Chlorphenamine
 | |
| Clinical data | |
|---|---|
| Trade names | Chlor-trimeton |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682543 |
| Pregnancy category |
|
| Routes of administration | Oral, IV, IM, SC |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 25 to 50% |
| Protein binding | 72% |
| Metabolism | Hepatic (CYP2D6) |
| Elimination half-life | 21–27 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.596 |
| Chemical and physical data | |
| Formula | C16H19ClN2 |
| Molar mass | 274.788 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Solubility in water | 0.55 g/100 mL, liquid mg/mL (20 °C) |
| |
| |
| | |
Chlorphenamine (INN) or chlorpheniramine (USAN, former BAN), commonly marketed in the form of chlorpheniramine maleate (Chlorphen-12[1]), is a first-generation alkylamine antihistamine used in the prevention of the symptoms of allergic conditions such as rhinitis and urticaria. Its sedative effects are relatively weak compared to other first-generation antihistamines. Chlorphenamine is one of the most commonly used antihistamines in small-animal veterinary practice. Although not generally approved as an antidepressant or anti-anxiety medication, chlorphenamine appears to have these properties as well.[2][3]
Chlorphenamine is part of a series of antihistamines including pheniramine (Naphcon) and its halogenated derivatives and others including fluorpheniramine, dexchlorpheniramine (Polaramine), brompheniramine (Dimetapp), dexbrompheniramine (Drixoral), deschlorpheniramine, dipheniramine (also known as triprolidine with the trade name Actifed), and iodopheniramine.
The halogenated alkylamine antihistamines all exhibit optical isomerism, and chlorphenamine in the indicated products is racemic chlorphenamine maleate, whereas dexchlorpheniramine is the dextrorotary stereoisomer.
Serotonergic and norepinephrinergic effects
In addition to being an histamine H1 receptor antagonist, chlorphenamine has been shown to work as a serotonin-norepinephrine reuptake inhibitor or SNRI.[4] A similar antihistamine, brompheniramine, led to the discovery of the SSRI zimelidine. Limited clinical evidence shows that it is comparable to several antidepressant medications in its ability to inhibit the reuptake of serotonin and also norepinephrine (noradrenaline).[5]
A study performed on Fischer 344/Brown Norway F1 hybrid rats showed that intraventricular administration of Chlorphenamine reduced fear-related behaviors and improved maze performance. It was also noted that long term administration of Chlorphenamine reduced age-related deficits in motor function.[6]
Combination medications
Chlorphenamine is often combined with phenylpropanolamine to form an allergy medication with both antihistamine and decongestant properties, though phenylpropenolamine is no longer available in the US after studies showed it increased the risk of stroke in young women. Chlorpheniramine remains available with no such risk. Brand names had included Demazin, Allerest 12 Hour, Codral Nighttime, Chlornade, Contac 12 Hour, Exchange Select Allergy Multi-Symptom, A. R. M. Allergy Relief, Ordrine, Ornade Spansules, Teldrin, Triaminic, and Tylenol Cold/Allergy.
Chlorphenamine is combined with a narcotic (hydrocodone) in the product Tussionex, which is indicated for treatment of cough and upper respiratory symptoms associated with allergy or cold in adults and children 6 years of age and older.[7] This combination is manufactured as a time-released formula, which allows for administration every 12 hours, versus the more common 4-to-6-hour regimen for other narcotic cough suppressants.
Chlorphenamine/dihydrocodeine immediate-release syrups are also marketed. The antihistamine is helpful in cases where allergy or common cold is the reason for the cough; it is also a potentiator of opioids, allowing enhanced suppression of cough, analgesia, and other effects from a given quantity of the drug by itself. In various places in the world, cough & cold preparations containing codeine and chlorphenamine are available.
In the drug Coricidin, chlorphenamine is combined with the cough suppressant dextromethorphan.
Adverse effects
The adverse effects include drowsiness, dizziness, confusion, constipation, anxiety, nausea, blurred vision, restlessness, decreased coordination, dry mouth, shallow breathing, hallucinations, irritability, problems with memory or concentration, tinnitus and trouble urinating.
Synthesis
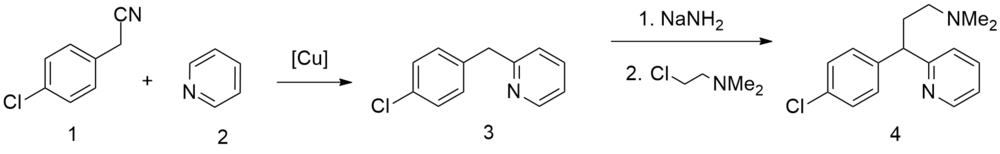
Chlorphenamine, 3-(p-chlorophenyl)-3-(2-pyridyl)propyldimethylamine, is synthesized in two ways. The first is from 4-chlorbenzylcyanide, which is reacted with 2-chloropyridine in the presence of sodium amide to form 4-chlorophenyl(2-pyridyl)acetonitrile. Alkylating this with 2-dimethylaminoethylchloride in the presence of sodium amide gives γ-(4-chlorphenyl)-γ-cyano-N,N-dimethyl-2-pyridinepropanamine, the hydrolysis and decarboxylation of which lead to chlorphenamine.

The second way is from pyridine, which undergoes alkylation by 4-chlorobenzylchloride,[8]
giving 2-(4-chlorobenzyl)pyridine. Alkylating this with 2-dimethylaminoethylchloride in the presence of sodium amide gives chlorphenamine.
See also
References
- ^ "Chlorphen-12".
- ^ Carlsson, Arvid; Lindqvist, Margit. "Central and peripheral monoaminergic membrane-pump blockade by some addictive analgesics and antihistamines". Journal of Pharmacy and Pharmacology. Journal of Pharmacy and Pharmacology. Retrieved 1 December 2013.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Gruetter CA, Lemke SM, Anestis DK, Szarek JL, Valentovic MA. "Potentiation of 5-hydroxytryptamine-induced contraction in rat aorta by chlorpheniramine, citalopram and fluoxetine". Department of Pharmacology, Marshall University School of Medicine, Huntington, WV. Retrieved 1 December 2013.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Carlsson, A.; Linqvist M. (1969). "Central and peripheral monoaminergic membrane-pump blockade by some addictive analgesics and antihistamines". Journal of Pharmacy and Pharmacology. 21 (7): 460–464. doi:10.1111/j.2042-7158.1969.tb08287.x. PMID 4390069.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hellbom, E. (2006). "Chlorpheniramine, selective serotonin-reuptake inhibitors (SSRIs) and over-the-counter (OTC) treatment". Medical Hypotheses. 66 (4): 689–690. doi:10.1016/j.mehy.2005.12.006. PMID 16413139.
- ^ Hasenöhrl, R. U.; Weth, K.; Huston, J. P. (1999). "Intraventricular infusion of the histamine H1 receptor antagonist chlorpheniramine improves maze performance and has anxiolytic-like effects in aged hybrid Fischer 344×Brown Norway rats". Experimental Brain Research. 128 (4): 435–440. doi:10.1007/s002210050866. PMID 10541737.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Tussionex® Pennkinetic® (hydrocodone polistirex and chlorpheniramine polistirex) Extended-Release Suspension" (PDF). UCB. 2011.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/ja01181a508, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/ja01181a508instead.
