Lefetamine: Difference between revisions
Smiles Aloud (talk | contribs) m sentence restructure |
|||
| Line 31: | Line 31: | ||
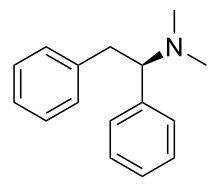
'''Lefetamine''' ('''Santenol''') is a [[drug]] which is both a [[stimulant]] and an [[analgesic]] with effects comparable to both [[morphine]] and [[methylphenidate]]. |
'''Lefetamine''' ('''Santenol''') is a [[drug]] which is both a [[stimulant]] and an [[analgesic]] with effects comparable to both [[morphine]] and [[methylphenidate]]. |
||
Lefetamine was invented in the 1940s<ref> Dodds EC, Lawson W, Simpson SA, Williams PC. Testing diphenylethylamine compounds for analgesic action. Journal of Physiology (1945); (104):47-51.</ref> and was widely abused in [[Japan]]<ref>K. Ogiu et al., J. Pharm. Soc. Japan 80, 283 (1960)</ref> during the 1950s.<ref>Mannelli P, Janiri L, De Marinis M, Tempesta E. Lefetamine: new abuse of an old drug--clinical evaluation of opioid activity. Drug and Alcohol Dependence. 1989 Oct;24(2):95-101.</ref> It has been researched for medicinal use but showed little advantage over other analgesics |
Lefetamine was invented in the 1940s<ref> Dodds EC, Lawson W, Simpson SA, Williams PC. Testing diphenylethylamine compounds for analgesic action. Journal of Physiology (1945); (104):47-51.</ref> and was widely abused in [[Japan]]<ref>K. Ogiu et al., J. Pharm. Soc. Japan 80, 283 (1960)</ref> during the 1950s.<ref>Mannelli P, Janiri L, De Marinis M, Tempesta E. Lefetamine: new abuse of an old drug--clinical evaluation of opioid activity. Drug and Alcohol Dependence. 1989 Oct;24(2):95-101.</ref> It has been researched for medicinal use but showed little advantage over other analgesics. Although it did seem promising for treating opiate withdrawal, it was not as good as [[buprenorphine]] for this purpose.<ref>Janiri L, Mannelli P, Persico AM, Serretti A, Tempesta E. Opiate detoxification of methadone maintenance patients using lefetamine, clonidine and buprenorphine. Drug and Alcohol Dependence. 1994 Oct;36(2):139-45.</ref> More recently it has been abused in [[Europe]], but it remains an obscure drug on the illicit market. |
||
Due to its [[chemical structure]], it binds to [[opioid receptor]]s while simultaneously [[reuptake inhibitor|inhibiting]] the [[reuptake]] of [[dopamine]] and [[norepinephrine]].<ref>Janiri L, Mannelli P, Pirrongelli C, Lo Monaco M, Tempesta E. Lephetamine abuse and dependence: clinical effects and withdrawal syndrome. British Journal of Addiction. 1989 Jan;84(1):89-95.</ref> Lefetamine has the necessary tertiary amine functional group required for opioid receptor recognition, as well as a [[phenethylamine]] skeleton (which is responsible for its [[amphetamine]]-like effects).<ref>Massa S, Di Santo R, Mai A, Artico M, Pantaleoni GC, Giorgi R, Coppolino MF. Pyrrylphenylethanones related to cathinone and lefetamine: synthesis and pharmacological activities. Archiv der Pharmazie (Weinheim). 1992 Jul;325(7):403-9.</ref> It's pyrrole analogues are also reported to have some [[NMDA antagonist]] effects and to have [[neurotoxic]] properties.<ref> Massa S, Stefancich G, Artico M, Corelli F, Silvestri R, Pantaleoni GC, Fanini D, Palumbo G, Giorgi R. Synthesis, neuropsychopharmacological effects and analgesic-antiinflammatory activities of pyrrole analogues of lefetamine. Farmaco. 1989 Sep;44(9):763-77.</ref> |
