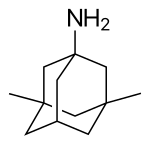
Memantine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Originally, Axura, Ebixa, Namenda; many generic names worldwide[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604006 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~100% |
| Metabolism | Hepatic (<10%) |
| Elimination half-life | 60–100 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.217.937 |
| Chemical and physical data | |
| Formula | C12H21N |
| Molar mass | 179.3 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Memantine is used to treat moderate to severe Alzheimer's disease. It acts on the glutamatergic system by blocking NMDA receptors. It was first synthesized by Eli Lilly and Company in 1968 as a potential agent to treat diabetes; the NMDA activity was discovered in the 1980s.
Medical use
Memantine is used to treat moderate-to-severe Alzheimer's disease, especially for people who are intolerant of or have a contraindication to AChE (acetylcholinesterase) inhibitors.[2][3]
Memantine has been associated with a moderate decrease in clinical deterioration[4] with only a small positive effect on cognition, mood, behavior, and the ability to perform daily activities in moderate to severe Alzheimer's disease.[5] There does not appear to be any benefit in mild disease.[6]
Adverse effects
Memantine is, in general, well-tolerated.[4] Common adverse drug reactions (≥1% of people) include confusion, dizziness, drowsiness, headache, insomnia, agitation, and/or hallucinations. Less common adverse effects include vomiting, anxiety, hypertonia, cystitis, and increased libido.[4][7]
Like many other NMDA antagonists, memantine behaves as a dissociative anesthetic at supratherapeutic doses.[8] Despite isolated reports, recreational use of memantine is rare due to the drug's long duration and limited availability.[8] Also memantine seems to lack most of the psychoactive effects recreational users are usually looking for, such as euphoria or hallucinations.[9]
Pharmacology
Glutamatergic (NMDA receptor)
A dysfunction of glutamatergic neurotransmission, manifested as neuronal excitotoxicity, is hypothesized to be involved in the etiology of Alzheimer's disease. Targeting the glutamatergic system, specifically NMDA receptors, offers a novel approach to treatment in view of the limited efficacy of existing drugs targeting the cholinergic system.[10]
Memantine is a low-affinity voltage-dependent uncompetitive antagonist at glutamatergic NMDA receptors.[11][12] By binding to the NMDA receptor with a higher affinity than Mg2+ ions, memantine is able to inhibit the prolonged influx of Ca2+ ions, particularly from extrasynaptic receptors, which forms the basis of neuronal excitotoxicity. The low affinity, uncompetitive nature, and rapid off-rate kinetics of memantine at the level of the NMDA receptor-channel, however, preserves the function of the receptor at synapses, as it can still be activated by physiological release of glutamate following depolarization of the presynaptic neuron.[13][14][15] The interaction of memantine with NMDA receptors plays a major role in the symptomatic improvement that the drug produces in Alzheimer's disease. However, there is no evidence as yet that the ability of memantine to protect against NMDA receptor-mediated excitotoxicity has a disease-modifying effect in Alzheimer's, although this has been suggested in animal models.[14]
Serotonergic (5-HT3 receptor)
Memantine acts as a non-competitive antagonist at the 5-HT3 receptor, with a potency similar to that for the NMDA receptor.[16] Many 5-HT3 antagonists function as antiemetics, however the clinical significance of this serotonergic activity in the treatment of Alzheimer's disease is unknown.
Cholinergic (nicotinic acetylcholine receptor)
Memantine acts as a non-competitive antagonist at different neuronal nicotinic acetylcholine receptors (nAChRs) at potencies possibly similar to the NMDA and 5-HT3 receptors, but this is difficult to ascertain with accuracy because of the rapid desensitization of nAChR responses in these experiments. It can be noted that memantine is an antagonist at Alpha-7 nAChR, which may contribute to initial worsening of cognitive function during early memantine treatment. Alpha-7 nAChR upregulates quickly in response to antagonism, which could explain the cognitive-enhancing effects of chronic memantine treatment.[17][18] It has been shown that the number of nicotinic receptors in the brain are reduced in Alzheimer's disease, even in the absence of a general decrease in the number of neurons, and nicotinic receptor agonists are viewed as interesting targets for anti-Alzheimer drugs.[19]
Dopaminergic (D2 receptor)
Memantine acts as an agonist at the dopamine D2 receptor with equal or slightly higher affinity than to the NMDA receptors.[20]
Sigmaergic (σ1 receptor)
It acts as an agonist at the σ1 receptor with a low Ki of 2.6 µm (2600 nm).[21] The consequences of this activity are unclear (as the role of sigma receptors in general is not yet that well understood) and memantine is probably too weak at the sigma binding site to exhibit significant agonist effects, only exhibiting partial agonism or antagonism. Some of memantine's adverse effects arise through this route[22].
