Progesterone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Utrogestan, Prometrium, Endometrin, Crinone, many others |
| Other names | Pregn-4-ene-3,20-dione[1][2] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604017 |
| Pregnancy category |
|
| Routes of administration | By mouth, transdermal, vaginal, rectal, intramuscular, subcutaneous, implant |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | OMP: <10%[4][5] |
| Protein binding | • Albumin: 80% • CBG: 18% • SHBG: <1% • Free: 1–2%[6][7] |
| Metabolism | Hepatic (CYP2C19, CYP3A4, CYP2C9, 5α-reductase, 3α-HSD, 17α-hydroxylase, 21-hydroxylase, 20α-HSD)[10][11] |
| Metabolites | • Pregnanediol • Pregnanetriol • 20α-Hydroxyprogesterone • 17α-Hydroxyprogesterone • Deoxycorticosterone • 5α-Dihydroprogesterone • Pregnanolone • Allopregnanolone |
| Elimination half-life | OMP: 16–18 hours[8][4][5] IM: 22–26 hours[5][9] SC: 13–18 hours[9] |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.318 |
| Chemical and physical data | |
| Formula | C21H30O2 |
| Molar mass | 314.46 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Specific rotation | [α]D |
| Melting point | 126 °C (259 °F) |
| |
| |
| | |
Progesterone (P4) is an endogenous steroid and progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species.[12] It belongs to a group of steroid hormones called the progestogens,[12] and is the major progestogen in the body. Progesterone is also a crucial metabolic intermediate in the production of other endogenous steroids, including the sex hormones and the corticosteroids, and plays an important role in brain function as a neurosteroid.[13]
It is on the WHO Model List of Essential Medicines, the most important medications needed in a basic health system.[14]
Biological function
Biological activity
Progesterone is the most important progestogen in the body, the result of its action as a potent agonist of the nuclear progesterone receptor (nPR) (with an affinity of KD = 1 nM[15]).[12] In addition, progesterone is an agonist of the more recently discovered membrane progesterone receptors (mPRs),[16] as well as a ligand of the PGRMC1 (progesterone receptor membrane component 1; formerly known as the σ2 receptor).[17] Moreover, progesterone is also known to be an antagonist of the σ1 receptor,[18][19] a negative allosteric modulator of the nACh receptors,[13] and a potent antagonist of the mineralocorticoid receptor (MR).[20] Progesterone prevents MR activation by binding to this receptor with an affinity exceeding even those of aldosterone and glucocorticoids such as cortisol and corticosterone,[20] and produces antimineralocorticoid effects, such as natriuresis, at physiological concentrations.[21] In addition, progesterone binds to and behaves as a partial agonist of the glucocorticoid receptor (GR), albeit with very low potency (EC50 >100-fold less relative to cortisol).[22][23]
Progesterone, through its neurosteroid active metabolites such as 5α-dihydroprogesterone and allopregnanolone, acts indirectly as a positive allosteric modulator of the GABAA receptor.[24]
Progesterone and some of its metabolites, such as 5β-dihydroprogesterone, are agonists of the pregnane X receptor (PXR),[25] albeit weakly so (EC50 >10 µM).[26] In accordance, progesterone induces several hepatic cytochrome P450 enzymes,[27] such as CYP3A4,[28][29] especially during pregnancy when concentrations are much higher than usual.[30] Perimenopausal women have been found to have greater CYP3A4 activity relative to men and postmenopausal women, and it has been inferred that this may be due to the higher progesterone levels present in perimenopausal women.[28]
Progesterone modulates the activity of CatSper (cation channels of sperm) voltage-gated Ca2+ channels. Since eggs release progesterone, sperm may use progesterone as a homing signal to swim toward eggs (chemotaxis). As a result, it has been suggested that substances that block the progesterone binding site on CatSper channels could potentially be used in male contraception.[31][32]
Progesterone binds extensively to plasma proteins, including albumin (50–54%) and transcortin (43–48%).[33] It has similar affinity for albumin relative to the PR.[15]
Interactions with other steroid hormones
Progesterone has a number of physiological effects that are amplified in the presence of estrogens. Estrogens through estrogen receptors (ERs) induce or upregulate the expression of the PR.[34] One example of this is in breast tissue, where estrogens allow progesterone to mediate lobuloalveolar development.[35][36][37]
Elevated levels of progesterone potently reduce the sodium-retaining activity of aldosterone, resulting in natriuresis and a reduction in extracellular fluid volume. Progesterone withdrawal, on the other hand, is associated with a temporary increase in sodium retention (reduced natriuresis, with an increase in extracellular fluid volume) due to the compensatory increase in aldosterone production, which combats the blockade of the mineralocorticoid receptor by the previously elevated level of progesterone.[38]
Reproductive system

Progesterone has key effects via non-genomic signalling on human sperm as they migrate through the female tract before fertilization occurs, though the receptor(s) as yet remain unidentified.[39] Detailed characterisation of the events occurring in sperm in response to progesterone has elucidated certain events including intracellular calcium transients and maintained changes,[40] slow calcium oscillations,[41] now thought to possibly regulate motility.[42] It is produced by the ovaries.[43] Interestingly, progesterone has also been shown to demonstrate effects on octopus spermatozoa.[44]
Progesterone is sometimes called the "hormone of pregnancy",[45] and it has many roles relating to the development of the fetus:
- Progesterone converts the endometrium to its secretory stage to prepare the uterus for implantation. At the same time progesterone affects the vaginal epithelium and cervical mucus, making it thick and impenetrable to sperm. Progesterone is anti-mitogenic in endometrial epithelial cells, and as such, mitigates the tropic effects of estrogen.[46] If pregnancy does not occur, progesterone levels will decrease, leading, in the human, to menstruation. Normal menstrual bleeding is progesterone-withdrawal bleeding. If ovulation does not occur and the corpus luteum does not develop, levels of progesterone may be low, leading to anovulatory dysfunctional uterine bleeding.
- During implantation and gestation, progesterone appears to decrease the maternal immune response to allow for the acceptance of the pregnancy.
- Progesterone decreases contractility of the uterine smooth muscle.[45]
- In addition progesterone inhibits lactation during pregnancy. The fall in progesterone levels following delivery is one of the triggers for milk production.
- A drop in progesterone levels is possibly one step that facilitates the onset of labor.
The fetus metabolizes placental progesterone in the production of adrenal steroids.
Breasts
Progesterone plays an important role in mammary gland development in females. In conjunction with prolactin, it mediates lobuloalveolar maturation of the breasts during pregnancy to allow for milk production, and thus lactation and breastfeeding after childbirth.[47] Estrogen is required for progesterone to mediate lobuloalveolar maturation, as it induces expression of the PR in breast tissue.[35][36][37] Moreover, it has been found that RANKL is a critical downstream mediator of progesterone-mediated lobuloalveolar development.[48] Knockout mice of RANKL show an almost identical mammary phenotype relative to PR knockout mice, including normal mammary ductal development but complete failure of the development of lobuloalveolar structures.[48]
Though to a far lesser extent than estrogen, which is the major mediator of breast ductal development (via ERα, specifically),[49][50] progesterone has been found to be involved in ductal development as well.[51] PR knockout mice or mice treated with the PR antagonist mifepristone show delayed but otherwise normal ductal development at puberty.[51] In addition, mice modified to have overexpression of PRA display ductal hyperplasia,[48] and progesterone induces ductal growth in mouse mammary gland.[51] Progesterone mediates ductal development mainly via the induction of amphiregulin, the same growth factor that estrogen primarily induces to mediate ductal development.[51] These findings suggest that, while not essential for full ductal development, progesterone seems to play a potentiating or accelerating role in estrogen-mediated ductal development, at least in mice.[51]
Progesterone also appears to be involved in the pathophysiology of breast cancer, though its role, and whether it is a promoter or inhibitor of breast cancer risk, has not been fully elucidated.[52] In any case, while certain synthetic progestins with androgenic effects such as medroxyprogesterone acetate and 19-nortestosterone derivatives including norethisterone acetate, norgestrel, and levonorgestrel have been found to significantly increase the risk of breast cancer in postmenopausal women in combination with estrogen as a component of hormone replacement therapy, the combination of natural progesterone or the pure, non-androgenic progestin dydrogesterone with estrogen has been found not to do so.[53][54] In fact, progesterone or dydrogesterone added to estrogen appear to decrease the risk of breast cancer relative to estrogen alone.[53]
Sexuality
Sex drive
Progesterone and its neurosteroid active metabolite allopregnanolone appear to be importantly involved in sex drive in females.[55]
Homosexuality
Dr. Diana Fleischman, of the University of Portsmouth, and colleagues examined the relationship between progesterone and sexual attitudes. Their research was published in the Archives of Sexual Behavior.[56] They found that women who have higher levels of progesterone are more likely to be open to the idea of engaging in sexual behaviour with other women.[57] Similarly, when heterosexual men are subtly reminded of the importance of having male friends and allies, they report more positive attitudes toward engaging in sexual behaviour with other men. This pattern is particularly dramatic in men who have high levels of progesterone.[58]
Nervous system
Progesterone, like pregnenolone and dehydroepiandrosterone (DHEA), belongs to an important group of endogenous steroids called neurosteroids. It can be synthesized within the central nervous system and also serves as a precursor to another major neurosteroid, allopregnanolone.
Neurosteroids are neuromodulators, and are neuroprotective, neurogenic, and regulate neurotransmission and myelination.[59] The effects of progesterone as a neurosteroid are mediated predominantly through its interactions with non-nuclear PRs, namely the mPRs and PGRMC1, as well as certain other receptors, such as the σ1 and nACh receptors.[citation needed]
Aging
Since most progesterone in males is created during testicular production of testosterone, and most in females by the ovaries, the shutting down (whether by natural or chemical means), or removal, of those inevitably causes a considerable reduction in progesterone levels. Previous concentration upon the role of progestogens in female reproduction, when progesterone was simply considered a "female hormone", obscured the significance of progesterone elsewhere in both sexes.
