E-selectin: Difference between revisions
No edit summary |
|||
| Line 6: | Line 6: | ||
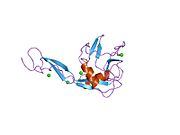
E selectin has a cassette structure: an N-terminal, [[C-type lectin]] domain, an epidermal-growth-factor (EGF)-like domain, 6 consensus repeat units, a transmembrane domain (TM) and an intracellular cytoplasmic tail (cyto). The three-dimensional structure of the ligand-binding region of human E-selectin has been determined at 2.0 Å resolution in 1994.<ref>{{cite journal|last=Graves|first=B.J.|coauthors=Crowther, R.L., Chandran, C., Rumberger, J.M., Li, S., Huang, K.S., Presky, D.H., Familletti, P.C., Wolitzky, B.A., Burns, D.K.|title=Insight into E-selectin/ligand interaction from the crystal structure and mutagenesis of the lec/EGF domains|journal=Nature|date=10|year=1994|month=Feb|pages=532-538}}</ref> The structure reveals limited contact between the two domains and a coordination of Ca2+ not predicted from other C-type lectins. Structure/function analysis indicates a defined region and specific amino-acid side chains that may be involved in ligand binding. The E-selectin bind weakly to sialyl LewisX sialyl LewisX (SLeX, NeuNAcα2,3Galβ1,4[Fucα1,3]GlcNAc) tetrasaccharide are solved in 2000.<ref>{{cite journal|last=Somers|first=WS|coauthors=Tang J, Shaw GD, Camphausen RT.|title=Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1.|journal=Cell|date=29|year=2001|month=Jun|volume=103|issue=3|pages=467-479}}</ref> |
E selectin has a cassette structure: an N-terminal, [[C-type lectin]] domain, an epidermal-growth-factor (EGF)-like domain, 6 consensus repeat units, a transmembrane domain (TM) and an intracellular cytoplasmic tail (cyto). The three-dimensional structure of the ligand-binding region of human E-selectin has been determined at 2.0 Å resolution in 1994.<ref>{{cite journal|last=Graves|first=B.J.|coauthors=Crowther, R.L., Chandran, C., Rumberger, J.M., Li, S., Huang, K.S., Presky, D.H., Familletti, P.C., Wolitzky, B.A., Burns, D.K.|title=Insight into E-selectin/ligand interaction from the crystal structure and mutagenesis of the lec/EGF domains|journal=Nature|date=10|year=1994|month=Feb|pages=532-538}}</ref> The structure reveals limited contact between the two domains and a coordination of Ca2+ not predicted from other C-type lectins. Structure/function analysis indicates a defined region and specific amino-acid side chains that may be involved in ligand binding. The E-selectin bind weakly to sialyl LewisX sialyl LewisX (SLeX, NeuNAcα2,3Galβ1,4[Fucα1,3]GlcNAc) tetrasaccharide are solved in 2000.<ref>{{cite journal|last=Somers|first=WS|coauthors=Tang J, Shaw GD, Camphausen RT.|title=Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1.|journal=Cell|date=29|year=2001|month=Jun|volume=103|issue=3|pages=467-479}}</ref> |
||
==Regulation== |
==Gene and Regulation== |
||
In humans, E-selectin is encoded by the SELE gene. Its CRD, EGF-like, CR repeat, and transmembrane domains are each encoded by separate exons, whereas the E-selectin cytosolic domain derives from two exons. The E-selectin locus flanks the L-selectin locus on chromosome 1.<ref>{{cite book|title=Essentials of Glycobiology|year=2009|publisher=Cold Spring Harbor Laboratory Press|isbn=9780879697709|url=http://www.ncbi.nlm.nih.gov/books/NBK20727/|edition=2nd|editor=Varki A, Richard D Cummings, Jeffrey D Esko, Hudson H Freeze, Pamela Stanley, Carolyn R Bertozzi, Gerald W Hart, and Marilynn E Etzler.|chapter=26}}</ref> |
|||
