From Wikipedia, the free encyclopedia
Chemical compound
Brompheniramine Trade names Bromfed, Dimetapp, Bromfenex, and others AHFS /Drugs.com Monograph MedlinePlus a682545 Routes of By mouth ATC code Legal status
AU :US :Unscheduled, OTC
Metabolism Hepatic Elimination half-life 24.9 ± 9.3 hours[ 1] Excretion Urine
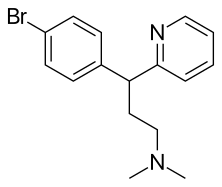
(R /S )-3-(4-Bromophenyl)-N ,N -dimethyl-3-pyridin-2-yl-propan-1-amine
CAS Number PubChem CID IUPHAR/BPS DrugBank ChemSpider UNII KEGG ChEBI ChEMBL CompTox Dashboard (EPA ) ECHA InfoCard 100.001.507 Formula C 16 H 19 Br N 2 Molar mass −1 3D model (JSmol )
Brc1ccc(cc1)C(c2ncccc2)CCN(C)C
InChI=1S/C16H19BrN2/c1-19(2)12-10-15(16-5-3-4-11-18-16)13-6-8-14(17)9-7-13/h3-9,11,15H,10,12H2,1-2H3
Y Key:ZDIGNSYAACHWNL-UHFFFAOYSA-N
Y (verify)
Brompheniramine , sold under the brand name Dimetapp among others, is an antihistamine drug of the propylamine (alkylamine) class.
It is indicated for the treatment of the symptoms of the common cold and allergic rhinitis , such as runny nose, itchy eyes, watery eyes, and sneezing.
It is a first-generation antihistamine and one of the drugs of highest anticholinergic activity.
It was patented in 1948 and came into medical use in 1955.[ 2]
Side effects
Brompheniramine's effects on the cholinergic system may include side-effects such as drowsiness, sedation, dry mouth, dry throat, blurred vision, and increased heart rate. It is listed as one of the drugs of highest anticholinergic activity in a study of anticholinergenic burden, including long-term cognitive impairment.[ 3]
Pharmacology
Brompheniramine works by acting as an antagonist of histamine H1 receptors . It also functions as a moderately effective anticholinergic agent, and is likely an antimuscarinic agent similar to other common antihistamines such as diphenhydramine .
Brompheniramine is metabolised by cytochrome P450s .
The halogenated alkylamine antihistamines all exhibit optic isomerism and brompheniramine products contain racaemic brompheniramine maleate whereas dexbrompheniramine (Drixoral) is the dextrorotary (right-handed) stereoisomer.[citation needed
Chemistry
Brompheniramine is part of a series of antihistamines including pheniramine (Naphcon) and its halogenated derivatives and others including fluorpheniramine , chlorpheniramine , dexchlorpheniramine (Polaramine), triprolidine (Actifed), and iodopheniramine. The halogenated alkylamine antihistamines all exhibit optical isomerism and brompheniramine products contain racemic brompheniramine maleate whereas dexbrompheniramine (Drixoral) is the dextrorotary (right-handed) stereoisomer.
Brompheniramine is an analog of chlorpheniramine . The only difference is that the chlorine atom in the benzene ring is replaced with a bromine atom. It is also synthesized in an analogous manner.[ 4] [ 5]
History
Based on this[which? knowledge, Arvid Carlsson and his colleagues, working at the Swedish company Astra AB , were able to derive the first marketed selective serotonin reuptake inhibitor , zimelidine , from brompheniramine.[ 6]
Names
Brand names include Bromfed, Dimetapp, Bromfenex, Dimetane, BPN, Lodrane. It is commonly marketed as its salt brompheniramine maleate .