Due to its [[chemical structure]], it binds to [[opioid receptor]]s while simultaneously [[reuptake inhibitor|inhibiting]] the [[reuptake]] of [[dopamine]] and [[norepinephrine]].<ref>Janiri L, Mannelli P, Pirrongelli C, Lo Monaco M, Tempesta E. Lephetamine abuse and dependence: clinical effects and withdrawal syndrome. British Journal of Addiction. 1989 Jan;84(1):89-95.</ref> Lefetamine has the necessary tertiary amine functional group required for opioid receptor recognition, as well as a [[phenethylamine]] skeleton (which is responsible for its [[amphetamine]]-like effects).<ref>Massa S, Di Santo R, Mai A, Artico M, Pantaleoni GC, Giorgi R, Coppolino MF. Pyrrylphenylethanones related to cathinone and lefetamine: synthesis and pharmacological activities. Archiv der Pharmazie (Weinheim). 1992 Jul;325(7):403-9.</ref> It's pyrrole analogues are also reported to have some [[NMDA antagonist]] effects and to have [[neurotoxic]] properties.<ref> Massa S, Stefancich G, Artico M, Corelli F, Silvestri R, Pantaleoni GC, Fanini D, Palumbo G, Giorgi R. Synthesis, neuropsychopharmacological effects and analgesic-antiinflammatory activities of pyrrole analogues of lefetamine. Farmaco. 1989 Sep;44(9):763-77.</ref> |
||
Revision as of 19:44, 12 January 2011
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H19N |
| Molar mass | 225.329 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Lefetamine (Santenol) is a drug which is both a stimulant and an analgesic with effects comparable to both morphine and methylphenidate.
Lefetamine was invented in the 1940s[1] and was widely abused in Japan[2] during the 1950s.[3] It has been researched for medicinal use but showed little advantage over other analgesics. Although it did seem promising for treating opiate withdrawal, it was not as good as buprenorphine for this purpose.[4] More recently it has been abused in Europe, but it remains an obscure drug on the illicit market.
Due to its chemical structure, it binds to opioid receptors while simultaneously inhibiting the reuptake of dopamine and norepinephrine.[5] Lefetamine has the necessary tertiary amine functional group required for opioid receptor recognition, as well as a phenethylamine skeleton (which is responsible for its amphetamine-like effects).[6] It's pyrrole analogues are also reported to have some NMDA antagonist effects and to have neurotoxic properties.[7]
See also
References
- ^ Dodds EC, Lawson W, Simpson SA, Williams PC. Testing diphenylethylamine compounds for analgesic action. Journal of Physiology (1945); (104):47-51.
- ^ K. Ogiu et al., J. Pharm. Soc. Japan 80, 283 (1960)
- ^ Mannelli P, Janiri L, De Marinis M, Tempesta E. Lefetamine: new abuse of an old drug--clinical evaluation of opioid activity. Drug and Alcohol Dependence. 1989 Oct;24(2):95-101.
- ^ Janiri L, Mannelli P, Persico AM, Serretti A, Tempesta E. Opiate detoxification of methadone maintenance patients using lefetamine, clonidine and buprenorphine. Drug and Alcohol Dependence. 1994 Oct;36(2):139-45.
- ^ Janiri L, Mannelli P, Pirrongelli C, Lo Monaco M, Tempesta E. Lephetamine abuse and dependence: clinical effects and withdrawal syndrome. British Journal of Addiction. 1989 Jan;84(1):89-95.
- ^ Massa S, Di Santo R, Mai A, Artico M, Pantaleoni GC, Giorgi R, Coppolino MF. Pyrrylphenylethanones related to cathinone and lefetamine: synthesis and pharmacological activities. Archiv der Pharmazie (Weinheim). 1992 Jul;325(7):403-9.
- ^ Massa S, Stefancich G, Artico M, Corelli F, Silvestri R, Pantaleoni GC, Fanini D, Palumbo G, Giorgi R. Synthesis, neuropsychopharmacological effects and analgesic-antiinflammatory activities of pyrrole analogues of lefetamine. Farmaco. 1989 Sep;44(9):763-77.
