CD74
HLA class II histocompatibility antigen gamma chain also known as HLA-DR antigens-associated invariant chain or CD74 (Cluster of Differentiation 74), is a protein that in humans is encoded by the CD74 gene.[5][6] The invariant chain (Abbreviated Ii) is a polypeptide involved in the formation and transport of MHC class II protein.[7] The cell surface form of the invariant chain is known as CD74.
Function
The nascent MHC class II protein in the rough ER binds a segment of the invariant chain (Ii; a trimer) in order to shape the peptide binding groove and prevent formation of a closed conformation. Binding to Ii might also prevent binding of peptides from the endogenous pathway to the groove of MHC class II. The invariant chain also facilitates MHC class II's export from the ER in a vesicle. The signal for endosomal targeting resides in the cytoplasmic tail of the invariant chain. This fuses with a late endosome containing the endocytosed proteins. It is then cleaved by cathepsin S (cathepsin L in cortical thymic epithelial cells), leaving only a small fragment called CLIP which blocks peptide binding until HLA-DM interacts with MHC II, releasing CLIP and allowing other peptides to bind. The stable MHC class-II is then presented on the cell surface.
Clinical significance
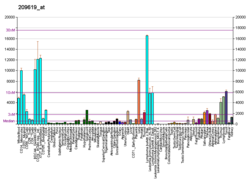
Found on a number of cancer cell types. Possible cancer therapy target. See Milatuzumab#CD74
Possible interactions
In limited cases, CD74 might interact with Macrophage migration inhibitory factor.[8]
See also
- Cluster of differentiation
- Milatuzumab the first Mab to target CD74
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000019582 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000024610 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Claesson L, Larhammar D, Rask L, Peterson PA (December 1983). "cDNA clone for the human invariant gamma chain of class II histocompatibility antigens and its implications for the protein structure". Proc. Natl. Acad. Sci. U.S.A. 80 (24): 7395–9. doi:10.1073/pnas.80.24.7395. PMC 389957. PMID 6324166.
- ^ Kudo J, Chao LY, Narni F, Saunders GF (December 1985). "Structure of the human gene encoding the invariant gamma-chain of class II histocompatibility antigens". Nucleic Acids Res. 13 (24): 8827–41. doi:10.1093/nar/13.24.8827. PMC 318954. PMID 3001652.
- ^ Cresswell P (1994). "Assembly, transport, and function of MHC class II molecules". Annu. Rev. Immunol. 12: 259–93. doi:10.1146/annurev.iy.12.040194.001355. PMID 8011283.
- ^
Shan ZX, Lin QX, Deng CY, Tan HH, Kuang SJ, Xiao DZ, Zhu JN, Fu YH, Yu XY (December 2009). "[Identification of the interactions between the truncated fragments of macrophage migration inhibitory factor and CD74 using a yeast two-hybrid system]". Nan Fang Yi Ke Da Xue Xue Bao (in Chinese). 29 (12): 2383–6, 2390. PMID 20034881.
Wang F, Shen X, Guo X, Peng Y, Liu Y, Xu S, Yang J (February 2010). "Spinal macrophage migration inhibitory factor contributes to the pathogenesis of inflammatory hyperalgesia in rats". Pain. 148 (2): 275–83. doi:10.1016/j.pain.2009.11.011. PMID 20005040.
Dobson SE, Augustijn KD, Brannigan JA, Schnick C, Janse CJ, Dodson EJ, Waters AP, Wilkinson AJ (December 2009). "The crystal structures of macrophage migration inhibitory factor from Plasmodium falciparum and Plasmodium berghei". Protein Sci. 18 (12): 2578–91. doi:10.1002/pro.263. PMC 2798171. PMID 19827093.
Piette C, Deprez M, Roger T, Noël A, Foidart JM, Munaut C (November 2009). "The Dexamethasone-induced Inhibition of Proliferation, Migration, and Invasion in Glioma Cell Lines Is Antagonized by Macrophage Migration Inhibitory Factor (MIF) and Can Be Enhanced by Specific MIF Inhibitors". J. Biol. Chem. 284 (47): 32483–92. doi:10.1074/jbc.M109.014589. PMC 2781663. PMID 19759012.
{{cite journal}}: CS1 maint: unflagged free DOI (link) Verjans E, Noetzel E, Bektas N, Schütz AK, Lue H, Lennartz B, Hartmann A, Dahl E, Bernhagen J (2009). "Dual role of macrophage migration inhibitory factor (MIF) in human breast cancer". BMC Cancer. 9: 230. doi:10.1186/1471-2407-9-230. PMC 2716369. PMID 19602265.{{cite journal}}: CS1 maint: unflagged free DOI (link)
Further reading
- Stove V, Verhasselt B (2006). "Modelling thymic HIV-1 Nef effects". Curr. HIV Res. 4 (1): 57–64. doi:10.2174/157016206775197583. PMID 16454711.