See also
References
^ Simons FE, Frith EM, Simons KJ (December 1982). "The pharmacokinetics and antihistaminic effects of brompheniramine". The Journal of Allergy and Clinical Immunology . 70 (6): 458–64. doi :10.1016/0091-6749(82)90009-4 . PMID 6128358 . ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery ISBN 9783527607495 ^ Salahudeen MJ; Duffull SB; Nishtala PS; et al. (2015-03-25). "Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review" . BMC Geriatrics . 15 (31): 31. doi :10.1186/s12877-015-0029-9 . PMC 4377853 PMID 25879993 . {{cite journal }}: CS1 maint: unflagged free DOI (link )^ L.A. Walter, U.S. patent 3,061,517
^ L.A. Walter, U.S. patent 3,030,371
^ Barondes, Samuel H. (2003). Better Than Prozac 39–40 . ISBN 0-19-515130-5
External links
Products Predecessors and People
Psychedelics (5-HT2A
Benzofurans Lyserg‐ Phenethyl‐
2C-x
3C-x 4C-x DOx HOT-x MDxx Mescaline (subst.) TMAs
TMA
TMA-2
TMA-3
TMA-4
TMA-5
TMA-6 Others
Piperazines Tryptamines
alpha -alkyltryptaminesx -DALT x -DET x -DiPT x -DMT
4,5-DHP-DMT 2,N,N-TMT 4-AcO-DMT 4-HO-5-MeO-DMT 4,N,N-TMT 4-Propionyloxy-DMT 5,6-diBr-DMT 5-AcO-DMT 5-Bromo-DMT 5-MeO-2,N ,N -TMT 5-MeO-4,N ,N -TMT 5-MeO-α,N,N-TMT 5-MeO-DMT 5-N ,N -TMT 7,N,N-TMT α,N,N-TMT (Bufotenin) 5-HO-DMT DMT Norbaeocystin (Psilocin) 4-HO-DMT (Psilocybin) 4-PO-DMT x -DPT Ibogaine-related x -MET x -MiPT Others
Others
Dissociatives (NMDAR antagonists )
Deliriants (mAChR antagonists ) Others
H1
Agonists Antagonists
Others: Atypical antipsychotics (e.g., aripiprazole , asenapine , brexpiprazole , brilaroxazine , clozapine , iloperidone , olanzapine , paliperidone , quetiapine , risperidone , ziprasidone , zotepine )Phenylpiperazine antidepressants (e.g., hydroxynefazodone , nefazodone , trazodone , triazoledione )Tetracyclic antidepressants (e.g., amoxapine , loxapine , maprotiline , mianserin , mirtazapine , oxaprotiline )Tricyclic antidepressants (e.g., amitriptyline , butriptyline , clomipramine , desipramine , dosulepin (dothiepin) , doxepin , imipramine , iprindole , lofepramine , nortriptyline , protriptyline , trimipramine )Typical antipsychotics (e.g., chlorpromazine , flupenthixol , fluphenazine , loxapine , perphenazine , prochlorperazine , thioridazine , thiothixene )
H2
H3
H4
DAT Tooltip Dopamine transporter (DRIs Tooltip Dopamine reuptake inhibitors )
NET Tooltip Norepinephrine transporter (NRIs Tooltip Norepinephrine reuptake inhibitors )
Others: Antihistamines (e.g., brompheniramine , chlorphenamine , pheniramine , tripelennamine )Antipsychotics (e.g., loxapine , ziprasidone )Arylcyclohexylamines (e.g., ketamine , phencyclidine )Dopexamine Ephenidine Ginkgo biloba Indeloxazine Nefazodone Opioids (e.g., desmetramadol , methadone , pethidine (meperidine) , tapentadol , tramadol , levorphanol )
SERT Tooltip Serotonin transporter (SRIs Tooltip Serotonin reuptake inhibitors )
Others: A-80426 Amoxapine Antihistamines (e.g., brompheniramine , chlorphenamine , dimenhydrinate , diphenhydramine , mepyramine (pyrilamine) , pheniramine , tripelennamine )Antipsychotics (e.g., loxapine , ziprasidone )Arylcyclohexylamines (e.g., 3-MeO-PCP , esketamine , ketamine , methoxetamine , phencyclidine )Cyclobenzaprine Delucemine Dextromethorphan Dextrorphan Efavirenz Hypidone Medifoxamine Mesembrine Mifepristone MIN-117 (WF-516) N-Me-5-HT Opioids (e.g., dextropropoxyphene , methadone , pethidine (meperidine) , levorphanol , tapentadol , tramadol )Roxindole
VMATs Tooltip Vesicular monoamine transporters Others
mAChRs Tooltip Muscarinic acetylcholine receptors
