Aripiprazole: Difference between revisions
MaynardClark (talk | contribs) m →Bipolar disorder: "stabilize" becomes "stabilizer" |
→Research: actual fucking chemistry knowledge. |
||
| Line 450: | Line 450: | ||
==Research== |
==Research== |
||
Perhaps owing to its mechanism of action relating to dopamine receptors, there is some evidence to suggest that aripiprazole blocks cocaine-seeking behavior in animal models without significantly affecting other rewarding behaviors (such as food self-administration).<ref>'Aripiprazole Blocks Reinstatement of Cocaine Seeking in an Animal Model of Relapse' Biological Psychiatry. Volume 61, Issue 5, Pages 582-590 (1 March 2007)http://www.journals.elsevierhealth.com/periodicals/bps/article/S0006-3223%2806%2900484-7/abstract</ref> The book ''Addiction Medicine'' mentions studies that suggest aripiprazole would be counter-therapeutic as treatment for methamphetamine dependency because it increased methamphetamine's stimulant and euphoric effects, and increased the baseline level of desire for methamphetamine.<ref>'Addiction Medicine:Science and Practice. Author: Bankole A. Johnson. url=http://books.google.com.br/books?id=zvbr4Zn9S9MC&pg=PA145&lpg=PA145&dq=dissulfiram+stop+craving+for+amphetamines&source=bl&ots=PkBZ_o4vXK&sig=J22yh5pVYFggqKMRaiJE0Ejmmgg&hl=pt-BR&ei=7v-xToz9LsLFgAeM3r2kAQ&sa=X&oi=book_result&ct=result&resnum=2&ved=0CC0Q6AEwAQ#v=onepage&q&f=false</ref> |
Perhaps owing to its mechanism of action relating to dopamine receptors, there is some evidence to suggest that aripiprazole blocks cocaine-seeking behavior in animal models without significantly affecting other rewarding behaviors (such as food self-administration).<ref>'Aripiprazole Blocks Reinstatement of Cocaine Seeking in an Animal Model of Relapse' Biological Psychiatry. Volume 61, Issue 5, Pages 582-590 (1 March 2007)http://www.journals.elsevierhealth.com/periodicals/bps/article/S0006-3223%2806%2900484-7/abstract</ref> The book ''Addiction Medicine'' mentions studies that suggest aripiprazole would be counter-therapeutic as treatment for methamphetamine dependency because it increased methamphetamine's stimulant and euphoric effects, and increased the baseline level of desire for methamphetamine.<ref>'Addiction Medicine:Science and Practice. Author: Bankole A. Johnson. url=http://books.google.com.br/books?id=zvbr4Zn9S9MC&pg=PA145&lpg=PA145&dq=dissulfiram+stop+craving+for+amphetamines&source=bl&ots=PkBZ_o4vXK&sig=J22yh5pVYFggqKMRaiJE0Ejmmgg&hl=pt-BR&ei=7v-xToz9LsLFgAeM3r2kAQ&sa=X&oi=book_result&ct=result&resnum=2&ved=0CC0Q6AEwAQ#v=onepage&q&f=false</ref> |
||
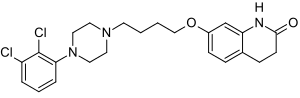
Also, 7-{4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy}-3,4-dihydroquinolin-2(1H)-one, as well as 4-[2-[4-(6-fluorobenzo[d]isoxazol-3-yl)- |
|||
1-piperidyl]ethyl]-3-methyl- |
|||
2,6-diazabicyclo[4.4.0]deca-1,3-dien-5-one, and 4-[4-(4-Chlorophenyl)-4-hydroxy-1-piperidyl]-1-(4-fluorophenyl)-butan-1-one, and |
|||
5-(2-chlorophenyl)-7-nitro-2,3-dihydro-1,4-benzodiazepin-2-one, |
|||
which may or may not be quite identical to abilify, Risperdal, haloperidol, and clonapin, |
|||
are definitely tools of an attempted series of war crimes [punishable under the Geneva conventions,]. anybody who knows anything about chemistry will be able to tell you this. |
|||
== References == |
== References == |
||
Revision as of 05:27, 7 April 2014
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Abilify |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603012 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral (via tablets, orodispersable tablets, and oral solution); intramuscular (including as a depot) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 87%[2][3][4][5] |
| Protein binding | >99%[2][3][4][5] |
| Metabolism | Hepatic (liver; mostly via CYP3A4 and CYP2D6[2][3][4][5]) |
| Elimination half-life | 75 hours (active metabolite is 94 hours)[2][3][4][5] |
| Excretion | Renal (27%; <1% unchanged), Faecal (60%; 18% unchanged)[2][3][4][5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.112.532 |
| Chemical and physical data | |
| Formula | C23H27Cl2N3O2 |
| Molar mass | 448.385 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Aripiprazole (/ˌɛər[invalid input: 'ɨ']ˈpɪprəzoʊl/ AIR-i-PIP-rə-zohl; brand names: Abilify, Aripiprex) is a partial dopamine agonist of the second generation (or atypical) class of antipsychotics that is primarily used in the treatment of schizophrenia, bipolar disorder, major depressive disorder (as an adjunct), tic disorders, and irritability associated with autism.[6] It was approved by the U.S. Food and Drug Administration (FDA) for schizophrenia on November 15, 2002 and the European Medicines Agency on 4 June 2004; for acute manic and mixed episodes associated with bipolar disorder on October 1, 2004; as an adjunct for major depressive disorder on November 20, 2007;[7] and to treat irritability in children with autism on 20 November 2009.[8] Likewise it was approved for use as a treatment for schizophrenia by the TGA of Australia in May 2003.[2] Aripiprazole was developed by Otsuka in Japan, and in the United States, Otsuka America markets it jointly with Bristol-Myers Squibb. Some doctors say that, because of promotion by the pharmaceutical industry, second-generation drugs like aripiprazole, which are appropriate for a few serious psychiatric disorders, are prescribed inappropriately for conditions it may not treat effectively, such as anxiety.[9]
Medical uses
Aripiprazole is primarily used for the treatment of schizophrenia or bipolar disorder.[10]
Schizophrenia
