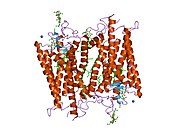
Rhodopsin
Rhodopsin (also known as visual purple) is a light-sensitive receptor protein involved in visual phototransduction. It is named after ancient Greek ῥόδον (rhódon) for “rose”, due to its pinkish color, and ὄψις (ópsis) for “sight”.[5] Rhodopsin is a biological pigment found in the rods of the retina and is a G-protein-coupled receptor (GPCR). Rhodopsin is extremely sensitive to light, and thus enables vision in low-light conditions.[6] When rhodopsin is exposed to light, it immediately photobleaches. In humans, it is regenerated fully in about 30 minutes; after which rods are more sensitive.[7]
Rhodopsin was discovered by Franz Christian Boll in 1876.[8][9]
Structure
Rhodopsin consists of a protein moiety also called scotopsin, which binds covalently a cofactor called retinal. Scotopsin is an opsin. Opsins are G protein coupled receptors and have seven transmembrane domains. The seven transmembrane domains form a pocket, where the retinal (as photoreactive chromophore) binds to a lysine residue in the seventh transmembrane domain. The retinal lies horizontally to the cell membrane. And the cell membrane lipid bilayer embeds half of the rhodopsin. Thousands of rhodopsin molecules are found in each outer segment disc of the host rod cell. Retinol is produced in the retina from Vitamin A, from dietary beta-carotene. Isomerization of 11-cis-retinal into all-trans-retinal by light induces a conformational change (bleaching) in opsin, continuing with metarhodopsin II, which activates the associated G protein transducin and triggers a Cyclic Guanosine Monophosphate, second messenger, cascade.[7][10][11]
Rhodopsin of the rods most strongly absorbs green-blue light and, therefore, appears reddish-purple, which is why it is also called "visual purple".[12] It is responsible for monochromatic vision in the dark.[7]

Several closely related opsins exist that differ only in a few amino acids and in the wavelengths of light that they absorb most strongly. Humans have eight different other opsins besides rhodopsin, as well as cryptochrome (light-sensitive, but not an opsin).[13][14]
The photopsins are found in the different types of the cone cells of the retina and are the basis of color vision. They have absorption maxima for yellowish-green (photopsin I), green (photopsin II), and bluish-violet (photopsin III) light. The remaining opsin (melanopsin) is found in photosensitive ganglion cells and absorbs blue light most strongly.
In rhodopsin, the aldehyde group of retinal is covalently linked to the amino group of a lysine residue on the protein in a protonated Schiff base (-NH+=CH-).[15] When rhodopsin absorbs light, its retinal cofactor isomerizes from the 11-cis to the all-trans configuration, and the protein subsequently undergoes a series of relaxations to accommodate the altered shape of the isomerized cofactor. The intermediates formed during this process were first investigated in the laboratory of George Wald, who received the Nobel prize for this research in 1967.[16] The photoisomerization dynamics has been subsequently investigated with time-resolved IR spectroscopy and UV/Vis spectroscopy. A first photoproduct called photorhodopsin forms within 200 femtoseconds after irradiation, followed within picoseconds by a second one called bathorhodopsin with distorted all-trans bonds. This intermediate can be trapped and studied at cryogenic temperatures, and was initially referred to as prelumirhodopsin.[17] In subsequent intermediates lumirhodopsin and metarhodopsin I, the Schiff's base linkage to all-trans retinal remains protonated, and the protein retains its reddish color. The critical change that initiates the neuronal excitation involves the conversion of metarhodopsin I to metarhodopsin II, which is associated with deprotonation of the Schiff's base and change in color from red to yellow.[18] The structure of rhodopsin has been studied in detail via x-ray crystallography on rhodopsin crystals. Several models (e.g., the bicycle-pedal mechanism, hula-twist mechanism) attempt to explain how the retinal group can change its conformation without clashing with the enveloping rhodopsin protein pocket.[19][20][21]
Recent data support that it is a functional monomer, instead of a dimer, which was the paradigm of G-protein-coupled receptors for many years.[22]
Phototransduction
Rhodopsin is an essential G-protein receptor in phototransduction.