Agonists Antagonists
3-Quinuclidinyl benzilate 4-DAMP Aclidinium bromide (+formoterol )Abediterol AF-DX 250 AF-DX 384 Ambutonium bromide Anisodamine Anisodine Antihistamines (first-generation) (e.g., brompheniramine , buclizine , captodiame , chlorphenamine (chlorpheniramine) , cinnarizine , clemastine , cyproheptadine , dimenhydrinate , dimetindene , diphenhydramine , doxylamine , meclizine , mequitazine , perlapine , phenindamine , pheniramine , phenyltoloxamine , promethazine , propiomazine , triprolidine )AQ-RA 741 Atropine Atropine methonitrate Atypical antipsychotics (e.g., clozapine , fluperlapine , olanzapine (+fluoxetine ), rilapine , quetiapine , tenilapine , zotepine )Benactyzine Benzatropine (benztropine) Benzilone Benzilylcholine mustard Benzydamine Bevonium BIBN 99 Biperiden Bornaprine Camylofin CAR-226,086 CAR-301,060 CAR-302,196 CAR-302,282 CAR-302,368 CAR-302,537 CAR-302,668 Caramiphen Cimetropium bromide Clidinium bromide Cloperastine CS-27349 Cyclobenzaprine Cyclopentolate Darifenacin DAU-5884 Desfesoterodine Dexetimide DIBD Dicycloverine (dicyclomine) Dihexyverine Difemerine Diphemanil metilsulfate Ditran Drofenine EA-3167 EA-3443 EA-3580 EA-3834 Emepronium bromide Etanautine Etybenzatropine (ethybenztropine) Fenpiverinium Fentonium bromide Fesoterodine Flavoxate Glycopyrronium bromide (+beclometasone/formoterol , +indacaterol , +neostigmine )Hexahydrodifenidol Hexahydrosiladifenidol Hexbutinol Hexocyclium Himbacine HL-031,120 Homatropine Imidafenacin Ipratropium bromide (+salbutamol )Isopropamide J-104,129 Hyoscyamine Mamba toxin 3 Mamba toxin 7 Mazaticol Mebeverine Meladrazine Mepenzolate Methantheline Methoctramine Methylatropine Methylhomatropine Methylscopolamine Metixene Muscarinic toxin 7 N-Ethyl-3-piperidyl benzilate N-Methyl-3-piperidyl benzilate Nefopam Octatropine methylbromide (anisotropine methylbromide) Orphenadrine Otenzepad (AF-DX 116) Otilonium bromide Oxapium iodide Oxitropium bromide Oxybutynin Oxyphencyclimine Oxyphenonium bromide PBID PD-102,807 PD-0298029 Penthienate Pethidine pFHHSiD Phenglutarimide Phenyltoloxamine Pipenzolate bromide Piperidolate Pirenzepine Piroheptine Pizotifen Poldine Pridinol Prifinium bromide Procyclidine Profenamine (ethopropazine) Propantheline bromide Propiverine Quinidine 3-Quinuclidinyl thiochromane-4-carboxylate Revefenacin Rociverine RU-47,213 SCH-57,790 SCH-72,788 SCH-217,443 Scopolamine (hyoscine) Scopolamine butylbromide (hyoscine butylbromide) Silahexacyclium Sofpironium bromide Solifenacin SSRIs Tooltip Selective serotonin reuptake inhibitors (e.g., femoxetine , paroxetine )Telenzepine Terodiline Tetracyclic antidepressants (e.g., amoxapine , maprotiline , mianserin , mirtazapine )Tiemonium iodide Timepidium bromide Tiotropium bromide Tiquizium bromide Tofenacin Tolterodine Tricyclic antidepressants (e.g., amitriptyline (+perphenazine ), amitriptylinoxide , butriptyline , cidoxepin , clomipramine , desipramine , desmethyldesipramine , dibenzepin , dosulepin (dothiepin) , doxepin , imipramine , lofepramine , nitroxazepine , northiaden (desmethyldosulepin) , nortriptyline , protriptyline , quinupramine , trimipramine )Tridihexethyl Trihexyphenidyl Trimebutine Tripitamine (tripitramine) Tropacine Tropatepine Tropicamide Trospium chloride Typical antipsychotics (e.g., chlorpromazine , chlorprothixene , cyamemazine (cyamepromazine) , loxapine , mesoridazine , thioridazine )Umeclidinium bromide (+vilanterol )WIN-2299 Xanomeline Zamifenacin
Precursors (and prodrugs )