The tendency for progesterone to have a regulatory effect, the presence of progesterone receptors in many types of body tissue, and the pattern of deterioration (or tumor formation) in many of those increasing in later years when progesterone levels have dropped, is prompting widespread research into the potential value of maintaining progesterone levels in both males and females.[citation needed]
Brain damage
Studies as far back as 1987 show that female sex hormones have an effect on the recovery of traumatic brain injury.[60] In these studies, it was first observed that pseudopregnant female rats had reduced edema after traumatic brain injury. Recent clinical trials have shown that among patients that have suffered moderate traumatic brain injury, those that have been treated with progesterone are more likely to have a better outcome than those who have not.[61]
Previous studies have shown that progesterone supports the normal development of neurons in the brain, and that the hormone has a protective effect on damaged brain tissue. It has been observed in animal models that females have reduced susceptibility to traumatic brain injury and this protective effect has been hypothesized to be caused by increased circulating levels of estrogen and progesterone in females.[62] A number of additional animal studies have confirmed that progesterone has neuroprotective effects when administered shortly after traumatic brain injury.[63] Encouraging results have also been reported in human clinical trials.[64][65]
Proposed mechanism
The mechanism of progesterone protective effects may be the reduction of inflammation that follows brain trauma.[66]
Damage incurred by traumatic brain injury is believed to be caused in part by mass depolarization leading to excitotoxicity. One way in which progesterone helps to alleviate some of this excitotoxicity is by blocking the voltage-dependent calcium channels that trigger neurotransmitter release.[67] It does so by manipulating the signaling pathways of transcription factors involved in this release. Another method for reducing the excitotoxicity is by up-regulating the GABAA, a widespread inhibitory neurotransmitter receptor.[68]
Progesterone has also been shown to prevent apoptosis in neurons, a common consequence of brain injury.[60] It does so by inhibiting enzymes involved in the apoptosis pathway specifically concerning the mitochondria, such as activated caspase 3 and cytochrome c.
Not only does progesterone help prevent further damage, it has also been shown to aid in neuroregeneration. One of the serious effects of traumatic brain injury includes edema. Animal studies show that progesterone treatment leads to a decrease in edema levels by increasing the concentration of macrophages and microglia sent to the injured tissue.[67][69] This was observed in the form of reduced leakage from the blood brain barrier in secondary recovery in progesterone treated rats. In addition, progesterone was observed to have antioxidant properties, reducing the concentration of oxygen free radicals faster than without.[68] There is also evidence that the addition of progesterone can also help remyelinate damaged axons due to trauma, restoring some lost neural signal conduction.[68] Another way progesterone aids in regeneration includes increasing the circulation of endothelial progenitor cells in the brain.[70] This helps new vasculature to grow around scar tissue which helps repair the area of insult.
Combination treatments
Vitamin D and progesterone separately have neuroprotective effects after traumatic brain injury, but when combined their effects are synergistic.[71] When used at their optimal respective concentrations, the two combined have been shown to reduce cell death more than when alone.
One study looks at a combination of progesterone with estrogen. Both progesterone and estrogen are known to have antioxidant-like qualities and are shown to reduce edema without injuring the blood-brain barrier. In this study, when the two hormones are administered alone it does reduce edema, but the combination of the two increases the water content, thereby increasing edema.[72]
Clinical trials
The clinical trials for progesterone as a treatment for traumatic brain injury have only recently begun. ProTECT, a phase II trial conducted in Atlanta at Grady Memorial Hospital in 2007, the first to show that progesterone reduces edema in humans. Since then, trials have moved on to phase III. The National Institute of Health began conducting a nationwide phase III trial in 2011 led by Emory University.[61] A global phase III initiative called SyNAPSe®, initiated in June 2010, is run by a U.S.-based private pharmaceutical company, BHR Pharma, and is being conducted in the United States, Argentina, Europe, Israel and Asia.[73][74] Approximately 1,200 patients with severe (Glasgow Coma Scale scores of 3-8), closed-head TBI will be enrolled in the study at nearly 150 medical centers.
Addiction
Progesterone enhances the function of serotonin receptors in the brain, so an excess or deficit of progesterone has the potential to result in significant neurochemical issues. This provides an explanation for why some people resort to substances that enhance serotonin activity such as nicotine, alcohol, and cannabis when their progesterone levels fall below optimal levels.[75]
- To examine the effects of progesterone on nicotine addiction, participants in one study were either treated orally with a progesterone treatment, or treated with a placebo. When treated with progesterone, participants exhibited enhanced suppression of smoking urges, reported higher ratings of “bad effects” from IV nicotine, and reported lower ratings of “drug liking”. These results suggest that progesterone not only alters the subjective effects of nicotine, but reduces the urge to smoke cigarettes.[76]
- Sex differences in hormone levels may induce women to respond differently than men to nicotine. When women undergo cyclic changes or different hormonal transition phases (menopause, pregnancy, adolescence), there are changes in their progesterone levels.[77] Therefore, females have an increased biological vulnerability to nicotine’s reinforcing effects compared to males and progesterone may be used to counter this enhanced vulnerability. This information supports the idea that progesterone can affect behavior.[75]
- Similar to nicotine, cocaine also increases the release of dopamine in the brain. The neurotransmitter is involved in the reward center and is one of the main neurotransmitters involved with substance abuse and reliance. In a study of cocaine users, it was reported that progesterone reduced craving and the feeling of being stimulated by cocaine. Thus, progesterone was suggested as an agent that decreases cocaine craving by reducing the dopaminergic properties of the drug.[78]
Other effects
- During pregnancy, progesterone is said to decrease irritability.[79]
- During pregnancy, progesterone helps to suppress immune responses of the mother to fetal antigens, which prevents rejection of the fetus.[79]
- Progesterone raises epidermal growth factor-1 (EGF-1) levels, a factor often used to induce proliferation, and used to sustain cultures, of stem cells.[citation needed]
- Progesterone increases core temperature (thermogenic function) during ovulation.[80]
- Progesterone reduces spasm and relaxes smooth muscle. Bronchi are widened and mucus regulated. (PRs are widely present in submucosal tissue.)
- Progesterone acts as an antiinflammatory agent and regulates the immune response.
- Progesterone reduces gall-bladder activity.[81]
- Progesterone normalizes blood clotting and vascular tone, zinc and copper levels, cell oxygen levels, and use of fat stores for energy.
- Progesterone may affect gum health, increasing risk of gingivitis (gum inflammation).[82]
- Progesterone appears to prevent endometrial cancer (involving the uterine lining) by regulating the effects of estrogen.
- Progesterone plays an important role in the signaling of insulin release and pancreatic function, and may affect the susceptibility to diabetes or gestational diabetes.[83][84]
- Progesterone may play a role in male behavior, such as in male aggression towards infants.[85]
Medical uses
The use of progesterone and its analogues have many medical applications, both to address acute situations and to address the long-term decline of natural progesterone levels. Because of the poor bioavailability of progesterone when taken by mouth, many synthetic progestins have been designed with improved bioavailability by mouth and have been used long before progesterone formulations became available.[86]
Prevention of preterm birth
Vaginally dosed progesterone is being investigated as potentially beneficial in preventing preterm birth in women at risk for preterm birth. The initial study by Fonseca suggested that vaginal progesterone could prevent preterm birth in women with a history of preterm birth.[87] According to a recent study, women with a short cervix that received hormonal treatment with a progesterone gel had their risk of prematurely giving birth reduced. The hormone treatment was administered vaginally every day during the second half of a pregnancy.[88] A subsequent and larger study showed that vaginal progesterone was no better than placebo in preventing recurrent preterm birth in women with a history of a previous preterm birth,[89] but a planned secondary analysis of the data in this trial showed that women with a short cervix at baseline in the trial had benefit in two ways: a reduction in births less than 32 weeks and a reduction in both the frequency and the time their babies were in intensive care.[90] In another trial, vaginal progesterone was shown to be better than placebo in reducing preterm birth prior to 34 weeks in women with an extremely short cervix at baseline.[91] An editorial by Roberto Romero discusses the role of sonographic cervical length in identifying patients who may benefit from progesterone treatment.[92] A meta-analysis published in 2011 found that vaginal progesterone cut the risk of premature births by 42 percent in women with short cervixes.[93] The meta-analysis, which pooled published results of five large clinical trials, also found that the treatment cut the rate of breathing problems and reduced the need for placing a baby on a ventilator.[94]
Other uses
- Progesterone is used for luteal support in assisted reproductive technology (ART) cycles such as in-vitro fertilization (IVF).
- Progesterone is used to control persistent anovulatory bleeding. It is also used to prepare uterine lining in infertility therapy and to support early pregnancy. Patients with recurrent pregnancy loss due to inadequate progesterone production may receive progesterone.
- Progesterone is also used in nonpregnant women with a delayed menstruation of one or more weeks, in order to allow the thickened endometrial lining to slough off. This process is termed a progesterone withdrawal bleed. The progesterone is taken orally for a short time (usually one week), after which the progesterone is discontinued and bleeding should occur.[citation needed]
- Progesterone can be used to treat catamenial epilepsy by supplementation during certain periods of the menstrual cycle.[95]
- Progesterone is being investigated as potentially beneficial in treating multiple sclerosis, since the characteristic deterioration of nerve myelin insulation halts during pregnancy, when progesterone levels are raised; deterioration commences again when the levels drop.
- Progesterone also has a role in skin elasticity and bone strength, in respiration, in nerve tissue and in female sexuality, and the presence of progesterone receptors in certain muscle and fat tissue may hint at a role in sexually dimorphic proportions of those.[96][infringing link?]
- Antiprogestins and selective progesterone receptor modulators (SPRM)s, such as mifepristone, can be used to prevent conception or induce medical abortions (note that methods of hormonal contraception do not contain progesterone but a progestin).
