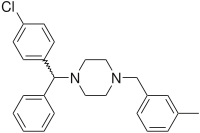
Meclizine
 | |
| Clinical data | |
|---|---|
| MedlinePlus | a682548 |
| Routes of administration | Oral, sublingual/buccal |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | hepatic |
| Elimination half-life | 6 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.008.477 |
| Chemical and physical data | |
| Formula | C25H27ClN2 |
| Molar mass | 390.948 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Boiling point | 230 °C (446 °F) |
| |
| |
| | |
Meclizine (INN,[1] or meclozine) is an antihistamine of the diphenylmethylpiperazine group considered to be an antiemetic. It is sold under the brand names Bonine, Bonamine, Antivert, Postafen, Sea Legs, and Dramamine II (Less Drowsy Formulation). Emesafene is a combination of meclizine (1/3) and pyridoxine (2/3). In Canada, Antivert Tab (which is no longer available) was a combination of meclizine and nicotinic acid.[2]
Classification
Meclizine is a first-generation antihistamine (nonselective H1 antagonist) of the piperazine class. It is structurally and pharmacologically similar to buclizine, cyclizine, and hydroxyzine, but has a shorter half-life of six hours compared to cyclizine and hydroxyzine with about 20 hours (though half-life should not be confused with duration). It is used as an antivertigo/antiemetic agent, specifically in the prevention and treatment of nausea, vomiting, and dizziness associated with motion sickness.[3] Meclizine is sometimes combined with opioids, especially ones of the open-chain class like methadone, dextropropoxyphene, and dipipanone (originally combined with meclizine's parent drug cyclizine, the brand name of this combination being Diconal).
Mechanism of action
Meclizine is an antagonist at H1 receptors. It possesses anticholinergic, central nervous system depressant, and local anesthetic effects. Its antiemetic and antivertigo effects are not fully understood, but its central anticholinergic properties are partially responsible. The drug depresses labyrinth excitability and vestibular stimulation, and it may affect the medullary chemoreceptor trigger zone.[3] Meclizine also is a dopamine antagonist at D1-like and D2-like receptors[citation needed], but does not cause catalepsy[note 1] in mice, perhaps because of its anticholinergic activity.[4]
Uses
Meclizine is approved by the U.S. Food and Drug Administration (FDA) to treat symptoms of motion sickness. Meclizine's safety and efficacy in children younger than 12 years old has not been established, therefore use in this population is not recommended. Also, meclizine should be taken with caution in the elderly (older than 65 years) because of increased risk of confusion and amnesia.[5]
Motion sickness
Meclizine is effective in inhibiting the symptoms of motion sickness, such as nausea, vomiting and dizziness.[medical citation needed]
The drug is also safe for treating nausea in pregnancy[6] and is a first-line therapy for this use.[7][8] Doxylamine is similarly safe. Meclizine may not be strong enough for especially sickening motion stimuli and second-line defenses should be tried in those cases.[9]
Vertigo
Meclizine is effective in relieving vertigo experienced as a result of inner ear infection.
Side effects
Some common side effects such as drowsiness, dry mouth, and tiredness may occur. Meclizine has been shown to have fewer dry mouth side effects than the traditional treatment for motion sickness, transdermal scopolamine.[10] A very serious allergic reaction to this drug is unlikely, but immediate medical attention should be sought if it occurs. Symptoms of a serious allergic reaction may include rash, itching, swelling, severe dizziness, and trouble breathing.[11]
Drowsiness
Drowsiness may result as a side effect of taking meclizine. Users are advised not to operate heavy machinery while under the influence. The consumption of alcohol while under the influence of meclizine may result in additional drowsiness.
Special considerations in the elderly
As with any anticholinergic agent, meclizine may cause confusion or aggravate symptoms in those with dementia in the geriatric population (older than 65 years). Therefore, caution should be used when administering meclizine to the elderly.[12]
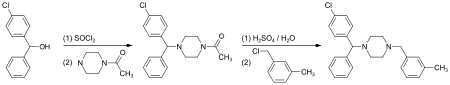
Synthesis
(4-Chlorphenyl)-phenylmethanol is halogenated with thionyl chloride before adding acetylpiperazine. The acetyl group is cleaved with diluted sulfuric acid. An N-alkylation of the piperazine ring with 3-methylbenzylchloride completes the synthesis.[13]
Alternatively, the last step can be replaced by a reductive N-alkylation with 3-methylbenzaldehyde. The reductive agent is hydrogen, and Raney nickel is used as a catalyst.[14][15]
Meclizine is obtained and used as a racemate, a 1:1 mixture of the two stereoisomers. Drug forms contain the racemic dihydrochloride.
Notes
References
- ^ Guidelines on the Use of INNs for Pharmaceutical Substances (1997). Accessed November, 2013 "Guidance on INN." WHO.
- ^ DrugBank. Drugbank: Drug card for Meclizine David Wishard: University of Alberta, Canada. Accessed November 7, 2010.
- ^ a b Clinical Pharmacology. Clinical Pharmacology, revised November 20, 2009, accessed November 7, 2010.[full citation needed]
- ^ a b Haraguchi K, Ito K, Kotaki H, Sawada Y, Iga T (1997). "Prediction of drug-induced catalepsy based on dopamine D1, D2, and muscarinic acetylcholine receptor occupancies". Drug Metabolism and Disposition. 25 (6): 675–684. PMID 9193868. Retrieved 2014-06-12.
- ^ MICROMEDEX 2.0. Accessed November 7, 2010.[full citation needed]
- ^ Källén B, Mottet I (2003). "Delivery outcome after the use of meclizine in early pregnancy" (PDF). European Journal of Epidemiology. 18 (7): 665–669. doi:10.1023/a:1024891618953. PMID 12952140. Retrieved 2010-09-17.
- ^ "Antiemetische Therapie bei Schwangerschaftserbrechen" [Antiemetic therapy in pregnancy]. Arznei-Telegramm (in German). 40: 87–89. 2009.
- ^ Embryotox: Meclozin Template:De icon
- ^ "Evaluation of Several Common Antimotion Sickness Medications and Recommendations Concerning Their Potential Usefulness During Special Operations". 2009. Retrieved 2016-04-18.
{{cite web}}: Unknown parameter|authors=ignored (help) - ^ Dahl E, Offer-Ohlsen D, Lillevold PE, Sandvik L. Transdermal scopolamine, oral meclizine, and placebo in motion sickness. Clinical Pharmacology And Therapeutics [Clin Pharmacol Ther] 1984 Jul; Vol. 36 (1), pp. 116-20. Available from: MEDLINE: Ipswich, MA. PMID 6734040
- ^ Meclizine - oral, Antivert, D-vert, Dramamine II. Accessed November 7, 2010.
- ^ Merck Manuals, Online Medical Library: Meclizine (Drug Information Provided by Lexi-Comp), revised January 2010, accessed November 7, 2010.
- ^ Organic Synthesis. Concepts and Methods. Wiley. 2003. p. 237. ISBN 978-3-527-30272-7.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ US 2 709 169 (UCB, 1955)
- ^ Pharmaceutical Substances. Synthesis, Patents, Applications (4 ed.). Thieme. 2001. ISBN 3-13-115134-X.
{{cite book}}: Unknown parameter|authors=ignored (help)