Transferrin receptor 1
Template:PBB Transferrin receptor protein 1 (TfR1), also known as Cluster of Differentiation 71 (CD71), is a protein that in humans is encoded by the TFRC gene.[1][2] TfR1 is required for iron import from transferrin into cells by endocytosis.[3][4]
Structure and function
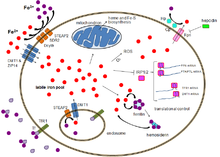
TfR1 is a transmembrane glycoprotein composed of two disulfide-linked monomers joined by two disulfide bonds. Each monomer binds one holo-transferrin molecule creating an iron-Tf-TfR complex which enters the cell by endocytosis.[5]
Clinical significance
TfR1 as a potential new target in cases of human leukemia & lymphoma. InatherYs, in Évry, France, developed a candidate drug, INA01 antibody (anti-CD71) that showed efficacy in pre-clinical studies in the therapy of two incurable orphan oncohematological diseases: the adult T cell leukemia (ATLL) caused by HTLV-1 and the Mantle cell lymphoma (MCL).[citation needed]
Interactions
TfR1 has been shown to interact with GABARAP[6] and HFE.[7][8]
See also
References
- ^ Sutherland R, Delia D, Schneider C, Newman R, Kemshead J, Greaves M (July 1981). "Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin". Proc. Natl. Acad. Sci. U.S.A. 78 (7): 4515–9. doi:10.1073/pnas.78.7.4515. PMC 319822. PMID 6270680.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rabin M, McClelland A, Kühn L, Ruddle FH (November 1985). "Regional localization of the human transferrin receptor gene to 3q26.2----qter". Am. J. Hum. Genet. 37 (6): 1112–6. PMC 1684729. PMID 3002171.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Aisen P (November 2004). "Transferrin receptor 1". Int. J. Biochem. Cell Biol. 36 (11): 2137–43. doi:10.1016/j.biocel.2004.02.007. PMID 15313461.
- ^ Moos T (November 2002). "Brain iron homeostasis". Danish Medical Bulletin. 49 (4): 279–301. PMID 12553165.
- ^ Speeckaert MM, Speeckaert R, Delanghe JR (December 2010). "Biological and clinical aspects of soluble transferrin receptor". Critical Reviews in Clinical Laboratory Sciences. 47 (5–6): 213–228. doi:10.3109/10408363.2010.550461. PMID 21391831.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Green F, O'Hare T, Blackwell A, Enns CA (May 2002). "Association of human transferrin receptor with GABARAP". FEBS Lett. 518 (1–3): 101–6. doi:10.1016/S0014-5793(02)02655-8. PMID 11997026.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Feder JN, Penny DM, Irrinki A, Lee VK, Lebrón JA, Watson N, Tsuchihashi Z, Sigal E, Bjorkman PJ, Schatzman RC (February 1998). "The hemochromatosis gene product complexes with the transferrin receptor and lowers its affinity for ligand binding". Proc. Natl. Acad. Sci. U.S.A. 95 (4): 1472–7. doi:10.1073/pnas.95.4.1472. PMC 19050. PMID 9465039.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ West AP, Bennett MJ, Sellers VM, Andrews NC, Enns CA, Bjorkman PJ (December 2000). "Comparison of the interactions of transferrin receptor and transferrin receptor 2 with transferrin and the hereditary hemochromatosis protein HFE". J. Biol. Chem. 275 (49): 38135–8. doi:10.1074/jbc.C000664200. PMID 11027676.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link)




