Cyamemazine: Difference between revisions
No edit summary |
|||
| Line 1: | Line 1: | ||
{{Drugbox |
|||
{{Expand|date=October 2008}} |
|||
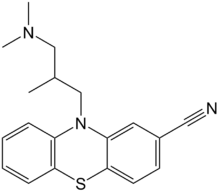
| IUPAC_name = 10-(3-dimethylamino-2-methyl-propyl)phenothiazine-2-carbonitrile |
|||
| image = Cyamemazine.png |
|||
| CAS_number = 3546-03-0 |
|||
| ATC_prefix = |
|||
| ATC_suffix = |
|||
| PubChem = 62865 |
|||
| DrugBank = |
|||
| ChemSpiderID = 56597 |
|||
| chemical_formula = C<sub>19</sub>H<sub>21</sub>N<sub>3</sub>S |
|||
| molecular_weight = 323.46 g/mol |
|||
| bioavailability = |
|||
| protein_bound = |
|||
| metabolism = |
|||
| elimination_half-life = |
|||
| excretion = |
|||
| pregnancy_category = |
|||
| legal_status = Rx-Only |
|||
| routes_of_administration = |
|||
}} |
|||
'''Cyamemazine''' ('''Tercian'''), also known as '''cyamepromazine''', is a [[typical antipsychotic]] [[drug]] of the [[phenothiazine]] [[chemical class|class]] which was introduced by [[Theraplix]] in [[France]] in 1972 and later in [[Portugal]] as well.<ref name="urlIndex nominum, international drug ... - Google Books">{{cite web | url = http://books.google.com/books?id=5GpcTQD_L2oC&lpg=PA280&dq=cyamemazine%20tercian&as_brr=3&pg=PA280#v=onepage&q=&f=false | title = Index nominum, international drug ... - Google Books | format = | work = | accessdate = }}</ref><ref name="isbn0-412-46630-9">{{cite book | author = David J. Triggle | title = Dictionary of Pharmacological Agents | publisher = Chapman & Hall/CRC | location = Boca Raton | year = 1996 | page = 534 | pages = 2700 | isbn = 0-412-46630-9 | oclc = | doi = | url = http://books.google.com/books?id=DeX7jgInYFMC&lpg=RA1-PA534&as_brr=3&pg=RA1-PA534#v=onepage&q=&f=false}}</ref><ref name="urlPharmaceutical manufacturing ... - Google Books">{{cite web | url = http://books.google.com/books?id=X2EyLsG4bcUC&lpg=PA397&dq=cyamemazine%20introduced&as_brr=3&pg=PA397#v=onepage&q=&f=false | title = Pharmaceutical manufacturing ... - Google Books | format = | work = | accessdate = }}</ref><ref name="pmid19393381">{{cite journal | author = Bret P, Bret MC, Queuille E | title = [Prescribing patterns of antipsychotics in 13 French psychiatric hospitals] | language = French | journal = L'Encéphale | volume = 35 | issue = 2 | pages = 129–38 | year = 2009 | month = April | pmid = 19393381 | doi = 10.1016/j.encep.2008.03.007 | url = http://www.masson.fr/masson/S0013-7006(08)00103-6}}</ref> It is used for the treatment of [[schizophrenia]] and, especially, for [[psychosis]]-associated [[anxiety]], due to its unique [[anxiolytic]] efficacy.<ref name="urlStahls Essential Psychopharmacology - Cambridge University Press">{{cite web | url = http://stahlonline.cambridge.org/prescribers_drug.jsf?page=0521683505c20_p115-120.html.therapeutics&name=Cyamemazine | title = Stahl's Essential Psychopharmacology - Cambridge University Press | format = | work = | accessdate = }}</ref><ref name="pmid11423169">{{cite journal | author = Bourin M, Nic Dhonnchadha BA, Claude Colombel M, Dib M, Hascoët M | title = Cyamemazine as an anxiolytic drug on the elevated plus maze and light/dark paradigm in mice | journal = Behavioural Brain Research | volume = 124 | issue = 1 | pages = 87–95 | year = 2001 | month = September | pmid = 11423169 | doi = | url = http://linkinghub.elsevier.com/retrieve/pii/S0166432801002388}}</ref> |
|||
10-(3-Dimethylamino-2-methylpropyl)-phenothiazine-2-carbonitrile. '''Cyamemazine''' is a [[sedative]] with [[antihistaminic]] and [[antispasmodic]] properties.<ref>[http://cancerweb.ncl.ac.uk/cgi-bin/omd?cyamemazine Cancer Web]</ref> |
|||