There is tentative evidence aripiprazole may be useful in schizophrenia; however, definitive conclusions are difficult to draw as there was a high rate of attrition during trials and there is a lack of outcome data regarding general functioning, behavior, mortality, economic outcomes, or cognitive functioning.[11][12] It is similar to other typical and atypical antipsychotics with respect to benefit.[11] Compared to typical antipsychotics, there is less extrapyramidal side effects, but higher rates of dizziness.[13] With respect to other atypicals, it is difficult to determine differences in adverse effects as data quality is poor.[14] The efficacy and tolerability of aripiprazole was in the middle range of 15 antipsychotics for efficacy, but had superior tolerability when compared to the other antipsychotic drugs (4th lowest SMD for weight gain, 5th lowest OR for extrapyramidal symptoms, lowest SMD for prolactin elevation, 2nd lowest OR for QTc prolongation, 5th lowest OR for sedation).[15] In a large (N=361) 12-week open-label (hence, no definitive conclusions can be drawn from this study alone) clinical trial, significant improvements in verbal memory and fluency were seen in patients with schizophrenia treated with aripiprazole.[16]
Bipolar disorder
When used by itself for bipolar disorder, aripiprazole does not appear to improve symptoms of depression,[17] although it may be useful in preventing mania.[18] Thus, it is often used in combination with an additional mood stabilizer; however, co-administration with a mood stabilizer increases the risk of extrapyramidal side effects.[19]
Major depression
Aripiprazole is an effective adjunct treatment for major depressive disorder. However, there is a greater rate of side effects as an adjunctive therapy (such as weight gain and akathisia).[20][21] Aripiprazole is the most efficacious antipsychotic to alleviate symptoms of treatment-resistant major depressive disorder (although not significantly).[22] Likewise, in a few earlier meta-analyses, similar results were obtained.[23][24] Aripiprazole may pharmacokinetically interact with some antidepressants, especially SSRIs. There are significant interactions with fluoxetine and paroxetine and lesser interactions with sertraline, escitalopram, citalopram and fluvoxamine, which inhibit CYP2D6, for which aripiprazole is a substrate.[2]
Autism
Short-term data (8 weeks) shows reduced irritability, hyperactivity, and stereotypy.[25] Adverse effects included weight gain, sleepiness, drooling and tremors.[25] Long-term outcomes are not clear.[25]
Regulatory Approval Status
| Regulatory Administration (Country)[26][27][28] | Schizophrenia | Acute Mania | Bipolar Maintenance | Major Depressive Disorder (as an adjunct) | Autism |
|---|---|---|---|---|---|
| Food and Drug Administration (US) | Yes | Yes | Yes (as an adjunct to lithium/valproate) | Yes | Yes |
| Therapeutic Goods Administration (AU) | Yes | Yes (as an adjunct to lithium/valproate) | Yes | No | No |
| Medicines and Healthcare products Regulatory Agency (UK) | Yes | No | Yes (to prevent mania) | No | No |
Side effects
Adverse effect incidences
In Adults[2][3][4][5][29]
- Very Common (>10% incidence) adverse effects
- Weight gain
- Headache
- Agitation
- Insomnia
- Anxiety
- Nausea & vomiting
- Akathisia — a sense of inner restlessness that presents itself with the inability to stay still
- Lightheadedness
- Constipation
- Common (1-10% incidence) adverse effects
- Dizziness
- Dyspepsia — indigestion
- Somnolence — which is usually mild and transient and less severe than that seen with most antipsychotics.[15]
- Fatigue
- Restlessness
- Dry mouth
- Extrapyramidal side effects (e.g. dystonia, parkinsonism, tremor, etc.)
- Orthostatic hypotension
- Musculoskeletal stiffness
- Abdominal discomfort
- Blurred vision
- Cough
- Pain
- Myalgia
- Rash
- Rhinitis
- Uncommon (0.1-1% incidence) adverse effects
- Leukopenia
- Neutropenia
- Thrombocytopenia
- Bradycardia (low heart rate)
- Palpitations
- Orthostatic hypotension
- Dry eye
- Photophobia
- Diplopia
- Eyelid oedema
- Photopsia
- Diarrhoea
- Gastritis
- Dysphagia
- Gastroesophageal reflux disease
- Swollen tongue
- Oesophagitis
- Hypoaesthesia oral
- Face oedema
- Gait disturbance
- Chills
- Discomfort
- Feeling abnormal
- Mobility decreased
- Self-mutilation
- Heart rate increased
- Blood glucose increased
- Pyrexia
- Blood prolactin increased
- Blood urea increased
- Electrocardiogram QT prolonged
- Blood bilirubin increased
- Hepatic enzyme increased
- Increased appetite
- Nocturia
- Polyuria
- Pollakiuria
- Incontinence
- Urinary retention
- Sexual dysfunction
- Amenorrhoea
- Pruritus (itchiness)
- Photosensitivity reaction
- Urticaria
- Rare (<0.1%) adverse effects include
- Neuroleptic malignant syndrome (Combination of fever, muscle stiffness, faster breathing, sweating, reduced consciousness, and sudden change in blood pressure and heart rate)
- Suicidal ideation and behaviour
- Depression
- Painful and/or sustained erection (Priapism)
- Seizures
- Rhabdomyolysis
- Agranulocytosis
- Cardiopulmonary failure
- Myocardial infarction (heart attack)
- Atrial flutter
- Supraventricular tachycardia
- Ventricular tachycardia
- Cardio-respiratory arrest
- Atrioventricular block
- Extrasystoles
- Sinus tachycardia
- Atrial fibrillation
- Angina pectoris
- Myocardial ischaemia
- Pancreatitis
- Diabetic ketoacidosis
- Prolonged QT interval (less common than with most other atypical antipsychotic drugs[15])
- Speech disorder
- Electrolyte abnormalities including hyponatraemia, hypokalaemia, hypocalcaemia, etc.