History
Memantine was first synthesized and patented by Eli Lilly and Company in 1968 as an anti-diabetic agent, but it was ineffective at lowering blood sugar. Later it was discovered to have CNS activity, and was developed by Merz for dementia in Germany; the NMDA activity was discovered after clinical trials had already begun. Memantine was first marketed for dementia in Germany in 1989 under the name Axura.[23]
In the US, some CNS activities were discovered at Children's Hospital of Boston in 1995, and Children's licensed patents covering uses of memantine outside the field of ophthalmology to Neurobiological Technologies (NTI) in 1995.[24] In 1998 NTI amended its agreement with Children's to allow Merz to take over development.[25]
In 2000 Merz partnered with Forest to develop the drug for Alzheimers in the U.S. under the name Namenda.[23]
In 2000 Merz partnered with Suntory for the Japanese market and with Lundbeck for other markets including Europe;[26] the drug was originally marketed by Lundbeck under the name Ebixa.[23]
Sales of the drug reached $1.8 billion for 2014.[27] The cost of Namenda was $269 to $489 a month in 2012.[28]
In February 2014 as the July 2015 patent expiration for memantine neared, Actavis, which had acquired Forest, announced that it was launching an extended release (XR) form of memantine that could be taken once a day instead of twice a day as needed with the then-current "immediate release" (IR) version, and that it intended to stop selling the IR version in August 2014 and withdraw the marketing authorization. This is a tactic to thwart generic competition called "product hopping". However the supply of the XR version ran short, so Actavis extended the deadline until the fall. In September 2014 the attorney general of New York, Eric Schneiderman, filed a lawsuit to compel Actavis to keep selling the IR version on the basis of antitrust law.[29][30]
In December 2014, a judge granted New York State its request and issued an injunction, preventing Actavis from withdrawing the IR version until generic versions could launch. Actavis appealed and in May a panel of the Second Circuit Court of Appeals upheld the injunction, and in June Actavis asked that its case be heard by the full Second Circuit panel.[31][32] In August 2015 Actavis' request was denied.[33]
Society and culture
As of August 2017 memantine was marketed under many brand names worldwide including Abixa, Adaxor, Admed, Akatinol, Alceba, Alios, Almenta, Alois, Alzant, Alzer, Alzia, Alzinex, Alzixa, Alzmenda, Alzmex, Axura, Biomentin, Carrier, Cogito, Cognomem, Conexine, Cordure, Dantex, Demantin, Demax, Dementa, Dementexa, Ebitex, Ebixa, Emantin, Emaxin, Esmirtal, Eutebrol, Evy, Ezemantis, Fentina, Korint, Lemix, Lindex, Lindex, Lucidex, Manotin, Mantine, Mantomed, Marbodin, Mardewel, Marixino, Maruxa, Maxiram, Melanda, Memabix, Memamed, Memando, Memantin, Memantina, Memantine, Mémantine, Memantinol, Memantyn, Memanvitae, Memanxa, Memanzaks, Memary, Memax, Memexa, Memigmin, Memikare, Memogen, Memolan, Memorel, Memorix, Memotec, Memox, Memxa, Mentikline, Mentium, Mentixa, Merandex, Merital, Mexia, Mimetix, Mirvedol, Modualz, Morysa, Namenda, Nemdatine, Nemdatine, Nemedan, Neumantine, Neuro-K, Neuroplus, Noojerone, Polmatine, Prilben, Pronervon, Ravemantine, Talentum, Timantila, Tingreks, Tonibral, Tormoro, Valcoxia, Vilimen, Vivimex, Vizarsin, Witgen, Xapimant, Xapimant, Ymana, Zalatine, Zemertinex, Zenmem, Zenmen, and Zimerz.[1]
It was also marketed in some countries as a combination drug with donepezil under the brands Namzaric, Neuroplus Dual, and Tonibral MD.[1]
Research
Memantine has been studied in dementia with Lewy bodies,[34][35] obsessive compulsive disorder,[36] generalized anxiety disorder, as an augmentation therapy for anxiety disorders,[37] ADHD,[38] as well as to help slowing down or even reversing the tolerance development to opioids.[39]
It has also been studied for nystagmus,[40] multiple sclerosis,[41] and migraine.[42]
See also
References
- ^ a b c "International brands for memantine". Drugs.com. Retrieved 7 August 2017.
- ^ Mount C, Downton C (July 2006). "Alzheimer disease: progress or profit?". Nat. Med. 12 (7): 780–4. doi:10.1038/nm0706-780. PMID 16829947.
- ^ NICE technology appraisal January 18, 2011 Azheimer's disease - donepezil, galantamine, rivastigmine and memantine (review): final appraisal determination
- ^ a b c Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006.
- ^ McShane, R; Areosa Sastre, A; Minakaran, N (19 April 2006). "Memantine for dementia". The Cochrane Database of Systematic Reviews (2): CD003154. doi:10.1002/14651858.CD003154.pub5. PMID 16625572.