Different from P-selectin, which is stored in vesicles called [[Weibel-Palade bodies]], E-selectin is not stored in the cell and has to be transcribed, translated, and transported to the cell surface. The production of E-selectin is stimulated by the expression of P-selectin which is stimulated by tumor necrosis factor α ([[tumor necrosis factor-alpha|TNFα]]), and it can also be stimulated by interleukin-1 ([[interleukin 1|IL-1]]) and lipopolysaccharide ([[lipopolysaccharide|LPS]]).<ref name="isbn0-8153-4101-6">{{cite book | author = Janeway C | title = Immunobiology: the immune system in health and disease | publisher = Garland Science | location = New York | year = 2005 | isbn = 0-8153-4101-6 }}</ref><ref name="Leeuwenberg_1992">{{cite journal | author = Leeuwenberg JF, Smeets EF, Neefjes JJ, Shaffer MA, Cinek T, Jeunhomme TM, Ahern TJ, Buurman WA | title = E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro | journal = Immunology | volume = 77 | issue = 4 | pages = 543–9 | year = 1992 | month = December | pmid = 1283598 | pmc = 1421640 | doi = }}</ref> It takes about two hours, after [[cytokine]] recognition, for E-selectin to be expressed on the endothelial cell's surface, with maximal expression of E-selectin occurring around 6-12 hours after cytokine recognition by the cell.<ref name="Leeuwenberg_1992"/> E-selectin production levels-out 24 hours after the cytokine signal, and continues to be expressed for three days. |
Different from P-selectin, which is stored in vesicles called [[Weibel-Palade bodies]], E-selectin is not stored in the cell and has to be transcribed, translated, and transported to the cell surface. The production of E-selectin is stimulated by the expression of P-selectin which is stimulated by tumor necrosis factor α ([[tumor necrosis factor-alpha|TNFα]]), and it can also be stimulated by interleukin-1 ([[interleukin 1|IL-1]]) and lipopolysaccharide ([[lipopolysaccharide|LPS]]).<ref name="isbn0-8153-4101-6">{{cite book | author = Janeway C | title = Immunobiology: the immune system in health and disease | publisher = Garland Science | location = New York | year = 2005 | isbn = 0-8153-4101-6 }}</ref><ref name="Leeuwenberg_1992">{{cite journal | author = Leeuwenberg JF, Smeets EF, Neefjes JJ, Shaffer MA, Cinek T, Jeunhomme TM, Ahern TJ, Buurman WA | title = E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro | journal = Immunology | volume = 77 | issue = 4 | pages = 543–9 | year = 1992 | month = December | pmid = 1283598 | pmc = 1421640 | doi = }}</ref> It takes about two hours, after [[cytokine]] recognition, for E-selectin to be expressed on the endothelial cell's surface, with maximal expression of E-selectin occurring around 6-12 hours after cytokine recognition by the cell.<ref name="Leeuwenberg_1992"/> E-selectin production levels-out 24 hours after the cytokine signal, and continues to be expressed for three days. |
||
Shear forces are also found to affect E-selectin expression. A high laminar shear enhances acute endothelial cell response to interleukin-1ß in naïve or shear-conditioned endothelial cells as may be found in the pathological setting of ischemia/reperfusion injury while conferring rapid E-selectin downregulation to protect against chronic inflammation.<ref>{{cite journal|last=Huang,|first=Ryan B.|coauthors=Eniola-Adefeso, Omolola|title=Shear stress modulation of IL-1β-induced E-selectin expression in human endothelial cells.|journal=PLoS One|date=24|year=2012|month=Feb|volume=7|issue=2|doi=10.1371/journal.pone.0031874}}</ref> |
|||
Phytoestrogens, plant compounds with estrogen-like biological activity, such as genistein, formononetin, biochanin A and daidzein, as well as the mix were found able to reduce E-selectin as well as VCAM-1and ICAM-1 on cell surface and in culture supernatant.<ref>{{cite journal|last=Andrade|first=CM|coauthors=Sá MF, Toloi MR.|title=Effects of phytoestrogens derived from soy bean on expression of adhesion molecules on HUVEC.|journal=Climacteric|year=2012|month=April|volume=15|issue=2}}</ref> |