- Thromboembolism
- Hypertension
- Dysphagia
- Oropharyngeal spasm
- Laryngospasm
- Hepatitis
- Jaundice
- Hypersalivation
- Chest pain
- Urinary retention or incontinence
- Alopecia (hair loss)
- Photosensitivity reaction
- Rash
- Xerostomia (when given by injection)
- Tardive dyskinesia (As with all antipsychotic medication, patients using aripiprazole may develop the permanent neurological disorder tardive dyskinesia.[30][31][32])
- Stroke
- Transient Ischaemic Attack
- Increased body temperature
- Angioedema
- Cardiorespiratory arrest
- Cardiorespiratory failure
Sudden unexplained death has been reported, however the frequency is unknown.[33]
- Common in children
- Feeling sleepy
- Headache
- Vomiting
- Fatigue
- Increased appetite
- Insomnia
- Nausea
- Stuffy nose
- Weight gain
- Uncontrolled movement such as restlessness, tremor muscle stiffness [34]
Discontinuation
Aripiprazole should be discontinued gradually, with careful consideration from the prescribing doctor, to avoid withdrawal symptoms or relapse.
The British National Formulary recommends a gradual withdrawal when discontinuing anti-psychotic treatment to avoid acute withdrawal syndrome or rapid relapse.[35] Due to compensatory changes at dopamine, serotonin, adrenergic and histamine receptor sites in the central nervous system, withdrawal symptoms can occur during abrupt or over-rapid reduction in dosage. Withdrawal symptoms reported to occur after discontinuation of antipsychotics include nausea, emesis, lightheadedness, diaphoresis, dyskinesia, orthostatic hypotension, tachycardia, rhabdomyolysis, nervousness, dizziness, headache, excessive non-stop crying, and anxiety.[36][37] Some have argued that additional somatic and psychiatric symptoms associated with dopaminergic super-sensitivity, including dyskinesia and acute psychosis, are common features of withdrawal in individuals treated with neuroleptics.[38][39][40][41] This has led some to suggest that the withdrawal process might itself be schizo-mimetic, producing schizophrenia-like symptoms even in previously healthy patients, indicating a possible pharmacological origin of mental illness in a yet unknown percentage of patients currently and previously treated with antipsychotics. This question is unresolved, and remains a highly controversial issue among professionals in the medical and mental health communities, as well the public.[42]
Overdosage
Children or adults who ingested acute overdoses have usually manifested central nervous system depression ranging from mild sedation to coma; serum concentrations of aripiprazole and dehydroaripiprazole in these patients were elevated by up to 3-4 fold over normal therapeutic levels, yet to date no deaths have been recorded.[43]
Drug interactions
Aripiprazole is a substrate of CYP2D6 and CYP3A4. Coadministration with medications that inhibit (e.g. paroxetine, fluoxetine) or induce (e.g. carbamazepine) these metabolic enzymes are known to increase and decrease, respectively, plasma levels of aripiprazole.[44] As such, anyone taking aripiprazole should be aware that their dosage of aripiprazole may need to be decreased.
Aripiprazole may change the subjective effects of alcohol. One study[45] found that aripiprazole increased the sedative effect and reduced the sense of euphoria normally associated with alcohol consumption. However, another alcohol study[46] found that there was no difference in subjective effect between a placebo group and a group taking aripiprazole.