Function
Metarhodopsin II activates the G protein transducin (Gt) to activate the visual phototransduction pathway. When transducin's α subunit is bound to GTP, it activates cGMP phosphodiesterase. cGMP phosphodiesterase hydrolyzes cGMP (breaks it down). cGMP can no longer activate cation channels. This leads to the hyperpolarization of photoreceptor cells and a change in the rate of transmitter release by these photoreceptor cells.
Deactivation
Meta II is deactivated rapidly after activating transducin by rhodopsin kinase and arrestin.[23] The rhodopsin pigment must be regenerated for further phototransduction to occur. This means replacing all-trans-retinal with 11-cis-retinal and the decay of Meta II is crucial in this process. During the decay of Meta II, the Schiff base link that normally holds all-trans-retinal and the apoprotein opsin is hydrolyzed and becomes Meta III. In the rod outer segment, Meta III decays into separate all-trans-retinal and opsin.[23] A second product of Meta II decay is an all-trans-retinal opsin complex in which the all-trans-retinal has been translocated to second binding sites. Whether the Meta II decay runs into Meta III or the all-trans-retinal opsin complex seems to depend on the pH of the reaction. Higher pH tends to drive the decay reaction towards Meta III.[23]
Rhodopsin and retinal disease
Mutation of the rhodopsin gene is a major contributor to various retinopathies such as retinitis pigmentosa. In general, the disease-causing protein aggregates with ubiquitin in inclusion bodies, disrupts the intermediate filament network, and impairs the ability of the cell to degrade non-functioning proteins, which leads to photoreceptor apoptosis.[24] Other mutations on rhodopsin lead to X-linked congenital stationary night blindness, mainly due to constitutive activation, when the mutations occur around the chromophore binding pocket of rhodopsin.[25] Several other pathological states relating to rhodopsin have been discovered including poor post-Golgi trafficking, dysregulative activation, rod outer segment instability and arrestin binding.[25]
Microbial rhodopsins
Some prokaryotes express proton pumps called bacteriorhodopsins, proteorhodopsins, and xanthorhodopsins to carry out phototrophy.[26] Like animal visual pigments, these contain a retinal chromophore (although it is an all-trans, rather than 11-cis form) and have seven transmembrane alpha helices; however, they are not coupled to a G protein. Prokaryotic halorhodopsins are light-activated chloride pumps.[26] Unicellular flagellate algae contain channelrhodopsins that act as light-gated cation channels when expressed in heterologous systems. Many other pro- and eukaryotic organisms (in particular, fungi such as Neurospora) express rhodopsin ion pumps or sensory rhodopsins of yet-unknown function. Very recently, a microbial rhodopsin with guanylyl cyclase activity has been discovered.[27] [28] While all microbial rhodopsins have significant sequence homology to one another, they have no detectable sequence homology to the G-protein-coupled receptor (GPCR) family to which animal visual rhodopsins belong. Nevertheless, microbial rhodopsins and GPCRs are possibly evolutionarily related, based on the similarity of their three-dimensional structures. Therefore, they have been assigned to the same superfamily in Structural Classification of Proteins (SCOP).[29]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000163914 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000030324 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Perception (2008), Guest Editorial Essay, Perception, p. 1
- ^ Litmann BJ, Mitchell DC (1996). "Rhodopsin structure and function". In Lee AG (ed.). Rhodopsin and G-Protein Linked Receptors, Part A (Vol 2, 1996) (2 Vol Set). Greenwich, Conn: JAI Press. pp. 1–32. ISBN 1-55938-659-2.
- ^ a b c Stuart JA, Brige RR (1996). "Characterization of the primary photochemical events in bacteriorhodopsin and rhodopsin". In Lee AG (ed.). Rhodopsin and G-Protein Linked Receptors, Part A (Vol 2, 1996) (2 Vol Set). Greenwich, Conn: JAI Press. pp. 33–140. ISBN 1-55938-659-2.
- ^ Encyclopedia of the Neurological Sciences. Academic Press. 29 April 2014. pp. 441–. ISBN 978-0-12-385158-1.