|||
== Ligands == |
== Ligands == |
||
| Line 24: | Line 30: | ||
== Pathological relevance == |
== Pathological relevance == |
||
In cases of elevated blood glucose levels, such as in sepsis, E-selectin expression is higher than normal, resulting in greater microvascular permeability. The greater permeability leads to [[edema]] of the skeletal [[endothelium]], resulting in skeletal muscle [[ischemia]] and eventually [[necrosis]]. This underlying pathology is the cause of the symptomatic disease [[Critical illness polyneuropathy|critical illness polyneuromyopathy]] (CIPNM).<ref name="pmid17038033">{{cite journal | author = Visser LH | title = Critical illness polyneuropathy and myopathy: clinical features, risk factors and prognosis | journal = Eur. J. Neurol. | volume = 13 | issue = 11 | pages = 1203–12 | year = 2006 | month = November | pmid = 17038033 | doi = 10.1111/j.1468-1331.2006.01498.x | url = }}</ref> Traditional Chinese herbal medicines, like [[berberine]] downregulate E-selectin.<ref name="Hu_2009">{{cite journal | author = Hu Y, Chen X, Duan H, Hu Y, Mu X | title = Chinese herbal medicinal ingredients inhibit secretion of IL-6, IL-8, E-selectin and TXB2 in LPS-induced rat intestinal microvascular endothelial cells | journal = Immunopharmacol Immunotoxicol | volume = 31 | issue = 4 | pages = 550–5 | year = 2009 | pmid = 19874221 | doi = 10.3109/08923970902814129 }}</ref> |
1. In cases of elevated blood glucose levels, such as in sepsis, E-selectin expression is higher than normal, resulting in greater microvascular permeability. The greater permeability leads to [[edema]] of the skeletal [[endothelium]], resulting in skeletal muscle [[ischemia]] and eventually [[necrosis]]. This underlying pathology is the cause of the symptomatic disease [[Critical illness polyneuropathy|critical illness polyneuromyopathy]] (CIPNM).<ref name="pmid17038033">{{cite journal | author = Visser LH | title = Critical illness polyneuropathy and myopathy: clinical features, risk factors and prognosis | journal = Eur. J. Neurol. | volume = 13 | issue = 11 | pages = 1203–12 | year = 2006 | month = November | pmid = 17038033 | doi = 10.1111/j.1468-1331.2006.01498.x | url = }}</ref> Traditional Chinese herbal medicines, like [[berberine]] downregulate E-selectin.<ref name="Hu_2009">{{cite journal | author = Hu Y, Chen X, Duan H, Hu Y, Mu X | title = Chinese herbal medicinal ingredients inhibit secretion of IL-6, IL-8, E-selectin and TXB2 in LPS-induced rat intestinal microvascular endothelial cells | journal = Immunopharmacol Immunotoxicol | volume = 31 | issue = 4 | pages = 550–5 | year = 2009 | pmid = 19874221 | doi = 10.3109/08923970902814129 }}</ref> |
||
2. E-selectin also plays a role in pathogen attachment. |
|||
Study shows the adherence of Porphyromonas gingivalis to human umbilical vein endothelial cells increases with the induction of E-selectin expression by TNF-α. An antibody to E-selectin and sialyl Lewis X suppressed P. gingivalis adherence to stimulated HUVECs. P. gingivalis mutants lacking OmpA-like proteins Pgm6/7 had reduced adherence to stimulated HUVECs, but fimbriae-deficient mutants were not affected. E-selecin-mediated P. gingivalis adherence activated endothelial exocytosis. These results suggest that the interaction between host E-selectin and pathogen Pgm6/7 mediates P. gingivalis adherence to endothelial cells and may trigger vascular inflammation.<ref>{{cite journal|last=Komatsu|first=T|coauthors=Nagano K, Sugiura S, Hagiwara M, Tanigawa N, Abiko Y, Yoshimura F, Furuichi Y, Matsushita K.|title=E-selectin Mediates Porphyromonas gingivalis Adherence to Human Endothelial Cells.|journal=Infection and Immunity|date=16|year=2012|month=April}}</ref> |