For the purpose of D2 blockage, aripiprazole, a partial agonist on D2 receptor site, should not be used with a full antagonist.[medical citation needed]
Pharmacology
Binding profile
Aripiprazole acts as an antagonist/inverse agonist (unless otherwise noted) of the following receptors and transporters:[47][48][49][50][51][52][53][54]
- 5-HT1A receptor (Ki = 5.6 nM) (partial agonist)
- 5-HT1B receptor (Ki = 832 nM)
- 5-HT1D receptor (Ki = 65.5 nM)
- 5-HT2A receptor (Ki = 8.7 nM)
- 5-HT2B receptor (Ki = 0.36 nM)
- 5-HT2C receptor (Ki = 22.4 nM) (partial agonist)
- 5-HT3 receptor (Ki = 628 nM)
- 5-HT5A receptor (Ki = 1240 nM)
- 5-HT6 receptor (Ki = 642 nM)
- 5-HT7 receptor (Ki = 10 nM) (weak partial agonist)
- D1 receptor (Ki = 1170 nM)
- D2 receptor (Ki = 1.6 nM) (partial agonist)
- D3 receptor (Ki = 5.4 nM) (partial agonist)
- D4 receptor (Ki = 514 nM) (partial agonist)
- D5 receptor (Ki = 2130 nM)
- α1A-adrenergic receptor (Ki = 25.9 nM)
- α1B-adrenergic receptor (Ki = 34.4 nM)
- α2A-adrenergic receptor (Ki= 74.1 nM)
- α2B-adrenergic receptor (Ki= 102 nM)
- α2C-adrenergic receptor (Ki= 37.6 nM)
- β1-adrenergic receptor (Ki = 141 nM)
- β2-adrenergic receptor (Ki = 163 nM)
- H1 receptor (Ki = 27.9 nM)
- M1 receptor (Ki = 6780 nM)
- M2 receptor (Ki = 3510 nM)
- M3 receptor (Ki = 4680 nM)
- M4 receptor (Ki = 1520 nM)
- M5 receptor (Ki = 2330 nM)
- SERT (Ki = 1080 nM)
- NET (Ki = 2090 nM)
- DAT (Ki = 3220 nM)
Aripiprazole's mechanism of action is different from those of the other FDA-approved atypical antipsychotics (e.g., clozapine, olanzapine, quetiapine, ziprasidone, and risperidone). Rather than antagonizing the D2 receptor, aripiprazole acts as a D2 partial agonist.[55][56] Aripiprazole is also a partial agonist at the 5-HT1A receptor, and like the other atypical antipsychotics displays an antagonist profile at the 5-HT2A receptor.[57][58] It also antagonizes the 5-HT7 receptor and acts as a partial agonist at the 5-HT2C receptor, both with high affinity. The latter action may underlie the minimal weight gain seen in the course of therapy.[59] Aripiprazole has moderate affinity for histamine, α-adrenergic, and D4 receptors as well as the serotonin transporter, while it has no appreciable affinity for cholinergic muscarinic receptors.[48]
D2 and D3 receptor occupancy levels are high, with average levels ranging between ~71% at 2 mg/day to ~96% at 40 mg/day.[60][61] Most atypical antipsychotics bind preferentially to extrastriatal receptors, but aripiprazole appears to be less preferential in this regard, as binding rates are high throughout the brain.[62]
Recently, it has been demonstrated that in 5-HT7 receptor knockout mice, aripiprazole does not reduce immobility time in the forced swim test (FST), and actually increases it.[63][64] This implicates 5-HT7 antagonism as playing a major role in aripiprazole's antidepressant effects, similarly to amisulpride.[63][64][65] Note, however, humans possess a splice variant not found in other mammals (the "d" isoform), while mice possess one not found in humans (the "c"). The significantly altered c-terminus observed in 5-HT7(d) results in a similar binding affinity to the other forms of this receptor, however, the "c" variant found in other mammals differs in affinity. This difference in expression means the receptor's function in modulating thalamic and hypothalamic output, and corresponding effect on fatigue perception and alertness may not be homologous in mice and humans.
Aripiprazole produces 2,3-dichlorophenylpiperazine (DCPP) as a metabolite in a similar fashion to the reactions of trazodone and nefazodone to give 3-chlorophenylpiperazine (mCPP) and the conversion of niaprazine to 4-fluorophenylpiperazine (pFPP).[66] It is unknown whether DCPP contributes to aripiprazole's pharmacology.[citation needed]
Pharmacokinetics
Aripiprazole displays linear kinetics and has an elimination half-life of approximately 75 hours. Steady-state plasma concentrations are achieved in about 14 days. Cmax (maximum plasma concentration) is achieved 3–5 hours after oral dosing. Bioavailability of the oral tablets is about 90% and the drug undergoes extensive hepatic metabolization (dehydrogenation, hydroxylation, and N-dealkylation), principally by the enzymes CYP2D6 and CYP3A4. Its only known active metabolite is dehydro-aripiprazole, which typically accumulates to approximately 40% of the aripiprazole concentration. The parenteral drug is excreted only in traces, and its metabolites, active or not, are excreted via feces and urine.[48] When dosed daily, brain concentrations of aripiprazole will increase for a period of 10–14 days, before reaching stable constant levels.[citation needed]
Society and culture
Regulator status
In the United States, the FDA has approved aripiprazole for the treatment of schizophrenia in adults and adolescents (aged 13–17), of manic and mixed episodes associated with Bipolar I (One) Disorder with or without psychotic features in adults, children and adolescents (aged 10–17),[67] of irritability associated with autism in pediatric patients (aged 6–17),[68] and of depression when used along with antidepressants in adults.[69]
Aripiprazole is also used off-label for schizophrenia in children (aged 10–12), and to treat dementia-related psychosis in geriatric patients, though Bristol-Myers Squibb was penalized for promoting such uses in the United States.[70]
Aripiprazole has been approved by the FDA for the treatment of acute manic and mixed episodes, in both pediatric patients aged 10–17 and in adults.[71]
In 2007, aripiprazole was approved by the FDA for the treatment of unipolar depression when used adjunctively with an antidepressant medication.[72] It has not been FDA-approved for use as monotherapy in unipolar depression.
