CD4
| CD4, Cluster of differentiation 4, extracellular | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 structure of t-cell surface glycoprotein cd4, monoclinic crystal form | |||||||||
| Identifiers | |||||||||
| Symbol | CD4-extrcel | ||||||||
| Pfam | PF09191 | ||||||||
| InterPro | IPR015274 | ||||||||
| SCOP2 | 1cid / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 230 | ||||||||
| OPM protein | 2klu | ||||||||
| CDD | cd07695 | ||||||||
| |||||||||

In molecular biology, CD4 (cluster of differentiation 4) is a glycoprotein found on the surface of immune cells such as T helper cells, monocytes, macrophages, and dendritic cells. It was discovered in the late 1970s and was originally known as leu-3 and T4 (after the OKT4 monoclonal antibody that reacted with it) before being named CD4 in 1984.[1] In humans, the CD4 protein is encoded by the CD4 gene.[2][3]
CD4+ T helper cells are white blood cells that are an essential part of the human immune system. They are often referred to as CD4 cells, T-helper cells or T4 cells. They are called helper cells because one of their main roles is to send signals to other types of immune cells, including CD8 killer cells, which then destroy the infectious particle. If CD4 cells become depleted, for example in untreated HIV infection, or following immune suppression prior to a transplant, the body is left vulnerable to a wide range of infections that it would otherwise have been able to fight.
Structure

Like many cell surface receptors/markers, CD4 is a member of the immunoglobulin superfamily.
It has four immunoglobulin domains (D1 to D4) that are exposed on the extracellular surface of the cell:
- D1 and D3 resemble immunoglobulin variable (IgV) domains.
- D2 and D4 resemble immunoglobulin constant (IgC) domains.
CD4 uses its D1 domain to interact with the β2-domain of MHC class II molecules. T cells expressing CD4 molecules (and not CD8) on their surface, therefore, are specific for antigens presented by MHC II and not by MHC class I (they are MHC class II-restricted). MHC class I contains Beta-2 microglobulin.
The short cytoplasmic/intracellular tail (C) of CD4 contains a special sequence of amino acids that allow it to interact with the lck molecule.
Function
CD4 is a co-receptor that assists the T cell receptor (TCR) in communicating with an antigen-presenting cell. Using its intracellular domain, CD4 amplifies the signal generated by the TCR by recruiting an enzyme, the tyrosine kinase Lck, which is essential for activating many molecular components of the signaling cascade of an activated T cell. Various types of T helper cells are thereby produced. CD4 also interacts directly with MHC class II molecules on the surface of the antigen-presenting cell using its extracellular domain. The extracellular domain adopts an immunoglobulin-like beta-sandwich with seven strands in 2 beta sheets, in a Greek key topology.[4]
During antigen presentation, both the TCR complex and CD4 are recruited to bind to different regions of the MHCII molecule (α1/β1 and β2, respectively) . Close proximity between the TCR complex and CD4 in this situation means the Lck kinase bound to the cytoplasmic tail of CD4 is able to tyrosine-phosphorylate the Immunoreceptor tyrosine activation motifs (ITAM) present on the cytoplasmic domains of CD3. Phosphorylated ITAM motifs on CD3 recruits and activates SH2 domain-containing protein tyrosine kinases (PTK) such as Zap70 to further mediate downstream signal transduction via tyrosine phosphorylation, leading to transcription factor activation including NF-κB and consequent T cell activation.
Other Interactions
CD4 has also been shown to interact with SPG21,[5] Lck[6][7][8][9][10] and Protein unc-119 homolog.[11]
Disease
HIV infection
HIV-1 uses CD4 to gain entry into host T-cells and achieves this through its viral envelope protein known as gp120.[12] The binding to CD4 creates a shift in the conformation of gp120 allowing HIV-1 to bind to a co-receptor expressed on the host cell. These co-receptors are chemokine receptors CCR5 or CXCR4. Following a structural change in another viral protein (gp41), HIV inserts a fusion peptide into the host cell that allows the outer membrane of the virus to fuse with the cell membrane.
HIV pathology
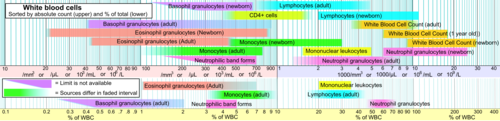
HIV infection leads to a progressive reduction in the number of T cells expressing CD4. Medical professionals refer to the CD4 count to decide when to begin treatment during HIV infection. Normal blood values are usually expressed as the number of cells per microliter (or cubic millimeter, mm3) of blood, with normal values for CD4 cells being 500-1200 cells/mm3.[13] A CD4 count measures the number of T cells expressing CD4. While CD4 counts are not a direct HIV test—e.g. they do not check the presence of viral DNA, or specific antibodies against HIV—they are used to assess the immune system of a patient. Patients often undergo treatments when the CD4 counts reach a level of 350 cells per microliter in Europe but usually around 500cpm in the US; people with less than 200 cells per microliter are at high risk of contracting AIDS defined illnesses. The newest National Institute of Health guidelines recommend treatment of any HIV-positive individuals, regardless of CD4 count[14] Medical professionals also refer to CD4 tests to determine efficacy of treatment.