- Progesterone is starting to be used in the treatment of the skin condition hidradenitis suppurativa.[citation needed]
- Progesterone is sometimes employed as a component of hormone replacement therapy for trans women.[97]
Interactions
There are several notable drug interactions with progesterone. Certain selective serotonin reuptake inhibitors (SSRIs) may increase the GABAA receptor-related central depressant effects of progesterone by enhancing its conversion into 5α-dihydroprogesterone and allopregnanolone via activation of 3α-HSD.[98] Progesterone potentiates the sedative effects of benzodiazepines and ethanol.[99] Notably, there is a case report of progesterone abuse alone with very high doses.[100] 5α-Reductase inhibitors such as finasteride and dutasteride, as well as inhibitors of 3α-HSD such as medroxyprogesterone acetate, inhibit the conversion of progesterone into its inhibitory neurosteroid metabolites, and for this reason, may have the potential to block or reduce its sedative effects.[101][102][103]
Progesterone is a weak but significant agonist of the PXR, and has been found to induce several hepatic cytochrome P450 enzymes, such as CYP3A4, especially when concentrations are high, such as with pregnancy range levels.[27][28][29][30] As such, progesterone may have the potential to accelerate the clearance of various drugs, especially with oral administration (which results in supraphysiological levels of progesterone in the liver), as well as with the high concentrations achieved with sufficient injection dosages.[citation needed]
Pharmacokinetics
Oral administration
The route of administration impacts the effects of progesterone. OMP has a wide inter-individual variability in absorption and bioavailability. In contrast, progestins are rapidly absorbed with a longer half-life than progesterone and maintain stable levels in the blood.[104] The absorption and bioavailability of OMP is increased approximately two-fold when it is taken with food.[105] Progesterone has a relatively short half-life in the body. As such, OMP is usually prescribed for twice or thrice-daily administration or once-daily administration when taken by injection.[8] Via the oral route, peak concentrations are seen about 2–3 hours after ingestion, and the half-life is about 16–18 hours.[8] Significantly elevated serum levels of progesterone are maintained for about 12 hours, and levels do not return to baseline until at least 24 hours have passed.[8] OMP is prescribed in divided doses .[106]
Progesterone, when taken orally, undergoes gastrointestinal (especially hepatic) metabolism to form hydroxylated metabolites, which in turn are metabolized into sulfate and glucuronide derivatives.[107] Enzymes involved in the hepatic metabolism of progesterone include, particularly, CYP2C19 and CYP3A4, as well as CYP2C9.[10][11]
Neurosteroids metabolites
A portion of progesterone is converted into 5α-dihydroprogesterone and allopregnanolone (a conversion that is catalyzed by the enzymes 5α-reductase and 3α-hydroxysteroid dehydrogenase (3α-HSD) and occurs in the liver, reproductive endocrine tissues, skin, and the brain),[108] which are neurosteroids and potent potentiators of GABAA receptors.[109][110] It is for this reason that common reported side effects of progesterone include dizziness, drowsiness or sedation, sleepiness, and fatigue, especially at high doses.[109][110] As a result, some physicians may instruct their patients to take their progesterone before bed.[109] Both oral and intramuscularly injected progesterone produce sedative effects, indicating that first-pass metabolism in the liver is not essential for the conversion to take place.[111][112][113] Moreover, the sedative effects occur in both men and women, indicating a lack of sex-specificity of the effects.[111]
Transdermal administration
Transdermal progesterone is about 5–7 times stronger than oral progesterone.[114] This is due to the fact transdermal administration bypasses first-pass metabolism.[114] As such, 20–30 mg/day transdermal progesterone is equivalent to about 100–200 mg/day oral progesterone.[114] Some researchers have reported that absorption of progesterone via the transdermal route is poor, impractical, and unsubstantiated, however they may have been measuring serum blood or urine levels.[115][116] Other studies have shown that transdermal absorption of progesterone cream is much higher when capillary blood or saliva testing is used.[117]
Vaginal administration
With vaginal and rectal administration, a 100 mg dose of progesterone results in peak levels at 4 hours and 8 hours after dosing, respectively, with the levels achieved being in the serum luteal phase range.[118] Following peak serum concentrations, there is a gradual decline in plasma levels, and after 24 hours, serum levels typical of the follicular phase are reached.[118]
Intramuscular injection
With intramuscular injection of 10 mg progesterone suspended in vegetable oil, maximum plasma concentrations (Cmax) are reached at approximately 8 hours after administration, and serum levels remain above baseline for about 24 hours.[33] Doses of 10 mg, 25 mg, and 50 mg via intramuscular injection result in mean maximum serum concentrations of 7 ng/mL, 28 ng/mL, and 50 ng/mL, respectively.[33] With intramuscular injection, a dose of 25 mg results in normal luteal phase serum levels of progesterone within 8 hours, and a 100 mg dose produces mid-pregnancy levels.[118] At these doses, serum levels of progesterone remain elevated above baseline for at least 48 hours,[118] with a half-life of about 22 hours.[9]
Due to the high concentrations achieved, progesterone by intramuscular injection at the usual clinical dose range is able to suppress gonadotropin secretion from the pituitary gland, demonstrating antigonadotropic efficacy (and therefore suppression of gonadal sex steroid production).[33]
Subcutaneous injection
Progesterone can also be administered alternatively via subcutaneous injection, with the new aqueous formulation Prolutex in Europe being intended specifically for once-daily administration by this route.[9][119][120] This formulation is rapidly absorbed and has been found to result in higher serum peak progesterone levels relative to intramuscular oil formulations.[120] In addition, subcutaneous injection of progesterone is considered to be easier, safer (less risk of injection site reactions), and less painful relative to intramuscular injection.[120] The terminal half-life of this formulation is 13 to 18 hours, which is similar to the terminal half-lives of OMP and intramuscular progesterone.[9]
Normal progesterone levels
For comparative purposes, mid-luteal serum levels of progesterone are above 5–9 ng/ml,[118] plasma levels in the first 4 to 8 weeks of pregnancy are 25–75 ng/ml,[15] and serum levels at term are typically around 200 ng/ml.[15] Production of progesterone in the body in late pregnancy is approximately 250 mg per day, 90% of which reaches maternal circulation.[79]
Chemistry

Like other steroids, progesterone consists of four interconnected cyclic hydrocarbons. Progesterone contains ketone and oxygenated functional groups, as well as two methyl branches. Like all steroid hormones, it is hydrophobic.
Sources
Animal
Progesterone is produced in high amounts in the ovaries (by the corpus luteum) from the onset of puberty to menopause, and is also produced in smaller amounts by the adrenal glands after the onset of adrenarche in both males and females. To a lesser extent, progesterone is produced in nervous tissue, especially in the brain, and in adipose (fat) tissue, as well.
During human pregnancy, progesterone is produced in increasingly high amounts by the ovaries and placenta. At first, the source is the corpus luteum that has been "rescued" by the presence of human chorionic gonadotropin (hCG) from the conceptus. However, after the 8th week, production of progesterone shifts to the placenta. The placenta utilizes maternal cholesterol as the initial substrate, and most of the produced progesterone enters the maternal circulation, but some is picked up by the fetal circulation and used as substrate for fetal corticosteroids. At term the placenta produces about 250 mg progesterone per day.
An additional animal source of progesterone is milk products. After consumption of milk products the level of bioavailable progesterone goes up.[121]
Plants
In at least one plant, Juglans regia, progesterone has been detected.[122] In addition, progesterone-like steroids are found in Dioscorea mexicana. Dioscorea mexicana is a plant that is part of the yam family native to Mexico.[123] It contains a steroid called diosgenin that is taken from the plant and is converted into progesterone.[124] Diosgenin and progesterone are also found in other Dioscorea species, as well as in other plants that are not closely related, such as fenugreek.
Another plant that contains substances readily convertible to progesterone is Dioscorea pseudojaponica native to Taiwan. Research has shown that the Taiwanese yam contains saponins — steroids that can be converted to diosgenin and thence to progesterone.[125]
Many other Dioscorea species of the yam family contain steroidal substances from which progesterone can be produced. Among the more notable of these are Dioscorea villosa and Dioscorea polygonoides. One study showed that the Dioscorea villosa contains 3.5% diosgenin.[126] Dioscorea polygonoides has been found to contain 2.64% diosgenin as shown by gas chromatography-mass spectrometry.[127] Many of the Dioscorea species that originate from the yam family grow in countries that have tropical and subtropical climates.[128]
Metabolism

Biosynthesis
In mammals, progesterone (6), like all other steroid hormones, is synthesized from pregnenolone (3), which in turn is derived from cholesterol (1) (see the upper half of the figure to the right).
Cholesterol (1) undergoes double oxidation to produce 20,22-dihydroxycholesterol (2). This vicinal diol is then further oxidized with loss of the side chain starting at position C-22 to produce pregnenolone (3). This reaction is catalyzed by cytochrome P450scc.
The conversion of pregnenolone to progesterone takes place in two steps. First, the 3-hydroxyl group is oxidized to a keto group (4) and second, the double bond is moved to C-4, from C-5 through a keto/enol tautomerization reaction.[129] This reaction is catalyzed by 3β-hydroxysteroid dehydrogenase/δ(5)-δ(4)isomerase.
Progesterone in turn (see lower half of figure to the right) is the precursor of the mineralocorticoid aldosterone, and after conversion to 17α-hydroxyprogesterone (another natural progestogen) of cortisol and androstenedione. Androstenedione can be converted to testosterone, estrone and estradiol.
Pregnenolone and progesterone can also be synthesized by yeast.[130]
Clearance
Progesterone is metabolized mainly in the liver.[33] It is reduced to a variety of active and inactive metabolites, including pregnanediol, pregnanetriol, and pregnanolone (pregnanedione).[33] These metabolites are subsequently conjugated into glucuronide and sulfate forms.[33] In urine, pregnanediol glucuronide is the major metabolite of progesterone.[15] It has been found to constitute about 30% of an injection of progesterone.[15]
Progesterone is reduced to its much less potent progestogen metabolite 20α-hydroxyprogesterone by the 20α-hydroxysteroid dehydrogenases AKR1C1 and AKR1C3.[131] In addition, progesterone is converted into its active progestogen and neurosteroid metabolite 5α-dihydroprogesterone by 5α-reductase, which in turn can be converted into the non-progestogen but even more potent neurosteroid allopregnanolone by 3α-hydroxysteroid dehydrogenase.[108] Progesterone is converted into 17α-hydroxyprogesterone and deoxycorticosterone by 17α-hydroxylase and 21-hydroxylase, respectively. 17α-Hydroxyprogesterone serves as a precursor for the androgens and estrogens, and deoxycorticosterone is a corticosteroid and a precursor for other corticosteroids as well.