Patent status
Otsuka's US patent on aripiprazole expires on October 20, 2014;[73] however, due to a pediatric extension, a generic will not become available until at least April 20, 2015.[71] Barr Laboratories (now Teva Pharmaceuticals) initiated a patent challenge under the Hatch-Waxman Act in March 2007.[74] On November 15, 2010, this challenge was rejected by a United States district court in New Jersey.[1][2]
Dosage forms
- Intramuscular injection, solution: 9.75 mg/mL (1.3 mL)
- Solution, oral: 1 mg/mL (150 mL) [contains propylene glycol, sucrose 400 mg/mL, and fructose 200 mg/mL; orange cream flavor]
- Tablet: 2 mg, 5 mg, 10 mg, 15 mg, 20 mg, 30 mg
- Tablet, orally disintegrating: 10 mg [contains phenylalanine 1.12 mg; creme de vanilla flavor]; 15 mg [contains phenylalanine 1.68 mg; creme de vanilla flavor]
Synthesis
Aripiprazole can be synthesized beginning with a dichloroaniline and bis(2-chloroethyl)amine:[75]
Research
Perhaps owing to its mechanism of action relating to dopamine receptors, there is some evidence to suggest that aripiprazole blocks cocaine-seeking behavior in animal models without significantly affecting other rewarding behaviors (such as food self-administration).[76] The book Addiction Medicine mentions studies that suggest aripiprazole would be counter-therapeutic as treatment for methamphetamine dependency because it increased methamphetamine's stimulant and euphoric effects, and increased the baseline level of desire for methamphetamine.[77]
Also, 7-{4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy}-3,4-dihydroquinolin-2(1H)-one, as well as 4-[2-[4-(6-fluorobenzo[d]isoxazol-3-yl)-
1-piperidyl]ethyl]-3-methyl- 2,6-diazabicyclo[4.4.0]deca-1,3-dien-5-one, and 4-[4-(4-Chlorophenyl)-4-hydroxy-1-piperidyl]-1-(4-fluorophenyl)-butan-1-one, and
5-(2-chlorophenyl)-7-nitro-2,3-dihydro-1,4-benzodiazepin-2-one, which may or may not be quite identical to abilify, Risperdal, haloperidol, and clonapin, are definitely tools of an attempted series of war crimes [punishable under the Geneva conventions,]. anybody who knows anything about chemistry will be able to tell you this.
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ a b c d e f g h "Product Information for ABILIFYTM Aripiprazole Tablets & Orally Disintegrating Tablets". TGA eBusiness Services. Bristol-Myers Squibb Australia Pty Ltd. 1 November 2012. Retrieved 22 October 2013.
- ^ a b c d e f "ABILIFY (aripiprazole) tablet ABILIFY (aripiprazole) solution ABILIFY DISCMELT (aripiprazole) tablet, orally disintegrating ABILIFY (aripiprazole) injection, solution [Otsuka America Pharmaceutical, Inc.]". DailyMed. Otsuka America Pharmaceutical, Inc. April 2013. Retrieved 22 October 2013.
- ^ a b c d e f "Abilify Tablets, Orodispersible Tablets, Oral Solution - Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Otsuka Pharmaceuticals (UK) Ltd. 20 September 2013. Retrieved 22 October 2013.
- ^ a b c d e f "ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS" (PDF). European Medicines Agency. Otsuka Pharmaceutical Europe Ltd. Retrieved 22 October 2013.
- ^ http://www.webmd.com/drugs/drug-64439-Abilify+Oral.aspx?drugid=64439&drugname=Abilify+Oral&source=1
- ^ Hitti, Miranda (20 November 2007). "FDA OKs Abilify for Depression". WebMD. Archived from the original on 5 December 2008. Retrieved 8 December 2008.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Keating, Gina (23 November 2009). "FDA OKs Abilify for child autism irritability". Reuters. Retrieved 22 September 2010.
- ^ A Call for Caution on Antipsychotic Drugs, By RICHARD A. FRIEDMAN, New York Times, September 24, 2012
- ^ "abilify". The American Society of Health-System Pharmacists. Retrieved 3 April 2011.
- ^ a b El-Sayeh, HG; Morganti, C (Apr 19, 2006). "Aripiprazole for schizophrenia". Cochrane database of systematic reviews (Online) (2): CD004578. doi:10.1002/14651858.CD004578.pub3. PMID 16625607.
- ^ Belgamwar, RB; El-Sayeh, HG (Aug 10, 2011). "Aripiprazole versus placebo for schizophrenia". Cochrane database of systematic reviews (Online) (8): CD006622. doi:10.1002/14651858.CD006622.pub2. PMID 21833956.
- ^ Bhattacharjee, J; El-Sayeh, HG (Jan 23, 2008). "Aripiprazole versus typicals for schizophrenia". Cochrane database of systematic reviews (Online) (1): CD006617. doi:10.1002/14651858.CD006617.pub2. PMID 18254107.
- ^ Khanna, P; Komossa, K; Rummel-Kluge, C; Hunger, H; Schwarz, S; El-Sayeh, HG; Leucht, S (Feb 28, 2013). "Aripiprazole versus other atypical antipsychotics for schizophrenia". Cochrane database of systematic reviews (Online). 2: CD006569. doi:10.1002/14651858.CD006569.pub4. PMID 23450570.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Leucht S, Cipriani A, Spineli L, Mavridis D, Örey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. The Lancet [Internet]. 2013 Jun 27 [cited 2013 Sep 17];382(9896) 951–62. Available from: http://www.sciencedirect.com/science/article/pii/S0140673613607333 Cite error: The named reference "Lancet" was defined multiple times with different content (see the help page).