- ^ Giese, Arthur C. (24 September 2013). Photophysiology: General Principles; Action of Light on Plants. Elsevier. p. 9. ISBN 978-1-4832-6227-7. Retrieved 23 September 2015.
- ^ Hofmann KP, Heck M (1996). "Light-induced protein-protein interactions on the rod photoreceptor disc membrane". In Lee AG (ed.). Rhodopsin and G-Protein Linked Receptors, Part A (Vol 2, 1996) (2 Vol Set). Greenwich, Conn: JAI Press. pp. 141–198. ISBN 1-55938-659-2.
- ^ Kolb H, Fernandez E, Nelson R, Jones BW (2010-03-01). "Webvision: Photoreceptors". University of Utah.
- ^ Rogers, Kara. "Rhodopsin". Encyclopedia Britannica. Britannica.com. Retrieved 30 January 2016.
- ^ Terakita, Akihisa (2005). "The opsins". Genome Biology. 6 (3): 213. doi:10.1186/gb-2005-6-3-213. ISSN 1465-6906. PMC 1088937. PMID 15774036.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Foley, Lauren E.; Gegear, Robert J.; Reppert, Steven M. (2011). "Human cryptochrome exhibits light-dependent magnetosensitivity". Nature Communications. 2: 356. doi:10.1038/ncomms1364. ISSN 2041-1723. PMC 3128388. PMID 21694704.
- ^ Bownds D, Wald G (Jan 1965). "Reaction of the rhodopsin chromophore with sodium borohydride". Nature. 205: 254–7. doi:10.1038/205254a0. PMID 14270706.
- ^ The Nobel Foundation. "The Nobel Prize in Physiology or Medicine 1967". Nobelprize.org. Nobel Media AB 2014. Retrieved 12 December 2015.
- ^ Yoshizawa T, Wald G (Mar 1963). "Pre-lumirhodopsin and the bleaching of visual pigments". Nature. 197 (Mar 30): 1279–86. doi:10.1038/1971279a0. PMID 14002749.
- ^ Matthews RG, Hubbard R, Brown PK, Wald G (Nov 1963). "Tautomeric forms of metarhodopsin". The Journal of General Physiology. 47: 215–40. doi:10.1085/jgp.47.2.215. PMC 2195338. PMID 14080814.
- ^ Nakamichi H, Okada T (Jun 2006). "Crystallographic analysis of primary visual photochemistry". Angewandte Chemie. 45 (26): 4270–3. doi:10.1002/anie.200600595. PMID 16586416.
- ^ Schreiber M, Sugihara M, Okada T, Buss V (Jun 2006). "Quantum mechanical studies on the crystallographic model of bathorhodopsin". Angewandte Chemie. 45 (26): 4274–7. doi:10.1002/anie.200600585. PMID 16729349.
- ^ Weingart O (Sep 2007). "The twisted C11=C12 bond of the rhodopsin chromophore--a photochemical hot spot". Journal of the American Chemical Society. 129 (35): 10618–9. doi:10.1021/ja071793t. PMID 17691730.
- ^ Chabre M, le Maire M (Jul 2005). "Monomeric G-protein-coupled receptor as a functional unit". Biochemistry. 44 (27): 9395–403. doi:10.1021/bi050720o. PMID 15996094.
- ^ a b c Heck M, Schädel SA, Maretzki D, Bartl FJ, Ritter E, Palczewski K, Hofmann KP (Jan 2003). "Signaling states of rhodopsin. Formation of the storage form, metarhodopsin III, from active metarhodopsin II". The Journal of Biological Chemistry. 278 (5): 3162–9. doi:10.1074/jbc.M209675200. PMC 1364529. PMID 12427735.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Saliba RS, Munro PM, Luthert PJ, Cheetham ME (Jul 2002). "The cellular fate of mutant rhodopsin: quality control, degradation and aggresome formation". Journal of Cell Science. 115 (Pt 14): 2907–18. PMID 12082151.
- ^ a b Mendes HF, van der Spuy J, Chapple JP, Cheetham ME (Apr 2005). "Mechanisms of cell death in rhodopsin retinitis pigmentosa: implications for therapy". Trends in Molecular Medicine. 11 (4): 177–85. doi:10.1016/j.molmed.2005.02.007. PMID 15823756.