Levels
| Person type | Reference range for blood test | ||
|---|---|---|---|
| Lower limit | Upper limit | Unit | |
| Female - menstrual cycle | (see diagram below) | ||
| Female - postmenopausal | <0.2[132] | 1[132] | ng/mL |
| <0.6[133] | 3[133] | nmol/L | |
| Female on oral contraceptives | 0.34[132] | 0.92[132] | ng/mL |
| 1.1[133] | 2.9[133] | nmol/L | |
| Males ≥16 years | 0.27[132] | 0.9[132] | ng/mL |
| 0.86[133] | 2.9[133] | nmol/L | |
| Female or male 1–9 years | 0.1[132] | 4.1[132] or 4.5[132] | ng/mL |
| 0.3[133] | 13[133] | nmol/L | |
In women, progesterone levels are relatively low during the preovulatory phase of the menstrual cycle, rise after ovulation, and are elevated during the luteal phase, as shown in diagram below. Progesterone levels tend to be < 2 ng/ml prior to ovulation, and > 5 ng/ml after ovulation. If pregnancy occurs, human chorionic gonadotropin is released maintaining the corpus luteum allowing it to maintain levels of progesterone. Between 7–9 weeks the placenta begins to produce progesterone in place of the corpus luteum, this process is named the luteal-placental shift.[134]
After the luteal-placental shift progesterone levels start to rise further and may reach 100-200 ng/ml at term. Whether a decrease in progesterone levels is critical for the initiation of labor has been argued and may be species-specific. After delivery of the placenta and during lactation, progesterone levels are very low.
Progesterone levels are relatively low in children and postmenopausal women.[135] Adult males have levels similar to those in women during the follicular phase of the menstrual cycle.
Blood test results should always be interpreted using the reference ranges provided by the laboratory that performed the results. Example reference ranges are listed below.

• The ranges denoted By biological stage may be used in closely monitored menstrual cycles in regard to other markers of its biological progression, with the time scale being compressed or stretched to how much faster or slower, respectively, the cycle progresses compared to an average cycle.
• The ranges denoted Inter-cycle variability are more appropriate to use in non-monitored cycles with only the beginning of menstruation known, but where the woman accurately knows her average cycle lengths and time of ovulation, and that they are somewhat averagely regular, with the time scale being compressed or stretched to how much a woman's average cycle length is shorter or longer, respectively, than the average of the population.
• The ranges denoted Inter-woman variability are more appropriate to use when the average cycle lengths and time of ovulation are unknown, but only the beginning of menstruation is given.
Chemical synthesis
Semisynthesis
Semisynthesis 1
An economical semisynthesis of progesterone from the plant steroid diosgenin isolated from yams was developed by Russell Marker in 1940 for the Parke-Davis pharmaceutical company.[136] This synthesis is known as the Marker degradation. Additional semisyntheses of progesterone have also been reported starting from a variety of steroids. For the example, cortisone can be simultaneously deoxygenated at the C-17 and C-21 position by treatment with iodotrimethylsilane in chloroform to produce 11-keto-progesterone (ketogestin), which in turn can be reduced at position-11 to yield progesterone.[137]

Semisynthesis 2
Progesterone can also be made from the stigmasterol found in soybean oil also. c.f. Percy Julian.

Total synthesis
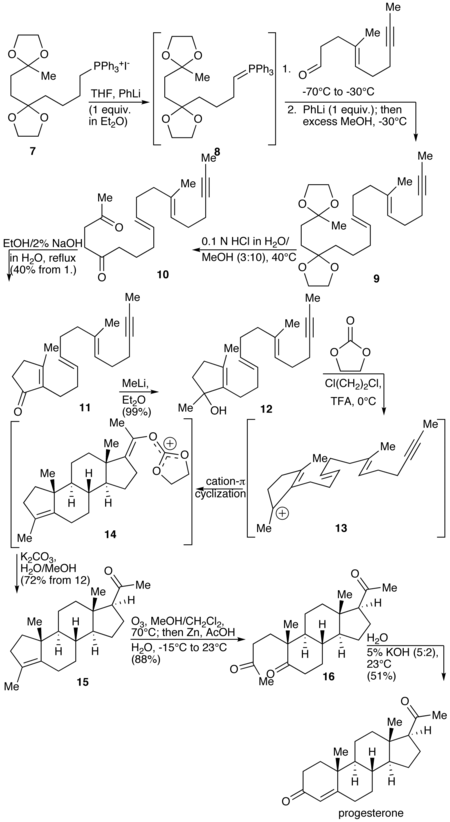
A total synthesis of progesterone was reported in 1971 by W.S. Johnson.[143] The synthesis begins with reacting the phosphonium salt 7 with phenyl lithium to produce the phosphonium ylide 8. The ylide 8 is reacted with an aldehyde to produce the alkene 9. The ketal protecting groups of 9 are hydrolyzed to produce the diketone 10, which in turn is cyclized to form the cyclopentenone 11. The ketone of 11 is reacted with methyl lithium to yield the tertiary alcohol 12, which in turn is treated with acid to produce the tertiary cation 13. The key step of the synthesis is the π-cation cyclization of 13 in which the B-, C-, and D-rings of the steroid are simultaneously formed to produce 14. This step resembles the cationic cyclization reaction used in the biosynthesis of steroids and hence is referred to as biomimetic. In the next step the enol orthoester is hydrolyzed to produce the ketone 15. The cyclopentene A-ring is then opened by oxidizing with ozone to produce 16. Finally, the diketone 17 undergoes an intramolecular aldol condensation by treating with aqueous potassium hydroxide to produce progesterone.[143]
History
The hormonal action of progesterone was discovered in 1929, following that of estrogen in 1923.[15][144][145] By 1931–1932, nearly pure crystalline material of high progestational activity had been isolated from the corpus luteum of animals, and by 1934, pure crystalline progesterone had been refined and obtained and the chemical structure of progesterone was determined[15][144] by professor Adolf Butenandt at the Chemisches Institut of Technical University in Gdańsk, who extracted this new compound from several thousand liters of urine[146]
Chemical synthesis of progesterone from stigmasterol and pregnanediol was accomplished later that year.[144][147] Up to this point, progesterone, known generically as corpus luteum hormone, had been being referred to by several groups by different names, including corporin, lutein, luteosterone, and progestin.[15][148] In 1935, at the time of the Second International Conference on the Standardization of Sex Hormones in London, England, a compromise was made between the groups and the name progesterone (progestational steroidal ketone) was created.[15][149] Shortly following its chemical synthesis, progesterone began being tested clinically in women.[144] In 1934, Schering introduced progesterone, along with estradiol (brand name Progynon) and testosterone (brand names Testoviron, Proviron), as a pharmaceutical drug, under the brand name Proluton.[150][151] It was originally administered by intramuscular injection because it is rapidly inactivated after oral ingestion.[152]
It was not until almost half a century later that a non-injected formulation of progesterone was marketed.[153] Micronization, similarly to the case of estradiol, allowed progesterone to be absorbed effectively via other routes of administration, but the micronization process was difficult for manufacturers for many years.[154] Oral micronized progesterone was finally marketed in France under the brand name Utrogestan in 1980,[151][155][156] and this was followed by the introduction of oral micronized progesterone in the United States under the brand name Prometrium in 1998.[154] In the early 1990s, vaginal micronized progesterone (brand names Crinone, Utrogestan, Endometrin)[157] was also marketed.[153]
Formulations
Progesterone is marketed under a large number of different brand names throughout the world.[158] Progesterone was approved by the United States Food and Drug Administration as vaginal gel on July 31, 1997,[159] a capsule to be taken by mouth on May 14, 1998[160] in an injection form on April 25, 2001[161] and as a vaginal insert on June 21, 2007.[162] Progesterone, when taken by mouth, has very poor pharmacokinetics, including low bioavailability (only about 10–15% reaches the bloodstream)[114] and a half-life of only about 5 minutes, unless it is micronized.[8][12] As such, it is sold in the form of oil-filled capsules containing micronized progesterone for oral use, termed oral micronized progesterone (OMP).[8][158] Progesterone is also available in the forms of vaginal or rectal suppositories or pessaries,[158] transdermally-administered gels or creams,[158][163] or via intramuscular or subcutaneous injection of a vegetable oil solution.[8][33][158]
Transdermal products made with progesterone USP (i.e., "natural progesterone") do not require a prescription. Some of these products also contain "wild yam extract" derived from Dioscorea villosa, but there is no evidence that the human body can convert its active ingredient (diosgenin, the plant steroid that is chemically converted to produce progesterone industrially[136]) into progesterone.[164][165]
See also
References
- ^ Adler, Norman; Pfaff, Donald; Goy, Robert W. (6 Dec 2012). Handbook of Behavioral Neurobiology Volume 7 Reproduction (1st ed.). New York: Plenum Press. p. 189. ISBN 978-1-4684-4834-4. Retrieved 4 July 2015.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "progesterone (CHEBI:17026)". ChEBI. European Molecular Biology Laboratory-EBI. Retrieved 4 July 2015.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ a b Stanczyk FZ (September 2002). "Pharmacokinetics and potency of progestins used for hormone replacement therapy and contraception". Reviews in Endocrine & Metabolic Disorders. 3 (3): 211–24. doi:10.1023/A:1020072325818. PMID 12215716.
- ^ a b c Simon JA, Robinson DE, Andrews MC, Hildebrand JR, Rocci ML, Blake RE, Hodgen GD (July 1993). "The absorption of oral micronized progesterone: the effect of food, dose proportionality, and comparison with intramuscular progesterone". Fertility and Sterility. 60 (1): 26–33. doi:10.1016/S0015-0282(16)56031-2. PMID 8513955.
- ^ Marc A. Fritz; Leon Speroff (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 44–. ISBN 978-1-4511-4847-3.