- ^ Bervoets, C; Morrens, M; Vansteelandt, K; Kok, F; de Patoul, A; Halkin, V; Pitsi, D; Constant, E; Peuskens, J; Sabbe, B (November 2012). "Effect of aripiprazole on verbal memory and fluency in schizophrenic patients : results from the ESCAPE study". CNS Drugs. 26 (11): 975–982. doi:10.1007/s40263-012-0003-4. PMID 23018547.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ De Fruyt, J; Deschepper, E; Audenaert, K; Constant, E; Floris, M; Pitchot, W; Sienaert, P; Souery, D; Claes, S (May 2012). "Second generation antipsychotics in the treatment of bipolar depression: a systematic review and meta-analysis". Journal of psychopharmacology (Oxford, England). 26 (5): 603–17. doi:10.1177/0269881111408461. PMID 21940761.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gitlin, M; Frye, MA (May 2012). "Maintenance therapies in bipolar disorders". Bipolar disorders. 14 Suppl 2: 51–65. doi:10.1111/j.1399-5618.2012.00992.x. PMID 22510036.
- ^ de Bartolomeis, A; Perugi, G (October 2012). "Combination of aripiprazole with mood stabilizers for the treatment of bipolar disorder: from acute mania to long-term maintenance". Expert opinion on pharmacotherapy. 13 (14): 2027–36. doi:10.1517/14656566.2012.719876. PMID 22946707.
- ^ Komossa, K; Depping, AM; Gaudchau, A; Kissling, W; Leucht, S (Dec 8, 2010). "Second-generation antipsychotics for major depressive disorder and dysthymia". Cochrane database of systematic reviews (Online) (12): CD008121. doi:10.1002/14651858.CD008121.pub2. PMID 21154393.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Spielmans, GI; Berman MI; Linardatos E; Rosenlicht NZ; Perry A; Tsai AC (Mar 12, 2013). "Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes". Cochrane database of systematic reviews (Online). 10 (3): CD008121. doi:10.1371/journal.pmed.1001403. PMID 23554581.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Spielmans GI, Berman MI, Linardatos E, Rosenlicht NZ, Perry A, Tsai AC. Adjunctive Atypical Antipsychotic Treatment for Major Depressive Disorder: A Meta-Analysis of Depression, Quality of Life, and Safety Outcomes. PLoS Med [Internet]. 2013 Mar [cited 2013 Sep 17];10(3). Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3595214/
- ^ Nelson MD, Papakostas MD. Atypical Antipsychotic Augmentation in Major Depressive Disorder: A Meta-Analysis of Placebo-Controlled Randomized Trials. Am J Psychiatry [Internet]. 2009 Sep 1 [cited 2013 Sep 17];166(9) 980–91. Available from: http://dx.doi.org/10.1176/appi.ajp.2009.09030312
- ^ Komossa K, Depping AM, Gaudchau A, Kissling W, Leucht S. Second-generation antipsychotics for major depressive disorder and dysthymia. Cochrane Database of Systematic Reviews [Internet]. John Wiley & Sons, Ltd; 2010 [cited 2013 Sep 17]. Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD008121.pub2/abstract
- ^ a b c Ching, H; Pringsheim, T (May 16, 2012). "Aripiprazole for autism spectrum disorders (ASD)". Cochrane database of systematic reviews (Online). 5: CD009043. doi:10.1002/14651858.CD009043.pub2. PMID 22592735.
- ^ Joint Formulary Committee. British National Formulary (BNF) 65. Pharmaceutical Pr; 2013.
- ^ Australian Medicines Handbook 2013 [Internet]. [cited 2013 Sep 30]. Available from: http://www.psa.org.au/shop/amh
- ^ Truven Health Analytics, Inc. DRUGDEX® System (Internet) [cited 2013 Jun 25]. Greenwood Village, CO: Thomsen Healthcare; 2013.
- ^ "Abilify Discmelt, Abilify Maintena (aripiprazole) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 22 October 2013.
- ^ Abbasian C, Power P (March 2009). "A case of aripiprazole and tardive dyskinesia". J Psychopharmacol (Oxford). 23 (2): 214–5. doi:10.1177/0269881108089591. PMID 18515468.
- ^ Zaidi SH, Faruqui RA (January 2008). "Aripiprazole is associated with early onset of Tardive Dyskinesia like presentation in a patient with ABI and psychosis". Brain Inj. 22 (1): 99–102. doi:10.1080/02699050701822493. PMID 18183513.
- ^ Maytal G, Ostacher M, Stern TA (June 2006). "Aripiprazole-related tardive dyskinesia". CNS Spectr. 11 (6): 435–9. PMID 16816781.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ http://www.medicines.org.uk/EMC/pdfviewer.aspx?isAttachment=true&documentid=16161
- ^ "ABILIFY (aripiprazole) [package insert]". Otsuka Pharmaceutical Co, Ltd. Retrieved 18 October 2012.
- ^ Group, BMJ, ed. (March 2009). "4.2.1". British National Formulary (57 ed.). United Kingdom: Royal Pharmaceutical Society of Great Britain. p. 192. ISBN 978-0-85369-845-6.
Withdrawal of antipsychotic drugs after long-term therapy should always be gradual and closely monitored to avoid the risk of acute withdrawal syndromes or rapid relapse.
{{cite book}}:|editor1-last=has generic name (help) - ^ Kim, DR.; Staab, JP. (May 2005). "Quetiapine discontinuation syndrome". Am J Psychiatry. 162 (5): 1020. doi:10.1176/appi.ajp.162.5.1020. PMID 15863814.
- ^ Michaelides, C.; Thakore-James, M.; Durso, R. (Jun 2005). "Reversible withdrawal dyskinesia associated with quetiapine". Mov Disord. 20 (6): 769–70. doi:10.1002/mds.20427. PMID 15747370.
- ^ Chouinard, G.; Jones, BD. (Jan 1980). "Neuroleptic-induced supersensitivity psychosis: clinical and pharmacologic characteristics". Am J Psychiatry. 137 (1): 16–21. PMID 6101522.