- ^ a b Bryant DA, Frigaard NU (Nov 2006). "Prokaryotic photosynthesis and phototrophy illuminated". Trends in Microbiology. 14 (11): 488–96. doi:10.1016/j.tim.2006.09.001. PMID 16997562.
- ^ Gao SQ, Nagpal J, Schneider MW, Kozjak-Pavlovic V, Nagel G, Gottschalk A (July 2015). "Optogenetic manipulation of cGMP in cells and animals by the tightly light-regulated guanylyl-cyclase opsin CyclOp". Nature Communications. 6 (8046). doi:10.1038/ncomms9046.
- ^ Scheib U, Stehfest K, Gee CE, Körschen HG, Fudim R, Oertner TG, Hegemann P (Aug 2015). "The rhodopsin-guanylyl cyclase of the aquatic fungus Blastocladiella emersonii enables fast optical control of cGMP signaling". Science Signaling. 8 (389): rs8. doi:10.1126/scisignal.aab0611. PMID 26268609.
- ^ "Superfamily: Bacterial photosystem II reaction centre, L and M subunits". SCOP.
Further reading
- Humphries P, Kenna P, Farrar GJ (May 1992). "On the molecular genetics of retinitis pigmentosa". Science. 256 (5058): 804–8. doi:10.1126/science.1589761. PMID 1589761.
- Edwards SC (Jul 1995). "Involvement of cGMP and calcium in the photoresponse in vertebrate photoreceptor cells". The Journal of the Florida Medical Association. 82 (7): 485–8. PMID 7673885.
- al-Maghtheh M, Gregory C, Inglehearn C, Hardcastle A, Bhattacharya S (1993). "Rhodopsin mutations in autosomal dominant retinitis pigmentosa". Human Mutation. 2 (4): 249–55. doi:10.1002/humu.1380020403. PMID 8401533.
- Garriga P, Manyosa J (Sep 2002). "The eye photoreceptor protein rhodopsin. Structural implications for retinal disease". FEBS Letters. 528 (1–3): 17–22. doi:10.1016/S0014-5793(02)03241-6. PMID 12297272.
- Mendes HF, van der Spuy J, Chapple JP, Cheetham ME (Apr 2005). "Mechanisms of cell death in rhodopsin retinitis pigmentosa: implications for therapy". Trends in Molecular Medicine. 11 (4): 177–85. doi:10.1016/j.molmed.2005.02.007. PMID 15823756.
- Inglehearn CF, Keen TJ, Bashir R, Jay M, Fitzke F, Bird AC, Crombie A, Bhattacharya S (Apr 1992). "A completed screen for mutations of the rhodopsin gene in a panel of patients with autosomal dominant retinitis pigmentosa". Human Molecular Genetics. 1 (1): 41–5. doi:10.1093/hmg/1.1.41. PMID 1301135.
- Farrar GJ, Findlay JB, Kumar-Singh R, Kenna P, Humphries MM, Sharpe E, Humphries P (Dec 1992). "Autosomal dominant retinitis pigmentosa: a novel mutation in the rhodopsin gene in the original 3q linked family". Human Molecular Genetics. 1 (9): 769–71. doi:10.1093/hmg/1.9.769. PMID 1302614.
- Robinson PR, Cohen GB, Zhukovsky EA, Oprian DD (Oct 1992). "Constitutively active mutants of rhodopsin". Neuron. 9 (4): 719–25. doi:10.1016/0896-6273(92)90034-B. PMID 1356370.
- Fujiki K, Hotta Y, Hayakawa M, Sakuma H, Shiono T, Noro M, Sakuma T, Tamai M, Hikiji K, Kawaguchi R (Jun 1992). "Point mutations of rhodopsin gene found in Japanese families with autosomal dominant retinitis pigmentosa (ADRP)". The Japanese Journal of Human Genetics. 37 (2): 125–32. doi:10.1007/BF01899733. PMID 1391967.