- ^ William J. Marshall; William J. Marshall (Ph. D.); S. K. Bangert (2008). Clinical Chemistry. Elsevier Health Sciences. pp. 192–. ISBN 0-7234-3455-7.
- ^ a b c d e f g Zutshi (1 January 2005). Hormones in Obstetrics and Gynaecology. Jaypee Brothers Publishers. p. 74. ISBN 978-81-8061-427-9.
- ^ a b c d e Cometti B (November 2015). "Pharmaceutical and clinical development of a novel progesterone formulation". Acta Obstetricia Et Gynecologica Scandinavica. 94 Suppl 161: 28–37. doi:10.1111/aogs.12765. PMID 26342177.
- ^ a b Yamazaki H, Shimada T (October 1997). "Progesterone and testosterone hydroxylation by cytochromes P450 2C19, 2C9, and 3A4 in human liver microsomes". Archives of Biochemistry and Biophysics. 346 (1): 161–9. doi:10.1006/abbi.1997.0302. PMID 9328296.
- ^ a b Gerard A. McKay; Matthew R. Walters (6 February 2013). Lecture Notes: Clinical Pharmacology and Therapeutics. John Wiley & Sons. p. 33. ISBN 978-1-118-34489-7.
- ^ a b c d King, Tekoa L.; Brucker, Mary C. (25 October 2010). Pharmacology for Women's Health. Jones & Bartlett Publishers. pp. 372–373. ISBN 978-1-4496-5800-7.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b Baulieu E, Schumacher M (2000). "Progesterone as a neuroactive neurosteroid, with special reference to the effect of progesterone on myelination". Steroids. 65 (10–11): 605–12. doi:10.1016/s0039-128x(00)00173-2. PMID 11108866.
- ^ "19th WHO Model List of Essential Medicines (April 2015)" (PDF). WHO. April 2015. Retrieved May 10, 2015.
- ^ a b c d e f g h i j J.B. Josimovich (11 November 2013). Gynecologic Endocrinology. Springer Science & Business Media. pp. 9, 25–29. ISBN 978-1-4613-2157-6.
- ^ Thomas P, Pang Y (2012). "Membrane progesterone receptors: evidence for neuroprotective, neurosteroid signaling and neuroendocrine functions in neuronal cells". Neuroendocrinology. 96 (2): 162–71. doi:10.1159/000339822. PMC 3489003. PMID 22687885.
- ^ Meyer C, Schmid R, Schmieding K, Falkenstein E, Wehling M (February 1998). "Characterization of high affinity progesterone-binding membrane proteins by anti-peptide antiserum". Steroids. 63 (2): 111–6. doi:10.1016/s0039-128x(97)00143-8. PMID 9516722.
- ^ Maurice T, Urani A, Phan VL, Romieu P (November 2001). "The interaction between neuroactive steroids and the sigma1 receptor function: behavioral consequences and therapeutic opportunities". Brain Research. Brain Research Reviews. 37 (1–3): 116–32. doi:10.1016/s0165-0173(01)00112-6. PMID 11744080.
- ^ Johannessen M, Fontanilla D, Mavlyutov T, Ruoho AE, Jackson MB (February 2011). "Antagonist action of progesterone at σ-receptors in the modulation of voltage-gated sodium channels". American Journal of Physiology. Cell Physiology. 300 (2): C328-37. doi:10.1152/ajpcell.00383.2010. PMC 3043630. PMID 21084640.
- ^ a b Rupprecht R, Reul JM, van Steensel B, Spengler D, Söder M, Berning B, Holsboer F, Damm K (October 1993). "Pharmacological and functional characterization of human mineralocorticoid and glucocorticoid receptor ligands". European Journal of Pharmacology. 247 (2): 145–54. doi:10.1016/0922-4106(93)90072-H. PMID 8282004.
- ^ Elger W, Beier S, Pollow K, Garfield R, Shi SQ, Hillisch A (2003). "Conception and pharmacodynamic profile of drospirenone". Steroids. 68 (10–13): 891–905. doi:10.1016/j.steroids.2003.08.008. PMID 14667981.
- ^ Attardi BJ, Zeleznik A, Simhan H, Chiao JP, Mattison DR, Caritis SN (2007). "Comparison of progesterone and glucocorticoid receptor binding and stimulation of gene expression by progesterone, 17-alpha hydroxyprogesterone caproate, and related progestins". Am. J. Obstet. Gynecol. 197 (6): 599.e1–7. doi:10.1016/j.ajog.2007.05.024. PMC 2278032. PMID 18060946.
- ^ Lei K, Chen L, Georgiou EX, Sooranna SR, Khanjani S, Brosens JJ, Bennett PR, Johnson MR (2012). "Progesterone acts via the nuclear glucocorticoid receptor to suppress IL-1β-induced COX-2 expression in human term myometrial cells". PloS One. 7 (11): e50167. doi:10.1371/journal.pone.0050167. PMID 23209664.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Paul SM, Purdy RH (March 1992). "Neuroactive steroids". FASEB Journal. 6 (6): 2311–22. PMID 1347506.
- ^ Kliewer SA, Goodwin B, Willson TM (2002). "The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism". Endocr. Rev. 23 (5): 687–702. doi:10.1210/er.2001-0038. PMID 12372848.
- ^ Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA (1998). "The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions". J. Clin. Invest. 102 (5): 1016–23. doi:10.1172/JCI3703. PMC 508967. PMID 9727070.
- ^ a b Nicholas A. Meanwell (8 December 2014). Tactics in Contemporary Drug Design. Springer. pp. 161–. ISBN 978-3-642-55041-6.
- ^ a b c Marianne J. Legato; John P. Bilezikian (2004). Principles of Gender-specific Medicine. Gulf Professional Publishing. pp. 146–. ISBN 978-0-12-440906-4.
- ^ a b Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 164–. ISBN 978-1-60913-345-0.
- ^ a b Estrogens—Advances in Research and Application: 2013 Edition: ScholarlyBrief. ScholarlyEditions. 21 June 2013. pp. 4–. ISBN 978-1-4816-7550-5.
- ^ Strünker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB (March 2011). "The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm". Nature. 471 (7338): 382–6. doi:10.1038/nature09769. PMID 21412338.
{{cite journal}}: Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help) - ^ Lishko PV, Botchkina IL, Kirichok Y (March 2011). "Progesterone activates the principal Ca2+ channel of human sperm". Nature. 471 (7338): 387–91. doi:10.1038/nature09767. PMID 21412339.
- ^ a b c d e f g h Progesterone - Drugs.com, retrieved 2015-08-23
- ^ Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P (May 1990). "Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B". The EMBO Journal. 9 (5): 1603–14. PMC 551856. PMID 2328727.
- ^ a b Sandra Z. Haslam; Janet R. Osuch (1 January 2006). Hormones and Breast Cancer in Post-Menopausal Women. IOS Press. p. 69. ISBN 978-1-58603-653-9.
- ^ a b Leonard R. Johnson (2003). Essential Medical Physiology. Academic Press. p. 770. ISBN 978-0-12-387584-6.
- ^ a b Jane Coad; Melvyn Dunstall (2011). Anatomy and Physiology for Midwives,with Pageburst online access,3: Anatomy and Physiology for Midwives. Elsevier Health Sciences. p. 413. ISBN 0-7020-3489-4.
- ^ Landau RL, Bergenstal DM, Lugibihl K, Kascht ME (October 1955). "The metabolic effects of progesterone in man". The Journal of Clinical Endocrinology and Metabolism. 15 (10): 1194–215. doi:10.1210/jcem-15-10-1194. PMID 13263410.
- ^ Correia JN, Conner SJ, Kirkman-Brown JC (May 2007). "Non-genomic steroid actions in human spermatozoa. "Persistent tickling from a laden environment"". Seminars in Reproductive Medicine. 25 (3): 208–19. doi:10.1055/s-2007-973433. PMID 17447210.
- ^ Kirkman-Brown JC, Bray C, Stewart PM, Barratt CL, Publicover SJ (June 2000). "Biphasic elevation of [Ca(2+)](i) in individual human spermatozoa exposed to progesterone". Developmental Biology. 222 (2): 326–35. doi:10.1006/dbio.2000.9729. PMID 10837122.
- ^ Kirkman-Brown JC, Barratt CL, Publicover SJ (March 2004). "Slow calcium oscillations in human spermatozoa". The Biochemical Journal. 378 (Pt 3): 827–32. doi:10.1042/BJ20031368. PMC 1223996. PMID 14606954.
- ^ Harper CV, Barratt CL, Publicover SJ (October 2004). "Stimulation of human spermatozoa with progesterone gradients to simulate approach to the oocyte. Induction of [Ca(2+)](i) oscillations and cyclical transitions in flagellar beating". The Journal of Biological Chemistry. 279 (44): 46315–25. doi:10.1074/jbc.M401194200. PMID 15322137.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Marieb, Elaine (2013). Anatomy & physiology. Benjamin-Cummings. p. 903. ISBN 9780321887603.
- ^ Tosti E, Di Cosmo A, Cuomo A, Di Cristo C, Gragnaniello G (May 2001). "Progesterone induces activation in Octopus vulgaris spermatozoa". Molecular Reproduction and Development. 59 (1): 97–105. doi:10.1002/mrd.1011. PMID 11335951.
- ^ a b Bowen R (2000-08-06). "Placental Hormones". Retrieved 2008-03-12.
- ^ Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S (2014). "Role of nuclear progesterone receptor isoforms in uterine pathophysiology". Human Reproduction Update. 21 (2): 155–73. doi:10.1093/humupd/dmu056. PMID 25406186.
- ^ Macias H, Hinck L (2012). "Mammary gland development". Wiley Interdisciplinary Reviews. Developmental Biology. 1 (4): 533–57. doi:10.1002/wdev.35. PMC 3404495. PMID 22844349.
- ^ a b c Hilton HN, Graham JD, Clarke CL (September 2015). "Minireview: Progesterone Regulation of Proliferation in the Normal Human Breast and in Breast Cancer: A Tale of Two Scenarios?". Molecular Endocrinology. 29 (9): 1230–42. doi:10.1210/me.2015-1152. PMID 26266959.