- ^ Miller, R.; Chouinard, G. (Nov 1993). "Loss of striatal cholinergic neurons as a basis for tardive and L-dopa-induced dyskinesias, neuroleptic-induced supersensitivity psychosis and refractory schizophrenia". Biol Psychiatry. 34 (10): 713–38. doi:10.1016/0006-3223(93)90044-E. PMID 7904833.
- ^ Chouinard, G.; Jones, BD.; Annable, L. (Nov 1978). "Neuroleptic-induced supersensitivity psychosis". Am J Psychiatry. 135 (11): 1409–10. PMID 30291.
- ^ Seeman, P.; Weinshenker, D.; Quiron, R.; Srivastava, LK.; Bhardwaj, SK.; Grandy, DK.; Premont, RT.; Sotnikova, TD.; Boksa, P.; El-Ghundi, M.; O'dowd, BF.; George, SR.; Perreault, ML.; Mannisto, PT; Robinson, S.; Palmiter, RD.; Tallerico, T. (Mar 2005). "Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis". Proc Natl Acad Sci U S A. 102 (9): 3513–8. doi:10.1073/pnas.0409766102. PMC 548961. PMID 15716360.
- ^ Moncrieff, J. (Jul 2006). "Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal-related relapse". Acta Psychiatr Scand. 114 (1): 3–13. doi:10.1111/j.1600-0447.2006.00787.x. PMID 16774655.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 105-106.
- ^ "Abilify (Aripiprazole) - Warnings and Precautions". DrugLib.com. 14 February 2007. Archived from the original on 4 December 2008. Retrieved 8 December 2008.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Kranzler, Henry R.; et al. (2008). "Effects of Aripiprazole on Subjective and Physiological Responses to Alcohol". Alcoholism: Clinical and Experimental Research. 32 (4): 573–579. doi:10.1111/j.1530-0277.2007.00608.x. PMC 3159685. PMID 18261195.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Konstantin Voronin, Patrick Randall, Hugh Myrick, Raymond Anton (2008). "ARIPIPRAZOLE EFFECTS ON ALCOHOL CONSUMPTION AND SUBJECTIVE REPORTS IN A CLINICAL LABORATORY PARADIGM: POSSIBLE INFLUENCE OF SELF-CONTROL". Alcoholism: Clinical and Experimental Research. 32 (11): 1954–1961. doi:10.1111/j.1530-0277.2008.00783.x. PMC 2588475. PMID 18782344.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Starrenburga, F.C.J.; J.P.A.M. Bogersb (April 2009). "How can antipsychotics cause diabetes mellitus? Insights based on receptor-binding profiles, humoral factors and transporter proteins". European Psychiatry. 24 (3): 164–170. doi:10.1016/j.eurpsy.2009.01.001. PMID 19285836.
- ^ a b c "Abilify (Aripiprazole) - Clinical Pharmacology". DrugLib.com. 14 February 2007. Retrieved 8 December 2008.
- ^ Brunton, Laurence (2011). Goodman & Gilman's The Pharmacological Basis of Therapeutics 12th Edition. China: McGraw-Hill. pp. 406–410. ISBN 978-0-07-162442-8.
- ^ "PDSP Ki Database". National Institute of Mental Health. Retrieved 30 June 2013.
- ^ Nguyen CT, Rosen JA, Bota RG. Aripiprazole Partial Agonism at 5-HT2C: A Comparison of Weight Gain Associated With Aripiprazole Adjunctive to Antidepressants With High Versus Low Serotonergic Activities. Prim Care Companion CNS Disord [Internet]. 2012 [cited 2013 Jul 22];14(5). Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3583771/
- ^ Newman-Tancredi A, Heusler P, Martel J-C, Ormière A-M, Leduc N, Cussac D. Agonist and antagonist properties of antipsychotics at human dopamine D4.4 receptors: G-protein activation and K+ channel modulation in transfected cells. The International Journal of Neuropsychopharmacology. 2008;11(03) 293–307. Available from: http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=1819032
- ^ Burstein ES, Ma J, Wong S, Gao Y, Pham E, Knapp AE, et al. Intrinsic Efficacy of Antipsychotics at Human D2, D3, and D4 Dopamine Receptors: Identification of the Clozapine Metabolite N-Desmethylclozapine as a D2/D3 Partial Agonist. J Pharmacol Exp Ther [Internet]. 2005 Dec 1 [cited 2013 Jul 22];315(3) 1278–87. Available from: http://jpet.aspetjournals.org/content/315/3/1278.full.pdf
- ^ Davies MA, Sheffler DJ, Roth BL. Aripiprazole: A Novel Atypical Antipsychotic Drug With a Uniquely Robust Pharmacology. CNS Drug Reviews [Internet]. 2004 [cited 2013 Jul 22];10(4) 317–36. Available from: http://onlinelibrary.wiley.com/doi/10.1111/j.1527-3458.2004.tb00030.x/pdf