Cyamemazine differs from other phenothiazine neuroleptics in that aside from the usual profile of [[dopamine receptor|dopamine]], [[Alpha-1 adrenergic receptor|α<sub>1</sub>-adrenergic]], [[H1 receptor|H<sub>1</sub>]], and [[muscarinic acetylcholine receptor|mACh receptor]] [[receptor_antagonist|antagonism]],<ref name="pmid12527336">{{cite journal | author = Hameg A, Bayle F, Nuss P, Dupuis P, Garay RP, Dib M | title = Affinity of cyamemazine, an anxiolytic antipsychotic drug, for human recombinant dopamine vs. serotonin receptor subtypes | journal = Biochemical Pharmacology | volume = 65 | issue = 3 | pages = 435–40 | year = 2003 | month = February | pmid = 12527336 | doi = | url = http://linkinghub.elsevier.com/retrieve/pii/S0006295202015150}}</ref> it additionally produces strong blockade of several [[serotonin receptor]]s, including [[5-HT2A receptor|5-HT<sub>2A</sub>]], [[5-HT2C receptor|5-HT<sub>2C</sub>]], and [[5-HT7 receptor|5-HT<sub>7</sub>]].<ref name="pmid12527336">{{cite journal | author = Hameg A, Bayle F, Nuss P, Dupuis P, Garay RP, Dib M | title = Affinity of cyamemazine, an anxiolytic antipsychotic drug, for human recombinant dopamine vs. serotonin receptor subtypes | journal = Biochemical Pharmacology | volume = 65 | issue = 3 | pages = 435–40 | year = 2003 | month = February | pmid = 12527336 | doi = | url = http://linkinghub.elsevier.com/retrieve/pii/S0006295202015150}}</ref><ref name="pmid10672635">{{cite journal | author = Alvarez-Guerra M, d'Alché-Birée F, Wolf WA, Vargas F, Dib M, Garay RP | title = 5-HT3- and 5-HT2C-antagonist properties of cyamemazine: significance for its clinical anxiolytic activity | journal = Psychopharmacology | volume = 147 | issue = 4 | pages = 412–7 | year = 2000 | month = January | pmid = 10672635 | doi = | url = http://link.springer.de/link/service/journals/00213/bibs/0147004/01470412.htm}}</ref><ref name="pmid12421652">{{cite journal | author = Alvarez-Guerra M, Hameg A, Bayle F, Dib M, Garay RP | title = 5-HT2A receptor antagonist properties of cyamemazine in rat and guinea pig smooth muscle | journal = European Journal of Pharmacology | volume = 454 | issue = 2-3 | pages = 235–9 | year = 2002 | month = November | pmid = 12421652 | doi = | url = http://linkinghub.elsevier.com/retrieve/pii/S0014299902024895}}</ref><ref name="pmid17936750">{{cite journal | author = Benyamina A, Arbus C, Nuss P, Garay RP, Neliat G, Hameg A | title = Affinity of cyamemazine metabolites for serotonin, histamine and dopamine receptor subtypes | journal = European Journal of Pharmacology | volume = 578 | issue = 2-3 | pages = 142–7 | year = 2008 | month = January | pmid = 17936750 | doi = 10.1016/j.ejphar.2007.09.025 | url = http://linkinghub.elsevier.com/retrieve/pii/S0014-2999(07)01053-9}}</ref> These actions have been implicated in cyamemazine's anxiolytic effects and lack of [[extrapyramidal symptom|extrapyramidal]] [[side effect]]s.<ref name="pmid12527336">{{cite journal | author = Hameg A, Bayle F, Nuss P, Dupuis P, Garay RP, Dib M | title = Affinity of cyamemazine, an anxiolytic antipsychotic drug, for human recombinant dopamine vs. serotonin receptor subtypes | journal = Biochemical Pharmacology | volume = 65 | issue = 3 | pages = 435–40 | year = 2003 | month = February | pmid = 12527336 | doi = | url = http://linkinghub.elsevier.com/retrieve/pii/S0006295202015150}}</ref><ref name="pmid10672635">{{cite journal | author = Alvarez-Guerra M, d'Alché-Birée F, Wolf WA, Vargas F, Dib M, Garay RP | title = 5-HT3- and 5-HT2C-antagonist properties of cyamemazine: significance for its clinical anxiolytic activity | journal = Psychopharmacology | volume = 147 | issue = 4 | pages = 412–7 | year = 2000 | month = January | pmid = 10672635 | doi = | url = http://link.springer.de/link/service/journals/00213/bibs/0147004/01470412.htm}}</ref> Despite its classification as a typical antipsychotic, cyamemazine actually behaves like an [[atypical antipsychotic|atypical]].<ref name="pmid12595954">{{cite journal | author = Peinado J, Hameg A, Garay RP, Bayle F, Nuss P, Dib M | title = Reduction of extracellular dopamine and metabolite concentrations in rat striatum by low doses of acute cyamemazine | journal = Naunyn-Schmiedeberg's Archives of Pharmacology | volume = 367 | issue = 2 | pages = 134–9 | year = 2003 | month = February | pmid = 12595954 | doi = 10.1007/s00210-002-0665-4 | url = http://dx.doi.org/10.1007/s00210-002-0665-4}}</ref> |