- ^ Jerome Frank Strauss; Robert L. Barbieri (13 September 2013). Yen and Jaffe's Reproductive Endocrinology. Elsevier Health Sciences. pp. 236–. ISBN 978-1-4557-2758-2.
- ^ Scaling AL, Prossnitz ER, Hathaway HJ (June 2014). "GPER mediates estrogen-induced signaling and proliferation in human breast epithelial cells and normal and malignant breast". Hormones & Cancer. 5 (3): 146–60. doi:10.1007/s12672-014-0174-1. PMC 4091989. PMID 24718936.
- ^ a b c d e Aupperlee MD, Leipprandt JR, Bennett JM, Schwartz RC, Haslam SZ (2013). "Amphiregulin mediates progesterone-induced mammary ductal development during puberty". Breast Cancer Research. 15 (3): R44. doi:10.1186/bcr3431. PMC 3738150. PMID 23705924.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Kuhl H, Schneider HP (August 2013). "Progesterone--promoter or inhibitor of breast cancer". Climacteric. 16 Suppl 1: 54–68. doi:10.3109/13697137.2013.768806. PMID 23336704.
- ^ a b Fournier A, Berrino F, Clavel-Chapelon F (2008). "Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study". Breast Cancer Res. Treat. 107 (1): 103–11. doi:10.1007/s10549-007-9523-x. PMC 2211383. PMID 17333341.
- ^ Campagnoli C, Clavel-Chapelon F, Kaaks R, Peris C, Berrino F (July 2005). "Progestins and progesterone in hormone replacement therapy and the risk of breast cancer". The Journal of Steroid Biochemistry and Molecular Biology. 96 (2): 95–108. doi:10.1016/j.jsbmb.2005.02.014. PMID 15908197.
- ^ Steven R. King (9 November 2012). Neurosteroids and the Nervous System. Springer Science & Business Media. pp. 44–46. ISBN 978-1-4614-5559-2.
- ^ Homosexuality may help us bond University of Portsmouth UoP News
- ^ Having homosexual thoughts 'is an essential part of human evolution' study suggests The Telegraph
- ^ Homosexuality May Have Evolved In Humans Because It Helps Us Bond, Scientists Say Huff Post
- ^ Schumacher M, Guennoun R, Robert F, Carelli C, Gago N, Ghoumari A, Gonzalez Deniselle MC, Gonzalez SL, Ibanez C, Labombarda F, Coirini H, Baulieu EE, De Nicola AF (June 2004). "Local synthesis and dual actions of progesterone in the nervous system: neuroprotection and myelination". Growth Hormone & IGF Research. 14 Suppl A: S18-33. doi:10.1016/j.ghir.2004.03.007. PMID 15135772.
- ^ a b Espinoza TR, Wright DW (2011). "The role of progesterone in traumatic brain injury". The Journal of Head Trauma Rehabilitation. 26 (6): 497–9. doi:10.1097/HTR.0b013e31823088fa. PMID 22088981.
- ^ a b Stein DG (September 2011). "Progesterone in the treatment of acute traumatic brain injury: a clinical perspective and update". Neuroscience. 191: 101–6. doi:10.1016/j.neuroscience.2011.04.013. PMID 21497181.
- ^ Roof RL, Hall ED (May 2000). "Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone". Journal of Neurotrauma. 17 (5): 367–88. doi:10.1089/neu.2000.17.367. PMID 10833057.
- ^ Gibson CL, Gray LJ, Bath PM, Murphy SP (February 2008). "Progesterone for the treatment of experimental brain injury; a systematic review". Brain. 131 (Pt 2): 318–28. doi:10.1093/brain/awm183. PMID 17715141.
- ^ Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, Salomone JP, Dent LL, Harris OA, Ander DS, Lowery DW, Patel MM, Denson DD, Gordon AB, Wald MM, Gupta S, Hoffman SW, Stein DG (April 2007). "ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury". Annals of Emergency Medicine. 49 (4): 391–402, 402.e1-2. doi:10.1016/j.annemergmed.2006.07.932. PMID 17011666.
- ^ Xiao G, Wei J, Yan W, Wang W, Lu Z (April 2008). "Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial". Critical Care. 12 (2): R61. doi:10.1186/cc6887. PMC 2447617. PMID 18447940.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Pan DS, Liu WG, Yang XF, Cao F (October 2007). "Inhibitory effect of progesterone on inflammatory factors after experimental traumatic brain injury". Biomedical and Environmental Sciences. 20 (5): 432–8. PMID 18188998.
- ^ a b Luoma JI, Stern CM, Mermelstein PG (August 2012). "Progesterone inhibition of neuronal calcium signaling underlies aspects of progesterone-mediated neuroprotection". The Journal of Steroid Biochemistry and Molecular Biology. 131 (1–2): 30–6. doi:10.1016/j.jsbmb.2011.11.002. PMC 3303940. PMID 22101209.
- ^ a b c Stein DG (March 2008). "Progesterone exerts neuroprotective effects after brain injury". Brain Research Reviews. 57 (2): 386–97. doi:10.1016/j.brainresrev.2007.06.012. PMC 2699575. PMID 17826842.
- ^ Herson PS, Koerner IP, Hurn PD (May 2009). "Sex, sex steroids, and brain injury". Seminars in Reproductive Medicine. 27 (3): 229–39. doi:10.1055/s-0029-1216276. PMC 2675922. PMID 19401954.
- ^ Li Z, Wang B, Kan Z, Zhang B, Yang Z, Chen J, Wang D, Wei H, Zhang JN, Jiang R (January 2012). "Progesterone increases circulating endothelial progenitor cells and induces neural regeneration after traumatic brain injury in aged rats". Journal of Neurotrauma. 29 (2): 343–53. doi:10.1089/neu.2011.1807. PMC 3261789. PMID 21534727.
- ^ Cekic M, Sayeed I, Stein DG (July 2009). "Combination treatment with progesterone and vitamin D hormone may be more effective than monotherapy for nervous system injury and disease". Frontiers in Neuroendocrinology. 30 (2): 158–72. doi:10.1016/j.yfrne.2009.04.002. PMC 3025702. PMID 19394357.
- ^ Khaksari M, Soltani Z, Shahrokhi N, Moshtaghi G, Asadikaram G (January 2011). "The role of estrogen and progesterone, administered alone and in combination, in modulating cytokine concentration following traumatic brain injury". Canadian Journal of Physiology and Pharmacology. 89 (1): 31–40. doi:10.1139/y10-103. PMID 21186375.
- ^ "Efficacy and Safety Study of Intravenous Progesterone in Patients With Severe Traumatic Brain Injury (SyNAPSe)". ClinicalTrials.gov. U.S. National Institutes of Health. Retrieved 2012-07-14.
- ^ "SyNAPse: The Global phase 3 study of progesterone in severe traumatic brain injury". BHR Pharma, LLC.
- ^ a b Lynch WJ, Sofuoglu M (December 2010). "Role of progesterone in nicotine addiction: evidence from initiation to relapse". Experimental and Clinical Psychopharmacology. 18 (6): 451–61. doi:10.1037/a0021265. PMC 3638762. PMID 21186920.
- ^ Sofuoglu M, Mitchell E, Mooney M (October 2009). "Progesterone effects on subjective and physiological responses to intravenous nicotine in male and female smokers". Human Psychopharmacology. 24 (7): 559–64. doi:10.1002/hup.1055. PMC 2785078. PMID 19743227.
- ^ Cosgrove KP, Esterlis I, McKee SA, Bois F, Seibyl JP, Mazure CM, Krishnan-Sarin S, Staley JK, Picciotto MR, O'Malley SS (April 2012). "Sex differences in availability of β2*-nicotinic acetylcholine receptors in recently abstinent tobacco smokers". Archives of General Psychiatry. 69 (4): 418–27. doi:10.1001/archgenpsychiatry.2011.1465. PMC 3508698. PMID 22474108.
- ^ Mello NK, Knudson IM, Kelly M, Fivel PA, Mendelson JH (October 2011). "Effects of progesterone and testosterone on cocaine self-administration and cocaine discrimination by female rhesus monkeys". Neuropsychopharmacology. 36 (11): 2187–99. doi:10.1038/npp.2011.130. PMC 3176575. PMID 21796112.
- ^ a b c Susan Blackburn (14 April 2014). Maternal, Fetal, & Neonatal Physiology. Elsevier Health Sciences. pp. 92–. ISBN 978-0-323-29296-2.
- ^ Template:GeorgiaPhysiology
- ^ Hould FS, Fried GM, Fazekas AG, Tremblay S, Mersereau WA (December 1988). "Progesterone receptors regulate gallbladder motility". The Journal of Surgical Research. 45 (6): 505–12. doi:10.1016/0022-4804(88)90137-0. PMID 3184927.
- ^ "Hormones and Oral Health". WebMD.
- ^ Picard F, Wanatabe M, Schoonjans K, Lydon J, O'Malley BW, Auwerx J (November 2002). "Progesterone receptor knockout mice have an improved glucose homeostasis secondary to beta -cell proliferation". Proceedings of the National Academy of Sciences of the United States of America. 99 (24): 15644–8. doi:10.1073/pnas.202612199. PMC 137770. PMID 12438645.
- ^ Brănişteanu DD, Mathieu C (March 2003). "Progesterone in gestational diabetes mellitus: guilty or not guilty?". Trends in Endocrinology and Metabolism. 14 (2): 54–6. doi:10.1016/S1043-2760(03)00003-1. PMID 12591170.
- ^ Schneider JS, Stone MK, Wynne-Edwards KE, Horton TH, Lydon J, O'Malley B, Levine JE (March 2003). "Progesterone receptors mediate male aggression toward infants". Proceedings of the National Academy of Sciences of the United States of America. 100 (5): 2951–6. doi:10.1073/pnas.0130100100. PMC 151447. PMID 12601162.
- ^ Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH (2008). "Classification and pharmacology of progestins" (PDF). Maturitas. 61 (1–2): 171–80. doi:10.1016/j.maturitas.2008.11.013. PMID 19434889.
