Butriptyline
 | |
| Clinical data | |
|---|---|
| Trade names | Evadyne, others |
| Other names | AY-62014[1] |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ?[2] |
| Protein binding | >90%[2] |
| Metabolism | Hepatic (N-demethylation) |
| Metabolites | Norbutriptyline[2] |
| Elimination half-life | 20 hours[2] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H27N |
| Molar mass | 293.446 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Butriptyline, sold under the brand name Evadyne among others, is a tricyclic antidepressant (TCA) that has been used in the United Kingdom and several other European countries for the treatment of depression but appears to no longer be marketed.[1][3][4][5][6] Along with trimipramine, iprindole, and amoxapine, it has been described as an "atypical" or "second-generation" TCA due to its relatively late introduction and atypical pharmacology.[7][8] It was very little-used compared to other TCAs, with the number of prescriptions dispensed only in the thousands.[9]
Medical uses
Butriptyline was used in the treatment of depression.[10] It was usually used at dosages of 150–300 mg/day.[11]
Side effects
Butriptyline is closely related to amitriptyline, and produces similar effects as other TCAs, but its side effects like sedation are said to be reduced in severity and it has a lower risk of interactions with other medications.[5][6][9]
Butriptyline has potent antihistamine effects, resulting in sedation and somnolence.[12] It also has potent anticholinergic effects,[13] resulting in side effects like dry mouth, constipation, urinary retention, blurred vision, and cognitive/memory impairment.[12] The drug has relatively weak effects as an alpha-1 blocker and has no effects as a norepinephrine reuptake inhibitor,[14][15] so is associated with little to no antiadrenergic and adrenergic side effects.[14][13][additional citation(s) needed]
Overdose
Pharmacology
Pharmacodynamics
| Site | Ki (nM) | Species | Ref |
|---|---|---|---|
| SERT | 1,360 4,300 10,000 (IC50) |
Human Rat Rat |
[15] [17] [18] |
| NET | 5,100 990 1,700 (IC50) |
Human Rat Rat |
[15] [17] [18] |
| DAT | 3,940 2,800 5,200 (IC50) |
Human Rat Rat |
[15] [17] [18] |
| 5-HT1A | 7,000 | Human | [19] |
| 5-HT2A | 380 | Human | [19] |
| 5-HT2C | ND | ND | ND |
| α1 | 570 | Human | [14] |
| α2 | 4,800 | Human | [14] |
| D2 | ND | ND | ND |
| H1 | 1.1 | Human | [14] |
| mACh | 35 | Human | [14] |
| Values are Ki (nM), unless otherwise noted. The smaller the value, the more strongly the drug binds to the site. | |||
In vitro, butriptyline is a strong antihistamine and anticholinergic, moderate 5-HT2 and α1-adrenergic receptor antagonist, and very weak or negligible monoamine reuptake inhibitor.[14][19][15][18] These actions appear to confer a profile similar to that of iprindole and trimipramine with serotonin-blocking effects as the apparent predominant mediator of mood-lifting efficacy.[20][18][17]
However, in small clinical trials, using similar doses, butriptyline was found to be similarly effective to amitriptyline and imipramine as an antidepressant, despite the fact that both of these TCAs are far stronger as both 5-HT2 antagonists and serotonin–norepinephrine reuptake inhibitors.[14][19][21] As a result, it may be that butriptyline has a different mechanism of action, or perhaps functions as a prodrug in the body to a metabolite with different pharmacodynamics.
Pharmacokinetics
Therapeutic concentrations of butriptyline are in the range of 60–280 ng/mL (204–954 nmol/L).[22] Its plasma protein binding is greater than 90%.[2]
Chemistry
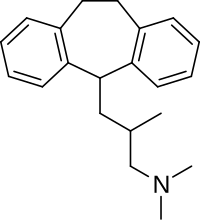
Butriptyline is a tricyclic compound, specifically a dibenzocycloheptadiene, and possesses three rings fused together with a side chain attached in its chemical structure.[23] Other dibenzocycloheptadiene TCAs include amitriptyline, nortriptyline, and protriptyline.[23] Butriptyline is an analogue of amitriptyline with an isobutyl side chain instead of a propylidene side chain.[9][24] It is a tertiary amine TCA, with its side chain-demethylated metabolite norbutriptyline being a secondary amine.[25][26] Other tertiary amine TCAs include amitriptyline, imipramine, clomipramine, dosulepin (dothiepin), doxepin, and trimipramine.[27][28] The chemical name of butriptyline is 3-(10,11-dihydro-5H-dibenzo[a,d]cycloheptene-5-yl)-N,N,2-trimethylpropan-1-amine and its free base form has a chemical formula of C21H27N with a molecular weight of 293.446 g/mol.[1] The drug has been used commercially both as the free base and as the hydrochloride salt.[1][3] The CAS Registry Number of the free base is 15686-37-0 and of the hydrochloride is 5585-73-9.[1][3]
History
Butriptyline was developed by Wyeth and introduced in the United Kingdom in either 1974 or 1975.[4][29][30]
Society and culture
Generic names
Butriptyline is the English and French generic name of the drug and its INN, BAN, and DCF, while butriptyline hydrochloride is its BANM and USAN.[1][3][10] Its generic name in Latin is butriptylinum, in German is butriptylin, and in Spanish is butriptylina.[3]
Brand names
Butriptyline has been marketed under the brand names Evadene, Evadyne, Evasidol, and Centrolese.[1][3][4]
Availability
Butriptyline has been marketed in Europe, including in the United Kingdom, Belgium, Luxembourg, Austria, and Italy.[3][4]
References
- ^ a b c d e f g J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 201–. ISBN 978-1-4757-2085-3.