- ^ Schneider, LS; Dagerman, KS; Higgins, JP; McShane, R (August 2011). "Lack of evidence for the efficacy of memantine in mild Alzheimer disease". Archives of Neurology. 68 (8): 991–8. doi:10.1001/archneurol.2011.69. PMID 21482915.
- ^ Joint Formulary Committee (2004). British National Formulary (47th ed.). London: BMA and the Royal Pharmaceutical Society of Great Britain. ISBN 0-85369-584-9.
- ^ a b Morris H, Wallach J (2014). "From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs". Drug Testing and Analysis. 6 (7–8): 614–32. doi:10.1002/dta.1620. PMID 24678061.
- ^ Swedberg MD, Ellgren M, Raboisson P (2014). "mGluR5 antagonist-induced psychoactive properties: MTEP drug discrimination, a pharmacologically selective non-NMDA effect with apparent lack of reinforcing properties". The Journal of Pharmacology and Experimental Therapeutics. 349 (1): 155–64. doi:10.1124/jpet.113.211185. PMID 24472725.
- ^ Cacabelos R, Takeda M, Winblad B (January 1999). "The glutamatergic system and neurodegeneration in dementia: preventive strategies in Alzheimer's disease". Int J Geriatr Psychiatry. 14 (1): 3–47. doi:10.1002/(SICI)1099-1166(199901)14:1<3::AID-GPS897>3.0.CO;2-7. PMID 10029935.
- ^ Rogawski, MA; Wenk GL (2003). "The neuropharmacological basis for the use of memantine in the treatment of Alzheimer's disease". CNS Drug Rev. 9 (3): 275–308. doi:10.1111/j.1527-3458.2003.tb00254.x. PMID 14530799.
- ^ Robinson, DM; Keating GM (2006). "Memantine: a review of its use in Alzheimer's disease". Drugs. 66 (11): 1515–34. doi:10.2165/00003495-200666110-00015. PMID 16906789.
- ^ Xia P, Chen HS, Zhang D, Lipton SA (2010). "Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses". J. Neurosci. 30 (33): 11246–11250. doi:10.1523/JNEUROSCI.2488-10.2010. PMC 2932667. PMID 20720132.
- ^ a b Parsons CG, Stöffler A, Danysz W (November 2007). "Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system — too little activation is bad, too much is even worse". Neuropharmacology. 53 (6): 699–723. doi:10.1016/j.neuropharm.2007.07.013. PMID 17904591.
- ^ Lipton SA (October 2007). "Pathologically activated therapeutics for neuroprotection". Nature Reviews Neuroscience. 8 (10): 803–8. doi:10.1038/nrn2229. PMID 17882256.
- ^ Rammes G, Rupprecht R, Ferrari U, Zieglgänsberger W, Parsons CG (2001). "The N-methyl-D-aspartate receptor channel blockers memantine, MRZ 2/579 and other amino-alkyl-cyclohexanes antagonise 5-HT(3) receptor currents in cultured HEK-293 and N1E-115 cell systems in a non-competitive manner". Neuroscience Letters. 306 (1–2): 81–4. doi:10.1016/S0304-3940(01)01872-9. PMID 11403963.
- ^ Buisson B, Bertrand D (1 March 1998). "Open-channel blockers at the human alpha4beta2 neuronal nicotinic acetylcholine receptor". Mol. Pharmacol. 53 (3): 555–63. doi:10.1124/mol.53.3.555. PMID 9495824.
- ^ Aracava Y, Pereira EF, Maelicke A, Albuquerque EX (March 2005). "Memantine blocks alpha7* nicotinic acetylcholine receptors more potently than n-methyl-D-aspartate receptors in rat hippocampal neurons". J Pharmacol Exp Ther. 312 (3): 1195–205. doi:10.1124/jpet.104.077172. PMID 15522999.
- ^ Gotti C, Clementi F (December 2004). "Neuronal nicotinic receptors: from structure to pathology". Prog Neurobiol. 74 (6): 363–96. doi:10.1016/j.pneurobio.2004.09.006. PMID 15649582.
- ^ Seeman P, Caruso C, Lasaga M (February 2008). "Memantine agonist action at dopamine D2High receptors". Synapse. 62 (2): 149–53. doi:10.1002/syn.20472. PMID 18000814.
- ^ Peeters M, Romieu P, Maurice T, Su TP, Maloteaux JM, Hermans E (April 2004). "Involvement of the sigma 1 receptor in the modulation of dopaminergic transmission by amantadine". Eur J Neurosci. 19 (8): 2212–20. doi:10.1111/j.0953-816X.2004.03297.x. PMID 15090047.