- ^ da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M (February 2003). "Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study". American Journal of Obstetrics and Gynecology. 188 (2): 419–24. doi:10.1067/mob.2003.41. PMID 12592250.
- ^ Harris, Gardiner (2011-05-02). "Hormone Is Said to Cut Risk of Premature Birth". New York Times. Retrieved 5 May 2011.
- ^ O'Brien JM, Adair CD, Lewis DF, Hall DR, Defranco EA, Fusey S, Soma-Pillay P, Porter K, How H, Schackis R, Eller D, Trivedi Y, Vanburen G, Khandelwal M, Trofatter K, Vidyadhari D, Vijayaraghavan J, Weeks J, Dattel B, Newton E, Chazotte C, Valenzuela G, Calda P, Bsharat M, Creasy GW (October 2007). "Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial". Ultrasound in Obstetrics & Gynecology. 30 (5): 687–96. doi:10.1002/uog.5158. PMID 17899572.
- ^ DeFranco EA, O'Brien JM, Adair CD, Lewis DF, Hall DR, Fusey S, Soma-Pillay P, Porter K, How H, Schakis R, Eller D, Trivedi Y, Vanburen G, Khandelwal M, Trofatter K, Vidyadhari D, Vijayaraghavan J, Weeks J, Dattel B, Newton E, Chazotte C, Valenzuela G, Calda P, Bsharat M, Creasy GW (October 2007). "Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial". Ultrasound in Obstetrics & Gynecology. 30 (5): 697–705. doi:10.1002/uog.5159. PMID 17899571.
- ^ Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH (August 2007). "Progesterone and the risk of preterm birth among women with a short cervix". The New England Journal of Medicine. 357 (5): 462–9. doi:10.1056/NEJMoa067815. PMID 17671254.
- ^ Romero R (October 2007). "Prevention of spontaneous preterm birth: the role of sonographic cervical length in identifying patients who may benefit from progesterone treatment". Ultrasound in Obstetrics & Gynecology. 30 (5): 675–86. doi:10.1002/uog.5174. PMID 17899585.
- ^ Hassan SS, Romero R, Vidyadhari D, Fusey S, Baxter JK, Khandelwal M, Vijayaraghavan J, Trivedi Y, Soma-Pillay P, Sambarey P, Dayal A, Potapov V, O'Brien J, Astakhov V, Yuzko O, Kinzler W, Dattel B, Sehdev H, Mazheika L, Manchulenko D, Gervasi MT, Sullivan L, Conde-Agudelo A, Phillips JA, Creasy GW (July 2011). "Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial". Ultrasound in Obstetrics & Gynecology. 38 (1): 18–31. doi:10.1002/uog.9017. PMC 3482512. PMID 21472815.
{{cite journal}}: Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help) - ^ "Progesterone helps cut risk of pre-term birth". Women's health. msnbc.com. 2011-12-14. Retrieved 2011-12-14.
- ^ Orrin Devinsky; Steven Schachter; Steven Pacia (1 January 2005). Complementary and Alternative Therapies for Epilepsy. Demos Medical Publishing. pp. 378–. ISBN 978-1-934559-08-6.
- ^ Sriram, D (2007). Medicinal Chemistry. New Delhi: Dorling Kindersley India Pvt. Ltd. p. 432. ISBN 81-317-0031-3.
- ^ World Professional Association for Transgender Health (September 2011), Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People, Seventh Version (PDF)
- ^ Pinna G, Agis-Balboa RC, Pibiri F, Nelson M, Guidotti A, Costa E (October 2008). "Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice". Neurochemical Research. 33 (10): 1990–2007. doi:10.1007/s11064-008-9718-5. PMID 18473173.
- ^ Babalonis S, Lile JA, Martin CA, Kelly TH (June 2011). "Physiological doses of progesterone potentiate the effects of triazolam in healthy, premenopausal women". Psychopharmacology. 215 (3): 429–39. doi:10.1007/s00213-011-2206-7. PMID 21350928.
- ^ "Progesterone abuse". Reactions Weekly. 599 (1). Springer International Publishing: 9. 1996. doi:10.2165/00128415-199605990-00031. ISSN 1179-2051.
- ^ Traish AM, Mulgaonkar A, Giordano N (June 2014). "The dark side of 5α-reductase inhibitors' therapy: sexual dysfunction, high Gleason grade prostate cancer and depression". Korean Journal of Urology. 55 (6): 367–79. doi:10.4111/kju.2014.55.6.367. PMC 4064044. PMID 24955220.
- ^ Meyer L, Venard C, Schaeffer V, Patte-Mensah C, Mensah-Nyagan AG (April 2008). "The biological activity of 3alpha-hydroxysteroid oxido-reductase in the spinal cord regulates thermal and mechanical pain thresholds after sciatic nerve injury". Neurobiology of Disease. 30 (1): 30–41. doi:10.1016/j.nbd.2007.12.001. PMID 18291663.
- ^ Pazol K, Wilson ME, Wallen K (June 2004). "Medroxyprogesterone acetate antagonizes the effects of estrogen treatment on social and sexual behavior in female macaques". The Journal of Clinical Endocrinology and Metabolism. 89 (6): 2998–3006. doi:10.1210/jc.2003-032086. PMC 1440328. PMID 15181090.
- ^ Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH (December 2003). "Classification and pharmacology of progestins". Maturitas. 46 Suppl 1: S7–S16. doi:10.1016/j.maturitas.2003.09.014. PMID 14670641.
- ^ Simon JA, Robinson DE, Andrews MC, Hildebrand JR, Rocci ML, Blake RE, Hodgen GD (July 1993). "The absorption of oral micronized progesterone: the effect of food, dose proportionality, and comparison with intramuscular progesterone". Fertility and Sterility. 60 (1): 26–33. PMID 8513955.
- ^ Integrative Medicine. Elsevier Health Sciences. 2012. p. 343. ISBN 1-4377-1793-4.
- ^ Sushma Deshmukh (30 September 2013). Infertility Management Made Easy. JP Medical Ltd. p. 273. ISBN 978-93-5090-531-9.
- ^ a b Reddy DS (2010). "Neurosteroids: endogenous role in the human brain and therapeutic potentials". Progress in Brain Research. 186: 113–37. doi:10.1016/B978-0-444-53630-3.00008-7. PMC 3139029. PMID 21094889.
- ^ a b c Rebekah Wang-Cheng; Joan M. Neuner; Vanessa M. Barnabei (2007). Menopause. ACP Press. p. 97. ISBN 978-1-930513-83-9.
- ^ a b Niels Bergemann; Anita Riecher-Rössler (27 December 2005). Estrogen Effects in Psychiatric Disorders. Springer Science & Business Media. p. 179. ISBN 978-3-211-27063-9.
- ^ a b Söderpalm AH, Lindsey S, Purdy RH, Hauger R, Wit de H (2004). "Administration of progesterone produces mild sedative-like effects in men and women". Psychoneuroendocrinology. 29 (3): 339–54. doi:10.1016/s0306-4530(03)00033-7. PMID 14644065.
- ^ de Wit H, Schmitt L, Purdy R, Hauger R (2001). "Effects of acute progesterone administration in healthy postmenopausal women and normally-cycling women". Psychoneuroendocrinology. 26 (7): 697–710. doi:10.1016/s0306-4530(01)00024-5. PMID 11500251.
- ^ van Broekhoven F, Bäckström T, Verkes RJ (2006). "Oral progesterone decreases saccadic eye velocity and increases sedation in women". Psychoneuroendocrinology. 31 (10): 1190–9. doi:10.1016/j.psyneuen.2006.08.007. PMID 17034954.
- ^ a b c d Nagrath Arun; Malhotra Narendra; Seth Shikha (15 December 2012). Progress in Obstetrics and Gynecology--3. Jaypee Brothers Medical Publishers Pvt. Ltd. pp. 370–. ISBN 978-93-5090-575-3.
- ^ Unfer V, Casini ML, Marelli G, Costabile L, Gerli S, Di Renzo GC (August 2005). "Different routes of progesterone administration and polycystic ovary syndrome: a review of the literature". Gynecological Endocrinology. 21 (2): 119–27. doi:10.1080/09513590500170049. PMID 16109599.
- ^ Elshafie MA, Ewies AA (2007). "Transdermal natural progesterone cream for postmenopausal women: inconsistent data and complex pharmacokinetics". Journal of Obstetrics and Gynaecology : the Journal of the Institute of Obstetrics and Gynaecology. 27 (7): 655–9. doi:10.1080/01443610701582727. PMID 17999287.
- ^ Du, JY; Sanchez, P; Kim, L; Azen, CG; Zava, DT; Stanczyk, FZ (November 2013). "Percutaneous progesterone delivery via cream or gel application in postmenopausal women: a randomized cross-over study of progesterone levels in serum, whole blood, saliva, and capillary blood". Menopause (New York, N.Y.). 20 (11): 1169–75. PMID 23652031.
- ^ a b c d e P.A. van Keep; W.H. Utian (6 December 2012). The Premenstrual Syndrome: Proceedings of a workshop held during the Sixth International Congress of Psychosomatic Obstetrics and Gynecology, Berlin, September 1980. Springer Science & Business Media. pp. 51–52. ISBN 978-94-011-6255-5.
- ^ Lockwood G, Griesinger G, Cometti B (2014). "Subcutaneous progesterone versus vaginal progesterone gel for luteal phase support in in vitro fertilization: a noninferiority randomized controlled study". Fertil. Steril. 101 (1): 112–119.e3. doi:10.1016/j.fertnstert.2013.09.010. PMID 24140033.
- ^ a b c Baker VL, Jones CA, Doody K, Foulk R, Yee B, Adamson GD, Cometti B, DeVane G, Hubert G, Trevisan S, Hoehler F, Jones C, Soules M (2014). "A randomized, controlled trial comparing the efficacy and safety of aqueous subcutaneous progesterone with vaginal progesterone for luteal phase support of in vitro fertilization". Hum. Reprod. 29 (10): 2212–20. doi:10.1093/humrep/deu194. PMC 4164149. PMID 25100106.