|||
| ⚫ | |||
<references/> |
|||
== See also == |
|||
| ⚫ | |||
* [[Typical antipsychotic]] |
|||
* [[Phenothiazine]] |
|||
| ⚫ | |||
{{Reflist|2}} |
|||
{{Antipsychotics}} |
|||
{{Adrenergics}} |
|||
{{Cholinergics}} |
|||
{{Dopaminergics}} |
|||
{{Histaminergics}} |
|||
{{Serotonergics}} |
|||
{{Tricyclics}} |
|||
| ⚫ | |||
[[Category:Phenothiazines]] |
|||
[[fr:Cyamémazine]] |
[[fr:Cyamémazine]] |
||
{{pharmacology-stub}} |
|||
Revision as of 20:14, 11 February 2010
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.020.541 |
| Chemical and physical data | |
| Formula | C19H21N3S |
| Molar mass | 323.46 g/mol |
Cyamemazine (Tercian), also known as cyamepromazine, is a typical antipsychotic drug of the phenothiazine class which was introduced by Theraplix in France in 1972 and later in Portugal as well.[1][2][3][4] It is used for the treatment of schizophrenia and, especially, for psychosis-associated anxiety, due to its unique anxiolytic efficacy.[5][6]
Cyamemazine differs from other phenothiazine neuroleptics in that aside from the usual profile of dopamine, α1-adrenergic, H1, and mACh receptor antagonism,[7] it additionally produces strong blockade of several serotonin receptors, including 5-HT2A, 5-HT2C, and 5-HT7.[7][8][9][10] These actions have been implicated in cyamemazine's anxiolytic effects and lack of extrapyramidal side effects.[7][8] Despite its classification as a typical antipsychotic, cyamemazine actually behaves like an atypical.[11]
See also
References
- ^ "Index nominum, international drug ... - Google Books".
- ^ David J. Triggle (1996). Dictionary of Pharmacological Agents. Boca Raton: Chapman & Hall/CRC. p. 534. ISBN 0-412-46630-9.
{{cite book}}: More than one of|pages=and|page=specified (help) - ^ "Pharmaceutical manufacturing ... - Google Books".
- ^ Bret P, Bret MC, Queuille E (2009). "[Prescribing patterns of antipsychotics in 13 French psychiatric hospitals]". L'Encéphale (in French). 35 (2): 129–38. doi:10.1016/j.encep.2008.03.007. PMID 19393381.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Stahl's Essential Psychopharmacology - Cambridge University Press".
- ^ Bourin M, Nic Dhonnchadha BA, Claude Colombel M, Dib M, Hascoët M (2001). "Cyamemazine as an anxiolytic drug on the elevated plus maze and light/dark paradigm in mice". Behavioural Brain Research. 124 (1): 87–95. PMID 11423169.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Hameg A, Bayle F, Nuss P, Dupuis P, Garay RP, Dib M (2003). "Affinity of cyamemazine, an anxiolytic antipsychotic drug, for human recombinant dopamine vs. serotonin receptor subtypes". Biochemical Pharmacology. 65 (3): 435–40. PMID 12527336.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Alvarez-Guerra M, d'Alché-Birée F, Wolf WA, Vargas F, Dib M, Garay RP (2000). "5-HT3- and 5-HT2C-antagonist properties of cyamemazine: significance for its clinical anxiolytic activity". Psychopharmacology. 147 (4): 412–7. PMID 10672635.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Alvarez-Guerra M, Hameg A, Bayle F, Dib M, Garay RP (2002). "5-HT2A receptor antagonist properties of cyamemazine in rat and guinea pig smooth muscle". European Journal of Pharmacology. 454 (2–3): 235–9. PMID 12421652.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Benyamina A, Arbus C, Nuss P, Garay RP, Neliat G, Hameg A (2008). "Affinity of cyamemazine metabolites for serotonin, histamine and dopamine receptor subtypes". European Journal of Pharmacology. 578 (2–3): 142–7. doi:10.1016/j.ejphar.2007.09.025. PMID 17936750.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Peinado J, Hameg A, Garay RP, Bayle F, Nuss P, Dib M (2003). "Reduction of extracellular dopamine and metabolite concentrations in rat striatum by low doses of acute cyamemazine". Naunyn-Schmiedeberg's Archives of Pharmacology. 367 (2): 134–9. doi:10.1007/s00210-002-0665-4. PMID 12595954.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