- Riberdy JM; Newcomb JR; Surman MJ; et al. (1992). "HLA-DR molecules from an antigen-processing mutant cell line are associated with invariant chain peptides". Nature. 360 (6403): 474–7. doi:10.1038/360474a0. PMID 1448172.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - Bakke O, Dobberstein B (1990). "MHC class II-associated invariant chain contains a sorting signal for endosomal compartments". Cell. 63 (4): 707–16. doi:10.1016/0092-8674(90)90137-4. PMID 2121367.
- Marks MS, Blum JS, Cresswell P (1990). "Invariant chain trimers are sequestered in the rough endoplasmic reticulum in the absence of association with HLA class II antigens". J. Cell Biol. 111 (3): 839–55. doi:10.1083/jcb.111.3.839. PMC 2116304. PMID 2391366.
- Spiro RC, Quaranta V (1989). "The invariant chain is a phosphorylated subunit of class II molecules". J. Immunol. 143 (8): 2589–94. PMID 2507633.
- O'Sullivan DM, Noonan D, Quaranta V (1987). "Four Ia invariant chain forms derive from a single gene by alternate splicing and alternate initiation of transcription/translation". J. Exp. Med. 166 (2): 444–60. doi:10.1084/jem.166.2.444. PMC 2189580. PMID 3036998.
- Genuardi M, Saunders GF (1988). "Localization of the HLA class II-associated invariant chain gene to human chromosome band 5q32". Immunogenetics. 28 (1): 53–6. doi:10.1007/BF00372530. PMID 3132422.
- O'Sullivan DM; Larhammar D; Wilson MC; et al. (1986). "Structure of the human Ia-associated invariant (gamma)-chain gene: identification of 5' sequences shared with major histocompatibility complex class II genes". Proc. Natl. Acad. Sci. U.S.A. 83 (12): 4484–8. doi:10.1073/pnas.83.12.4484. PMC 323758. PMID 3459184.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - Koch N, Hämmerling GJ (1986). "Ia-associated invariant chain is fatty acylated before addition of sialic acid". Biochemistry. 24 (22): 6185–90. doi:10.1021/bi00343a023. PMID 3866610.
- Claesson L, Peterson PA (1983). "Association of human gamma chain with class II transplantation antigens during intracellular transport". Biochemistry. 22 (13): 3206–13. doi:10.1021/bi00282a026. PMID 6576808.
- Strubin M, Mach B, Long EO (1984). "The complete sequence of the mRNA for the HLA-DR-associated invariant chain reveals a polypeptide with an unusual transmembrane polarity". EMBO J. 3 (4): 869–72. PMC 557440. PMID 6586420.
- Kvist S; Wiman K; Claesson L; et al. (1982). "Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens". Cell. 29 (1): 61–9. doi:10.1016/0092-8674(82)90090-3. PMID 6955026.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - Machamer CE, Cresswell P (1983). "Biosynthesis and glycosylation of the invariant chain associated with HLA-DR antigens". J. Immunol. 129 (6): 2564–9. PMID 6982931.
- Ghosh P, Amaya M, Mellins E, Wiley DC (1995). "The structure of an intermediate in class II MHC maturation: CLIP bound to HLA-DR3". Nature. 378 (6556): 457–62. doi:10.1038/378457a0. PMID 7477400.
- Bijlmakers MJ, Benaroch P, Ploegh HL (1994). "Mapping functional regions in the lumenal domain of the class II- associated invariant chain". J. Exp. Med. 180 (2): 623–9. doi:10.1084/jem.180.2.623. PMC 2191624. PMID 7519244.
- Brown JH; Jardetzky TS; Gorga JC; et al. (1993). "Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1". Nature. 364 (6432): 33–9. doi:10.1038/364033a0. PMID 8316295.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - Naujokas MF; Morin M; Anderson MS; et al. (1993). "The chondroitin sulfate form of invariant chain can enhance stimulation of T cell responses through interaction with CD44". Cell. 74 (2): 257–68. doi:10.1016/0092-8674(93)90417-O. PMID 8343954.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - Roche PA; Teletski CL; Stang E; et al. (1993). "Cell surface HLA-DR-invariant chain complexes are targeted to endosomes by rapid internalization". Proc. Natl. Acad. Sci. U.S.A. 90 (18): 8581–5. doi:10.1073/pnas.90.18.8581. PMC 47401. PMID 8397411.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help)
External links
- CD74+protein,+human at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Overview at Davidson College (student generated)
- School of Crystallography The Invariant Chain