- ^ Goodson III WH, Handagama P, Moore II DH, Dairkee S (2007-12-13). "Milk products are a source of dietary progesterone". 30th Annual San Antonio Breast Cancer Symposium. pp. abstract # 2028. Retrieved 2008-03-12.
- ^ Pauli GF, Friesen JB, Gödecke T, Farnsworth NR, Glodny B (March 2010). "Occurrence of progesterone and related animal steroids in two higher plants". Journal of Natural Products. 73 (3): 338–45. doi:10.1021/np9007415. PMID 20108949.
- ^ Applezweig N (May 1969). "Steroids". Chemical Week. 104: 57–72. PMID 12255132.
- ^ Noguchi E, Fujiwara Y, Matsushita S, Ikeda T, Ono M, Nohara T (September 2006). "Metabolism of tomato steroidal glycosides in humans". Chemical & Pharmaceutical Bulletin. 54 (9): 1312–4. doi:10.1248/cpb.54.1312. PMID 16946542.
- ^ Yang DJ, Lu TJ, Hwang LS (October 2003). "Isolation and identification of steroidal saponins in Taiwanese yam cultivar (Dioscorea pseudojaponica Yamamoto)". Journal of Agricultural and Food Chemistry. 51 (22): 6438–44. doi:10.1021/jf030390j. PMID 14558759.
- ^ Hooker E (2004). "Final report of the amended safety assessment of Dioscorea Villosa (Wild Yam) root extract". International Journal of Toxicology. 23 Suppl 2: 49–54. doi:10.1080/10915810490499055. PMID 15513824.
- ^ Niño J, Jiménez DA, Mosquera OM, Correa YM (2007). "Diosgenin quantification by HPLC in a Dioscorea polygonoides tuber collection from colombian flora". Journal of the Brazilian Chemical Society. 18 (5): 1073–1076. doi:10.1590/S0103-50532007000500030.
- ^ Myoda T, Nagai T, Nagashima T (2005). Properties of starches in yam (Dioscorea spp.) tuber. pp. 105–114. ISBN 81-308-0003-9.
{{cite book}}:|journal=ignored (help) - ^ Dewick, Paul M. (2002). Medicinal natural products: a biosynthetic approach. New York: Wiley. p. 244. ISBN 0-471-49641-3.
- ^ Duport C, Spagnoli R, Degryse E, Pompon D (February 1998). "Self-sufficient biosynthesis of pregnenolone and progesterone in engineered yeast". Nature Biotechnology. 16 (2): 186–9. doi:10.1038/nbt0298-186. PMID 9487528.
- ^ Beranič N, Gobec S, Rižner TL (2011). "Progestins as inhibitors of the human 20-ketosteroid reductases, AKR1C1 and AKR1C3". Chem. Biol. Interact. 191 (1–3): 227–33. doi:10.1016/j.cbi.2010.12.012. PMID 21182831.
- ^ a b c d e f g h i Progesterone Reference Ranges, Performed at the Clinical Center at the National Institutes of Health, Bethesda MD, 03Feb09
- ^ a b c d e f g h Converted from mass values using molar mass of 314.46 g/mol
- ^ Csapo AI, Pulkkinen MU, Wiest WG (1973). "Effects of lutectomy and progestreone replacement therapy in early pregnancy patients". Am J Obstet Gynecol. 115: 759–65.
- ^ NIH Clinical Center (2004-08-16). "Progesterone Historical Reference Ranges". United States National Institutes of Health. Retrieved 2008-03-12.
- ^ a b c Marker RE, Krueger J (1940). "Sterols. CXII. Sapogenins. XLI. The Preparation of Trillin and its Conversion to Progesterone". J. Am. Chem. Soc. 62 (12): 3349–3350. doi:10.1021/ja01869a023.
- ^ Numazawa M, Nagaoka M, Kunitama Y (September 1986). "Regiospecific deoxygenation of the dihydroxyacetone moiety at C-17 of corticoid steroids with iodotrimethylsilane". Chemical & Pharmaceutical Bulletin. 34 (9): 3722–6. doi:10.1248/cpb.34.3722. PMID 3815593.
- ^ Heyl FW (1950). "Progesterone from 3-Acetoxybisnor-5-cholenaldehyde and 3-Ketobisnor-4-cholenaldehyde". Journal of the American Chemical Society. 72 (6): 2617–2619. doi:10.1021/ja01162a076.
- ^ Slomp G (1958). "Ozonolysis. II. 1 The Effect of Pyridine on the Ozonolysis of 4,22-Stigmastadien-3-one 2". Journal of the American Chemical Society. 80 (4): 915–921. doi:10.1021/ja01537a041.
- ^ Sundararaman P, Djerassi C (October 1977). "A convenient synthesis of progesterone from stigmasterol". The Journal of Organic Chemistry. 42 (22): 3633–4. doi:10.1021/jo00442a044. PMID 915584.
- ^ "Nova Transcripts: Forgotten Genius". PBS.org. February 6, 2007.
- ^ "Giants of the Past". lipidlibrary.aocs.org.
- ^ a b c Johnson WS, Gravestock MB, McCarry BE (August 1971). "Acetylenic bond participation in biogenetic-like olefinic cyclizations. II. Synthesis of dl-progesterone". Journal of the American Chemical Society. 93 (17): 4332–4. doi:10.1021/ja00746a062. PMID 5131151.
- ^ a b c d Elsimar M. Coutinho; Sheldon Jerome Segal (1999). Is Menstruation Obsolete?. Oxford University Press. pp. 31–. ISBN 978-0-19-513021-8.
- ^ Anne Walker (7 March 2008). The Menstrual Cycle. Routledge. pp. 49–. ISBN 978-1-134-71411-7.
- ^ Piosik, R. (2003). "Adolf Butenandt und sein Wirken an der Technischen Hochschule Danzig". Chemkon. 10 (3): 135. doi:10.1002/ckon.200390038.
- ^ Benson Ginsburg (6 December 2012). Premenstrual Syndrome: Ethical and Legal Implications in a Biomedical Perspective. Springer Science & Business Media. pp. 274–. ISBN 978-1-4684-5275-4.
- ^ Sir Humphry Davy Rolleston (1936). The Endocrine Organs in Health and Disease: With an Historical Review. Oxford University Press, H. Milford. p. 406.
- ^ Allen WM (October 1970). "Progesterone: how did the name originate?". Southern Medical Journal. 63 (10): 1151–5. doi:10.1097/00007611-197010000-00012. PMID 4922128.
- ^ Barbara Seaman (4 January 2011). The Greatest Experiment Ever Performed on Women: Exploding the Estrogen Myth. Seven Stories Press. pp. 27–. ISBN 978-1-60980-062-8.
- ^ a b Simon JA (December 1995). "Micronized progesterone: vaginal and oral uses". Clinical Obstetrics and Gynecology. 38 (4): 902–14. doi:10.1097/00003081-199538040-00024. PMID 8616985.
- ^ Gerald, Michael (2013). The Drug Book. New York, New York: Sterling Publishing. p. 186. ISBN 9781402782640.
- ^ a b Mark V. Sauer (1 March 2013). Principles of Oocyte and Embryo Donation. Springer Science & Business Media. pp. 7, 118. ISBN 978-1-4471-2392-7.
- ^ a b Mary Jane Minkin; Carol V. Wright (2005). A Woman's Guide to Menopause & Perimenopause. Yale University Press. pp. 143–. ISBN 978-0-300-10435-6.
- ^ Ruan X, Mueck AO (November 2014). "Systemic progesterone therapy--oral, vaginal, injections and even transdermal?". Maturitas. 79 (3): 248–55. doi:10.1016/j.maturitas.2014.07.009. PMID 25113944.
- ^ Csech J, Gervais C (September 1982). "[Utrogestan]". Soins. Gynécologie, Obstétrique, Puériculture, Pédiatrie (in French) (16): 45–6. PMID 6925387.
- ^ Racowsky C, Schlegel PN, Fauser BC, Carrell D (7 June 2011). Biennial Review of Infertility. Springer Science & Business Media. pp. 84–85. ISBN 978-1-4419-8456-2.
- ^ a b c d e Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. p. 880. ISBN 978-3-88763-075-1.
- ^ "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations: 020701". Food and Drug Administration. 2010-07-02. Retrieved 2010-07-07.
- ^ "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations: 019781". Food and Drug Administration. 2010-07-02. Retrieved 2010-07-07.
- ^ "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations: 075906". Food and Drug Administration. 2010-07-02. Retrieved 2010-07-07.
- ^ "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations: 022057". Food and Drug Administration. 2010-07-02. Retrieved 2010-07-07.
- ^ Lark, Susan (1999). Making the Estrogen Decision. McGraw-Hill Professional. p. 22. ISBN 9780879836962.
- ^ Zava DT, Dollbaum CM, Blen M (March 1998). "Estrogen and progestin bioactivity of foods, herbs, and spices". Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine. 217 (3): 369–78. doi:10.3181/00379727-217-44247. PMID 9492350.
- ^ Komesaroff PA, Black CV, Cable V, Sudhir K (June 2001). "Effects of wild yam extract on menopausal symptoms, lipids and sex hormones in healthy menopausal women". Climacteric. 4 (2): 144–50. doi:10.1080/713605087. PMID 11428178.
External links
- Progesterone MS Spectrum
- Progesterone at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Kimball JW (2007-05-27). "Progesterone". Kimball's Biology Pages. Retrieved 2008-06-18.
- Antimineralocorticoids
- Antigonadotropins
- GABAA receptor positive allosteric modulators
- Glucocorticoids
- Glycine receptor antagonists
- Neuroprotective agents
- Neurosteroids
- Hormones of the hypothalamus-pituitary-adrenal axis
- Hormones of the hypothalamus-pituitary-gonad axis
- Hormones of the ovary
- Hormones of the placenta
- Hormones of the suprarenal cortex
- Hormones of the brain
- Hormones of the pregnant female
- Human hormones
- Pregnane X receptor agonists
- Progestogens
- Sedatives
- Sex hormones
- Sigma antagonists
- Total synthesis
- Steroid hormones
- Human female endocrine system