Other diseases
CD4 continues to be expressed in most neoplasms derived from T helper cells. It is therefore possible to use CD4 immunohistochemistry on tissue biopsy samples to identify most forms of peripheral T cell lymphoma and related malignant conditions.[15] The antigen has also been associated with a number of autoimmune diseases such as vitiligo and type I diabetes mellitus.[16]
T-cells play a large part in autoinflammatory diseases.[17] When testing a drug's efficacy or studying diseases, it is helpful to quantify the amount of T-cells. on fresh-frozen tissue with CD4+, CD8+, and CD3+ T-cell markers (which stain different markers on a T-cell - giving different results).[18]
See also
References
- ^ Bernard A, Boumsell L, Hill C (1984). "Joint Report of the First International Workshop on Human Leucocyte Differentiation Antigens by the Investigators of the Participating Laboratories". In Bernard A, Boumsell L, Dausset J, Milstein C, Schlossman SF (ed.). Leucocyte typing: human leucocyte differentiation antigens detected by monoclonal antibodies: specification, classification, nomenclature. Berlin: Springer. pp. 45–48. doi:10.1007/978-3-642-68857-7_3. ISBN 0-387-12056-4.
Report on the first international references workshop sponsored by INSERM, WHO and IUIS
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Isobe M, Huebner K, Maddon PJ, Littman DR, Axel R, Croce CM (June 1986). "The gene encoding the T-cell surface protein T4 is located on human chromosome 12". Proc. Natl. Acad. Sci. U.S.A. 83 (12): 4399–402. doi:10.1073/pnas.83.12.4399. PMC 323740. PMID 3086883.
- ^ Ansari-Lari MA, Muzny DM, Lu J, Lu F, Lilley CE, Spanos S, Malley T, Gibbs RA (April 1996). "A gene-rich cluster between the CD4 and triosephosphate isomerase genes at human chromosome 12p13". Genome Res. 6 (4): 314–26. doi:10.1101/gr.6.4.314. PMID 8723724.
- ^ Brady RL, Dodson EJ, Dodson GG, Lange G, Davis SJ, Williams AF, Barclay AN (May 1993). "Crystal structure of domains 3 and 4 of rat CD4: relation to the NH2-terminal domains". Science. 260 (5110): 979–83. doi:10.1126/science.8493535. PMID 8493535.
- ^ Zeitlmann L, Sirim P, Kremmer E, Kolanus W (Mar 2001). "Cloning of ACP33 as a novel intracellular ligand of CD4". J. Biol. Chem. 276 (12): 9123–32. doi:10.1074/jbc.M009270200. PMID 11113139.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Rudd CE, Trevillyan JM, Dasgupta JD, Wong LL, Schlossman SF (September 2010). "Pillars article: the CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. 1988". J. Immunol. 185 (5): 2645–9. PMC 3791413. PMID 20724730.
- ^ Rudd CE, Trevillyan JM, Dasgupta JD, Wong LL, Schlossman SF (July 1988). "The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes". Proc. Natl. Acad. Sci. U.S.A. 85 (14): 5190–4. doi:10.1073/pnas.85.14.5190. PMC 281714. PMID 2455897.
- ^ Barber EK, Dasgupta JD, Schlossman SF, Trevillyan JM, Rudd CE (May 1989). "The CD4 and CD8 antigens are coupled to a protein-tyrosine kinase (p56lck) that phosphorylates the CD3 complex". Proc. Natl. Acad. Sci. U.S.A. 86 (9): 3277–81. doi:10.1073/pnas.86.9.3277. PMC 287114. PMID 2470098.
- ^ Hawash IY, Hu XE, Adal A, Cassady JM, Geahlen RL, Harrison ML (April 2002). "The oxygen-substituted palmitic acid analogue, 13-oxypalmitic acid, inhibits Lck localization to lipid rafts and T cell signaling". Biochim. Biophys. Acta. 1589 (2): 140–50. doi:10.1016/S0167-4889(02)00165-9. PMID 12007789.
- ^ Foti M, Phelouzat MA, Holm A, Rasmusson BJ, Carpentier JL (February 2002). "p56Lck anchors CD4 to distinct microdomains on microvilli". Proc. Natl. Acad. Sci. U.S.A. 99 (4): 2008–13. doi:10.1073/pnas.042689099. PMC 122310. PMID 11854499.
- ^ Gorska MM, Stafford SJ, Cen O, Sur S, Alam R (February 2004). "Unc119, a Novel Activator of Lck/Fyn, Is Essential for T Cell Activation". J. Exp. Med. 199 (3): 369–79. doi:10.1084/jem.20030589 (inactive 2015-10-29). PMC 2211793. PMID 14757743.