- Olsson JE, Gordon JW, Pawlyk BS, Roof D, Hayes A, Molday RS, Mukai S, Cowley GS, Berson EL, Dryja TP (Nov 1992). "Transgenic mice with a rhodopsin mutation (Pro23His): a mouse model of autosomal dominant retinitis pigmentosa". Neuron. 9 (5): 815–30. doi:10.1016/0896-6273(92)90236-7. PMID 1418997.
- Andréasson S, Ehinger B, Abrahamson M, Fex G (Sep 1992). "A six-generation family with autosomal dominant retinitis pigmentosa and a rhodopsin gene mutation (arginine-135-leucine)". Ophthalmic Paediatrics and Genetics. 13 (3): 145–53. doi:10.3109/13816819209046483. PMID 1484692.
- Inglehearn CF, Lester DH, Bashir R, Atif U, Keen TJ, Sertedaki A, Lindsey J, Jay M, Bird AC, Farrar GJ (Mar 1992). "Recombination between rhodopsin and locus D3S47 (C17) in rhodopsin retinitis pigmentosa families". American Journal of Human Genetics. 50 (3): 590–7. PMC 1684283. PMID 1539595.
- Fishman GA, Stone EM, Gilbert LD, Sheffield VC (May 1992). "Ocular findings associated with a rhodopsin gene codon 106 mutation. Glycine-to-arginine change in autosomal dominant retinitis pigmentosa". Archives of Ophthalmology. 110 (5): 646–53. doi:10.1001/archopht.1992.01080170068026. PMID 1580841.
- Keen TJ, Inglehearn CF, Lester DH, Bashir R, Jay M, Bird AC, Jay B, Bhattacharya SS (Sep 1991). "Autosomal dominant retinitis pigmentosa: four new mutations in rhodopsin, one of them in the retinal attachment site". Genomics. 11 (1): 199–205. doi:10.1016/0888-7543(91)90119-Y. PMID 1765377.
- Dryja TP, Hahn LB, Cowley GS, McGee TL, Berson EL (Oct 1991). "Mutation spectrum of the rhodopsin gene among patients with autosomal dominant retinitis pigmentosa". Proceedings of the National Academy of Sciences of the United States of America. 88 (20): 9370–4. doi:10.1073/pnas.88.20.9370. PMC 52716. PMID 1833777.
- Gal A, Artlich A, Ludwig M, Niemeyer G, Olek K, Schwinger E, Schinzel A (Oct 1991). "Pro-347-Arg mutation of the rhodopsin gene in autosomal dominant retinitis pigmentosa". Genomics. 11 (2): 468–70. doi:10.1016/0888-7543(91)90159-C. PMID 1840561.
- Sung CH, Davenport CM, Hennessey JC, Maumenee IH, Jacobson SG, Heckenlively JR, Nowakowski R, Fishman G, Gouras P, Nathans J (Aug 1991). "Rhodopsin mutations in autosomal dominant retinitis pigmentosa". Proceedings of the National Academy of Sciences of the United States of America. 88 (15): 6481–5. doi:10.1073/pnas.88.15.6481. PMC 52109. PMID 1862076.
- Jacobson SG, Kemp CM, Sung CH, Nathans J (Sep 1991). "Retinal function and rhodopsin levels in autosomal dominant retinitis pigmentosa with rhodopsin mutations". American Journal of Ophthalmology. 112 (3): 256–71. doi:10.1016/s0002-9394(14)76726-1. PMID 1882937.
- Sheffield VC, Fishman GA, Beck JS, Kimura AE, Stone EM (Oct 1991). "Identification of novel rhodopsin mutations associated with retinitis pigmentosa by GC-clamped denaturing gradient gel electrophoresis". American Journal of Human Genetics. 49 (4): 699–706. PMC 1683182. PMID 1897520.
External links
- Rhodopsin at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Kolb H, Fernandez E, Nelson R, Jones BW (2010-03-01). "Webvision Home Page: The organization of the retina and visual system". University of Utah.
- The Rhodopsin Protein
- Photoisomerization of rhodopsin, animation.
- Rhodopsin and the eye, summary with pictures.
- UMich Orientation of Proteins in Membranes families/superfamily-6 - Calculated spatial positions of rhodopsin-like proteins in membrane