- ^ citation needed
- ^ a b c Witt A, Macdonald N, Kirkpatrick P. Memantine hydrochloride. Nat Rev Drug Discov. 2004 Feb;3(2):109-10. PMID 15040575
- ^ "Form 10-KSB For the fiscal year ended June 30, 1996". SEC Edgar. 30 September 1996. NTI-Childrens' license is included in the filing.
- ^ Delevett, Peter (9 January 2000). "Cash is king, focus is queen". Silicon Valley Business Journal.
- ^ Staff, the Pharma Letter. August 15, 2000 Lundbeck signs memantine licensing agreement for Merz+Co
- ^ Drugs.com Namenda Sales Data. February 2014.
- ^ ConsumerReportsHealth. Evaluating Prescription Drugs Used to Treat: Alzheimer’s Disease. Comparing Effectiveness, Safety, and Price. Updated in May 2012.
- ^ Pollack, Andrew (15 September 2014). "Forest Laboratories' Namenda Is Focus of Lawsuit". The New York Times.
- ^ Capati, Vincent C.; Kesselheim, Aaron S. (29 March 2016). "Drug Product Life-Cycle Management as Anticompetitive Behavior: The Case of Memantine". Journal of Managed Care & Specialty Pharmacy. 22 (4): 339–344. doi:10.18553/jmcp.2016.22.4.339. ISSN 2376-0540.
- ^ "Actavis Confirms Appeals Court Ruling Requiring Continued Distribution of Namenda IR". Actavis. 22 May 2015.
- ^ Gurrieri, Vin (9 June 2015). "Actavis, Others Plotted To Delay Generic Namenda, Suit Says - Law360". Law360.
- ^ LoBiondo, George A. (12 August 2015). "Second Circuit Denies Petition for Actavis Rehearing | David Kleban". Patterson Belknap Webb & Tyler LLP.
- ^ Aarsland, D; Ballard, C; Walker, Z; Bostrom, F; Alves, G; Kossakowski, K; Leroi, I; Pozo-Rodriguez, F; Minthon, L; Londos, E (July 2009). "Memantine in patients with Parkinson's disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial". Lancet Neurology. 8 (7): 613–8. doi:10.1016/S1474-4422(09)70146-2. PMID 19520613.
- ^ Johansson, C; Ballard, C; Hansson, O; Palmqvist, S; Minthon, L; Aarsland, D; Londos, E (February 2011). "Efficacy of memantine in PDD and DLB: an extension study including washout and open-label treatment". International journal of geriatric psychiatry. 26 (2): 206–13. doi:10.1002/gps.2516. PMID 20665553.
- ^ Haghighi M, Jahangard L, Mohammad-Beigi H, Bajoghli H, Hafezian H, Rahimi A, Afshar H, Holsboer-Trachsler E, Brand S (2013). "In a double-blind, randomized and placebo-controlled trial, adjuvant memantine improved symptoms in inpatients suffering from refractory obsessive-compulsive disorders (OCD)". Psychopharmacology. 228 (4): 633–40. doi:10.1007/s00213-013-3067-z. PMID 23525525.
- ^ Schwartz, Thomas L.; Siddiqui, Umar A.; Raza, Shafi (2012). "Memantine as an Augmentation Therapy for Anxiety Disorders". Case Reports in Psychiatry. 2012: 1–3. doi:10.1155/2012/749796.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Hosenbocus S, Chahal R (2013). "Memantine: a review of possible uses in child and adolescent psychiatry". Journal of the Canadian Academy of Child and Adolescent Psychiatry. 22 (2): 166–71. PMC 3647634. PMID 23667364.
- ^ Bisaga A, Comer SD, Ward AS, Popik P, Kleber HD, Fischman MW (2001). "The NMDA antagonist memantine attenuates the expression of opioid physical dependence in humans". Psychopharmacology. 157 (1): 1–10. doi:10.1007/s002130100739. PMID 11512037.
- ^ Corbett J (September 2007). "Memantine/Gabapentin for the treatment of congenital nystagmus". Curr Neurol Neurosci Rep. 7 (5): 395–6. doi:10.1007/s11910-007-0061-z. PMID 17764629.
- ^ Villoslada P, Arrondo G, Sepulcre J, Alegre M, Artieda J (December 2008). "Memantine induces reversible neurologic impairment in patients with MS". Neurology. 72 (19): 1630–3. doi:10.1212/01.wnl.0000342388.73185.80. PMID 19092106.
- ^ Borghol, Amne; Kirkwood A; Hawawini F (May 2010). "Memantine for the Treatment of Migraine". US Pharm. 35 (5): 28–35.
Further reading
- Lipton SA (2005). "The molecular basis of memantine action in Alzheimer's disease and other neurologic disorders: low-affinity, uncompetitive antagonism". Current Alzheimer research. 2 (2): 155–65. doi:10.2174/1567205053585846. PMID 15974913.
External links