- ^ Lawler CP; et al. (1999). "Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes". Neuropsychopharmacology. 20 (6): 612–27. doi:10.1016/S0893-133X(98)00099-2. PMID 10327430.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Burstein ES, Ma J, Wong S, Gao Y, Pham E, Knapp AE, Nash NR, Olsson R, Davis RE, Hacksell U, Weiner DM, Brann MR (December 2005). "Intrinsic Efficacy of Antipsychotics at Human D2, D3, and D4 Dopamine Receptors: Identification of the Clozapine Metabolite N-Desmethylclozapine as a D2/D3 Partial Agonist". J Pharmacol Exp Ther. 315 (3): 1278–87. doi:10.1124/jpet.105.092155. PMID 16135699.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jordan, S; et al. (2002). "The antipsychotic aripiprazole is a potent, partial agonist at the human 5-HT1A receptor". Eur J Pharmacol. 441 (3): 137–140. doi:10.1016/S0014-2999(02)01532-7. PMID 12063084.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Shapiro, DA; et al. (2003). "Aripiprazole, A Novel Atypical Antipsychotic Drug with a Unique and Robust Pharmacology". Neuropsychopharmacology. 28 (8): 1400–1411. doi:10.1038/sj.npp.1300203. PMID 12784105.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Zhang JY, Kowal DM, Nawoschik SP, Lou Z, Dunlop J (February 2006). "Distinct functional profiles of aripiprazole and olanzapine at RNA edited human 5-HT2C receptor isoforms". Biochem Pharmacol. 71 (4): 521–9. doi:10.1016/j.bcp.2005.11.007. PMID 16336943.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kegeles, LS; et al. (2008). "Dose–Occupancy Study of Striatal and Extrastriatal Dopamine D2 Receptors by Aripiprazole in Schizophrenia with PET and [18F]Fallypride". Neuropsychopharmacology. 33 (13): 3111–3125. doi:10.1038/npp.2008.33. PMID 18418366.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Yokoi F, Gründer G, Biziere K, Stephane M, Dogan AS, Dannals RF, Ravert H, Suri A, Bramer S, Wong DF (August 2002). "Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride". Neuropsychopharmacology. 27 (2): 248–59. doi:10.1016/S0893-133X(02)00304-4. PMID 12093598.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "In This Issue". Am J Psychiatry. 165 (8): A46. August 2008. doi:10.1176/appi.ajp.2008.165.8.A46.
- ^ a b Hedlund PB (October 2009). "The 5-HT7 receptor and disorders of the nervous system: an overview". Psychopharmacology. 206 (3): 345–54. doi:10.1007/s00213-009-1626-0. PMC 2841472. PMID 19649616.
- ^ a b Sarkisyan G, Roberts AJ, Hedlund PB (January 2010). "The 5-HT7 receptor as a mediator and modulator of antidepressant-like behavior". Behavioural Brain Research. 209 (1): 99–108. doi:10.1016/j.bbr.2010.01.022. PMC 2832919. PMID 20097233.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Abbas AI, Hedlund PB, Huang XP, Tran TB, Meltzer HY, Roth BL (July 2009). "Amisulpride is a potent 5-HT7 antagonist: relevance for antidepressant actions in vivo". Psychopharmacology. 205 (1): 119–28. doi:10.1007/s00213-009-1521-8. PMC 2821721. PMID 19337725.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Caccia S (August 2007). "N-dealkylation of arylpiperazine derivatives: disposition and metabolism of the 1-aryl-piperazines formed". Current Drug Metabolism. 8 (6): 612–22. doi:10.2174/138920007781368908. PMID 17691920.
- ^ "Abilify Receives Approval for Expanded Use in Children, Teens". Psych Central. Retrieved 2012-07-16.
- ^ "Abilify Gets FDA Approval For Autism Irritability". Furious Seasons. Retrieved 2012-07-16.
- ^ "FDA OKs Abilify for Depression : Antipsychotic Drug Approved for Use in Addition to Antidepressants for Treating Depression". WebMD. Retrieved 2012-07-16.
- ^ "Bristol-Myers Squibb to Pay More Than $515 Million to Resolve Allegations of Illegal Drug Marketing and Pricing". US Department of Justice. Retrieved 2012-07-16.
- ^ a b "Patent and Exclusivity Search Results". Electronic Orange Book. US Food and Drug Administration. Retrieved 8 December 2008.
- ^ http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021436s21,021713s16,021729s8,021866s8lbl.pdfSection 2.3 pp 7-8
- ^ US 5006528, Oshiro, Yasuo; Sato, Seiji & Kurahashi, Nobuyuki, "Carbostyril derivatives", published October 20, 1989
- ^ "Barr Confirms Filing an Application with a Paragraph IV Certification for ABILIFY(R) Tablets" (Press release). Barr Pharmaceuticals, Inc. 2007-03-20. Retrieved 2008-12-23.
- ^ U.S. patent 5,006,528
- ^ 'Aripiprazole Blocks Reinstatement of Cocaine Seeking in an Animal Model of Relapse' Biological Psychiatry. Volume 61, Issue 5, Pages 582-590 (1 March 2007)http://www.journals.elsevierhealth.com/periodicals/bps/article/S0006-3223%2806%2900484-7/abstract
- ^ 'Addiction Medicine:Science and Practice. Author: Bankole A. Johnson. url=http://books.google.com.br/books?id=zvbr4Zn9S9MC&pg=PA145&lpg=PA145&dq=dissulfiram+stop+craving+for+amphetamines&source=bl&ots=PkBZ_o4vXK&sig=J22yh5pVYFggqKMRaiJE0Ejmmgg&hl=pt-BR&ei=7v-xToz9LsLFgAeM3r2kAQ&sa=X&oi=book_result&ct=result&resnum=2&ved=0CC0Q6AEwAQ#v=onepage&q&f=false
Aripiprazole-induced oculogyric crisis (acute dystonia).Jyotik T Bhachech.Journal of Pharmacology and Pharmacotherapeutics. Jul-Sept 2012,3:279-81.http://www.jpharmacol.com/article.asp?issn=0976-500X;year=2012;volume=3;issue=3;spage=279;epage=281;aulast=Bhachech;type=0