{{cite journal}}: CS1 maint: DOI inactive as of October 2015 (link) - ^ Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA (June 1998). "Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody". Nature. 393 (6686): 648–59. doi:10.1038/31405. PMID 9641677.
- ^ Bofill M, Janossy G, Lee CA, MacDonald-Burns D, Phillips AN, Sabin C, Timms A, Johnson MA, Kernoff PB (May 1992). "Laboratory control values for CD4 and CD8 T lymphocytes. Implications for HIV-1 diagnosis". Clin. Exp. Immunol. 88 (2): 243–52. doi:10.1111/j.1365-2249.1992.tb03068.x. PMC 1554313. PMID 1349272.
- ^ "Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents" (PDF). AIDSinfo. U.S. Department of Health & Human Services. 2013-02-13.
- ^ Kumarasen Cooper; Anthony S-Y. Leong (2003). Manual of diagnostic antibodies for immunohistology. London: Greenwich Medical Media. p. 65. ISBN 1-84110-100-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Zamani M, Tabatabaiefar MA, Mosayyebi S, Mashaghi A, Mansouri P (July 2010). "Possible association of the CD4 gene polymorphism with vitiligo in an Iranian population". Clin. Exp. Dermatol. 35 (5): 521–4. doi:10.1111/j.1365-2230.2009.03667.x. PMID 19843086.
- ^ Ciccarelli F, De Martinis M, Ginaldi L (2014). "An update on autoinflammatory diseases". Curr. Med. Chem. 21 (3): 261–9. doi:10.2174/09298673113206660303. PMC 3905709. PMID 24164192.
- ^ "550280 - BD Biosciences". BD Biosciences. Becton Dickinson.
Further reading
- Miceli MC, Parnes JR (1993). "Role of CD4 and CD8 in T cell activation and differentiation". Adv. Immunol. Advances in Immunology. 53: 59–122. doi:10.1016/S0065-2776(08)60498-8. ISBN 978-0-12-022453-1. PMID 8512039.
- Geyer M, Fackler OT, Peterlin BM (2001). "Structure–function relationships in HIV-1 Nef". EMBO Rep. 2 (7): 580–5. doi:10.1093/embo-reports/kve141. PMC 1083955. PMID 11463741.
- Greenway AL, Holloway G, McPhee DA, Ellis P, Cornall A, Lidman M (2004). "HIV-1 Nef control of cell signalling molecules: multiple strategies to promote virus replication". J. Biosci. 28 (3): 323–35. doi:10.1007/BF02970151. PMID 12734410.
- Bénichou S, Benmerah A (2003). "[The HIV nef and the Kaposi-sarcoma-associated virus K3/K5 proteins: "parasites"of the endocytosis pathway]". Med Sci (Paris). 19 (1): 100–6. doi:10.1051/medsci/2003191100. PMID 12836198.
- Leavitt SA, SchOn A, Klein JC, Manjappara U, Chaiken IM, Freire E (2004). "Interactions of HIV-1 proteins gp120 and Nef with cellular partners define a novel allosteric paradigm". Curr. Protein Pept. Sci. 5 (1): 1–8. doi:10.2174/1389203043486955. PMID 14965316.
- Tolstrup M, Ostergaard L, Laursen AL, Pedersen SF, Duch M (2004). "HIV/SIV escape from immune surveillance: focus on Nef". Curr. HIV Res. 2 (2): 141–51. doi:10.2174/1570162043484924. PMID 15078178.
- Hout DR, Mulcahy ER, Pacyniak E, Gomez LM, Gomez ML, Stephens EB (2005). "Vpu: a multifunctional protein that enhances the pathogenesis of human immunodeficiency virus type 1". Curr. HIV Res. 2 (3): 255–70. doi:10.2174/1570162043351246. PMID 15279589.
- Joseph AM, Kumar M, Mitra D (2005). "Nef: "necessary and enforcing factor" in HIV infection". Curr. HIV Res. 3 (1): 87–94. doi:10.2174/1570162052773013. PMID 15638726.
- Anderson JL, Hope TJ (2005). "HIV accessory proteins and surviving the host cell". Current HIV/AIDS reports. 1 (1): 47–53. doi:10.1007/s11904-004-0007-x. PMID 16091223.
- Li L, Li HS, Pauza CD, Bukrinsky M, Zhao RY (2006). "Roles of HIV-1 auxiliary proteins in viral pathogenesis and host-pathogen interactions". Cell Res. 15 (11–12): 923–34. doi:10.1038/sj.cr.7290370. PMID 16354571.
- Stove V, Verhasselt B (2006). "Modelling thymic HIV-1 Nef effects". Curr. HIV Res. 4 (1): 57–64. doi:10.2174/157016206775197583. PMID 16454711.
External links
- CD1+Antigen at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Mouse CD Antigen Chart
- Human CD Antigen Chart
- *Human Immunodeficiency Virus Glycoprotein 120