut (PDF), retrieved 15 September 2017
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z Lapin, I. (2001). "Phenibut (beta-phenyl-GABA): A tranquilizer and nootropic drug". CNS Drug Reviews. 7 (4): 471–481. doi:10.1111/j.1527-3458.2001.tb00211.x. PMID 11830761.
- ^ a b c d e f g h i Zvejniece L, Vavers E, Svalbe B, Veinberg G, Rizhanova K, Liepins V, Kalvinsh I, Dambrova M (2015). "R-phenibut binds to the α2-δ subunit of voltage-dependent calcium channels and exerts gabapentin-like anti-nociceptive effects". Pharmacol. Biochem. Behav. 137: 23–9. doi:10.1016/j.pbb.2015.07.014. PMID 26234470.
- ^ a b c d e f g h i j k Регистр лекарственных средств России ([Russian Medicines Register]). "Фенибут (Phenybutum)" [Fenibut (Phenybutum)] (HTML). Retrieved 15 September 2017.
- ^ a b Sivchik, V.V.; Grygoryan, H.O.; Survilo, V.L.; Trukhachova, T.V. (2012), Синтез γ-амино-β-фенилмасляной кислоты (фенибута) [[Synthesis of β-phenyl-γ-aminobutyric acid (phenibut)] (PDF)
- ^ Bowery NG, Hill DR, Hudson AL (1983). "Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes". Br. J. Pharmacol. 78 (1): 191–206. PMC 2044790. PMID 6297646.
- ^ a b GABAb Receptor Pharmacology: A Tribute to Norman Bowery: A Tribute to Norman Bowery. Academic Press. 21 September 2010. pp. 25–. ISBN 978-0-12-378648-7.
- ^ a b Dambrova M, Zvejniece L, Liepinsh E, Cirule H, Zharkova O, Veinberg G, Kalvinsh I (2008). "Comparative pharmacological activity of optical isomers of phenibut". Eur. J. Pharmacol. 583 (1): 128–34. doi:10.1016/j.ejphar.2008.01.015. PMID 18275958.
- ^ Allan, R.D.; Bates, M.C.; Drew, C.A.; Duke, R.K.; Hambley, T.W.; Johnston, G.A.R.; Mewett, K.N.; Spence, I. (1990). "A new synthesis resolution and in vitro activities of (R)- and (S)-β-Phenyl-Gaba". Tetrahedron. 46 (7): 2511–2524. doi:10.1016/S0040-4020(01)82032-9. ISSN 0040-4020.
- ^ Zyablitseva, Evgeniya A.; Kositsyn, Nikolay S.; Shul'gina, Galina I. (2013). "The Effects of Agonists of Ionotropic GABAA and Metabotropic GABAB Receptors on Learning". The Spanish journal of psychology. 12 (01): 12–20. doi:10.1017/S1138741600001438. ISSN 1138-7416.
- ^ a b Froestl W (2010). "Chemistry and pharmacology of GABAB receptor ligands". Adv. Pharmacol. 58: 19–62. doi:10.1016/S1054-3589(10)58002-5. PMID 20655477.
- ^ Vavers E, Zvejniece L, Svalbe B, Volska K, Makarova E, Liepinsh E, Rizhanova K, Liepins V, Dambrova M (2016). "The neuroprotective effects of R-phenibut after focal cerebral ischemia". Pharmacol. Res. 113 (Pt B): 796–801. doi:10.1016/j.phrs.2015.11.013. PMID 26621244.
- ^ a b c d Belozertseva I, Nagel J, Valastro B, Franke L, Danysz W (2016). "Optical isomers of phenibut inhibit [H(3)]-Gabapentin binding in vitro and show activity in animal models of chronic pain". Pharmacol Rep. 68 (3): 550–4. doi:10.1016/j.pharep.2015.12.004. PMID 26894962.
- ^ Schifano F, Orsolini L, Duccio Papanti G, Corkery JM (2015). "Novel psychoactive substances of interest for psychiatry". World Psychiatry. 14 (1): 15–26. doi:10.1002/wps.20174. PMC 4329884. PMID 25655145.
- ^ Khaunina, R. A.; Lapin, I. P. (1976). "Fenibut, a new tranquilizer". Pharmaceutical Chemistry Journal. 10 (12): 1703–1705. doi:10.1007/BF00760021. ISSN 0091-150X.