- ^ a b c d e Florencio Zaragoza Dörwald (4 February 2013). Lead Optimization for Medicinal Chemists: Pharmacokinetic Properties of Functional Groups and Organic Compounds. John Wiley & Sons. pp. 313–. ISBN 978-3-527-64565-7.
- ^ a b c d e f g Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. ISBN 3-88763-075-0.
- ^ a b c d William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia. Elsevier. pp. 777–. ISBN 978-0-8155-1856-3.
- ^ a b Helmut Buschmann (16 April 2007). Antidepressants, Antipsychotics, Anxiolytics: From Chemistry and Pharmacology to Clinical Application. Wiley. pp. 180–. ISBN 978-3-527-31058-6.
- ^ a b Eugene S. Paykel (1992). Handbook of Affective Disorders. Guilford Press. pp. 339–. ISBN 978-0-89862-674-2.
- ^ Seth (18 November 2009). Textbook Of Pharmacology. Elsevier India. pp. 119–. ISBN 978-81-312-1158-8.
- ^ Bhattacharya (2003). Pharmacology, 2/e. Elsevier India. pp. 292–. ISBN 978-81-8147-009-6.
- ^ a b c J. K. Aronson (2009). Meyler's Side Effects of Psychiatric Drugs. Elsevier. pp. 7, 18, 31. ISBN 978-0-444-53266-4.
- ^ a b I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 58–. ISBN 978-94-011-4439-1.
- ^ J. K. Wing; Lorna Wing (29 October 1982). Handbook of Psychiatry: Volume 3, Psychoses of Uncertain Aetiology. CUP Archive. pp. 167–. ISBN 978-0-521-28438-7.
- ^ a b Gillman PK (July 2007). "Tricyclic antidepressant pharmacology and therapeutic drug interactions updated". British Journal of Pharmacology. 151 (6): 737–48. doi:10.1038/sj.bjp.0707253. PMC 2014120. PMID 17471183.
- ^ a b Marco Mumenthaler; P. A. van Zwieten; Jean Marie Farcot (1990). Treatment of Chronic Pain: Possibilities, Limitations, and Long-term Follow-up. CRC Press. pp. 114–. ISBN 978-3-7186-5027-9.
- ^ a b c d e f g h Richelson E, Nelson A (1984). "Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro". J. Pharmacol. Exp. Ther. 230 (1): 94–102. PMID 6086881.
- ^ a b c d e Tatsumi M, Groshan K, Blakely RD, Richelson E (1997). "Pharmacological profile of antidepressants and related compounds at human monoamine transporters". Eur. J. Pharmacol. 340 (2–3): 249–58. doi:10.1016/s0014-2999(97)01393-9. PMID 9537821.
- ^ Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- ^ a b c d Richelson E, Pfenning M (September 1984). "Blockade by antidepressants and related compounds of biogenic amine uptake into rat brain synaptosomes: most antidepressants selectively block norepinephrine uptake". European Journal of Pharmacology. 104 (3–4): 277–86. doi:10.1016/0014-2999(84)90403-5. PMID 6499924.
- ^ a b c d e Randrup A, Braestrup C (August 1977). "Uptake inhibition of biogenic amines by newer antidepressant drugs: relevance to the dopamine hypothesis of depression". Psychopharmacology. 53 (3): 309–14. doi:10.1007/BF00492370. PMID 408861.
- ^ a b c d Wander TJ, Nelson A, Okazaki H, Richelson E (1986). "Antagonism by antidepressants of serotonin S1 and S2 receptors of normal human brain in vitro". Eur. J. Pharmacol. 132 (2–3): 115–21. doi:10.1016/0014-2999(86)90596-0. PMID 3816971.
- ^ Jaramillo J, Greenberg R (February 1975). "Comparative pharmacological studies on butriptyline and some related standard tricyclic antidepressants". Canadian Journal of Physiology and Pharmacology. 53 (1): 104–12. doi:10.1139/y75-014. PMID 166748.
- ^ Yong Zhou (22 October 2013). Drugs in Psychiatric Practice. Elsevier. pp. 194–. ISBN 978-1-4831-9193-5.
- ^ Forensic Science Progress. Springer Science & Business Media. 6 December 2012. pp. 24–. ISBN 978-3-642-73058-0.
- ^ a b Michael S Ritsner (15 February 2013). Polypharmacy in Psychiatry Practice, Volume I: Multiple Medication Use Strategies. Springer Science & Business Media. pp. 270–271. ISBN 978-94-007-5805-6.
- ^ Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 604–. ISBN 978-1-60913-345-0.
- ^ Neal R. Cutler; John J. Sramek; Prem K. Narang (20 September 1994). Pharmacodynamics and Drug Development: Perspectives in Clinical Pharmacology. John Wiley & Sons. pp. 160–. ISBN 978-0-471-95052-3.
- ^ Pavel Anzenbacher; Ulrich M. Zanger (23 February 2012). Metabolism of Drugs and Other Xenobiotics. John Wiley & Sons. pp. 302–. ISBN 978-3-527-64632-6.
- ^ Patricia K. Anthony (2002). Pharmacology Secrets. Elsevier Health Sciences. pp. 39–. ISBN 1-56053-470-2.
- ^ Philip Cowen; Paul Harrison; Tom Burns (9 August 2012). Shorter Oxford Textbook of Psychiatry. OUP Oxford. pp. 532–. ISBN 978-0-19-162675-3.
- ^ K. Ghose (11 November 2013). Antidepressants for Elderly People. Springer. pp. 182–. ISBN 978-1-4899-3436-9.
- ^ Richard C. Dart (2004). Medical Toxicology. Lippincott Williams & Wilkins. pp. 836–. ISBN 978-0-7817-2845-4.