|||
3.Acute coronary syndrome |
|||
The immunohistochemical expressions of E-selectin and PECAM-1 were significantly increased at intima in vulnerable plaques of ACS group, especially in neovascular endothelial cells, and positively correlated with inflammatory cell density, suggesting that PECAM-1 and E-selectin might play an important role in inflammatory reaction and development of vulnerable plaque. E-selectin Ser128Arg polymorphism is associated with ACS, and it might be a risk factor for ACS.<ref>{{cite journal|last=Fang|first=F|coauthors=Zhang W, Yang L, Wang Z, Liu DG.|title=PECAM-1 and E-selectin expression in vulnerable plague and their relationships to myocardial Leu125Val polymorphism of PECAM-1 and Ser128Arg polymorphism of E-selectin in patients with acute coronary syndrome|journal=Zhonghua Xin Xue Guan Bing Za Zhi|date=39|year=2011|month=December}}</ref> |
|||
4. Nicotine-mediated induction of E-selectin in aortic endothelial cells requires Src kinase and E2F1 transcriptional activity. |
|||
Smoking is highly correlated with enhanced likelihood of atherosclerosis by inducing endothelial dysfunction. In endothelial cells, various cell-adhesion molecules including E-selectin, are shown to be upregulated upon exposure to nicotine, the addictive component of tobacco smoke. Nicotine-stimulated adhesion of monocytes to endothelial cells is dependent on the activation of α7-nAChRs, β-Arr1 and cSrc regulated increase in E2F1-mediated transcription of E-selectin gene. Therefore, agents such as RRD-251 that can target activity of E2F1 may have potential therapeutic benefit against cigarette smoke induced atherosclerosis.<ref>{{cite journal|last=Alamanda|first=V|coauthors=Singh S, Lawrence NJ, Chellappan SP.|title=Nicotine-mediated induction of E-selectin in aortic endothelial cells requires Src kinase and E2F1 transcriptional activity.|journal=Biochemical and Biophysical Research Communication|date=3|year=2012|month=Feb}}</ref> |
|||
| ⚫ | 5. E-selectin is also an emerging biomarker for the metastatic potential of some cancers including colorectal cancer and recurrences.<ref name="Sato_2010">{{cite journal | author = Sato H, Usuda N, Kuroda M, Hashimoto S, Maruta M, Maeda K | title = Significance of serum concentrations of E-selectin and CA19-9 in the prognosis of colorectal cancer | journal = Jpn. J. Clin. Oncol. | volume = 40 | issue = 11 | pages = 1073–80 | year = 2010 | month = November | pmid = 20576794 | doi = 10.1093/jjco/hyq095 }}</ref> |
||
It's also found that E-selectin expression increased in human ruptured cerebral aneurysm tissues. E-selectin might be an important factor involved in the process of cerebral aneurysm formation and rupture, by promoting inflammation and weakening cerebral artery walls.<ref>{{cite journal|last=Jia|first=W|coauthors=Wang R , Zhao J , Liu IY , Zhang D , Wang X, Han X .|title=E-selectin expression increased in human ruptured cerebral aneurysm tissues.|journal=Canadian Journal Neurological Sciences|year=2011|month=Nov}}</ref> |
|||
| ⚫ | E-selectin is also an emerging biomarker for the metastatic potential of some cancers including colorectal cancer and recurrences.<ref name="Sato_2010">{{cite journal | author = Sato H, Usuda N, Kuroda M, Hashimoto S, Maruta M, Maeda K | title = Significance of serum concentrations of E-selectin and CA19-9 in the prognosis of colorectal cancer | journal = Jpn. J. Clin. Oncol. | volume = 40 | issue = 11 | pages = 1073–80 | year = 2010 | month = November | pmid = 20576794 | doi = 10.1093/jjco/hyq095 }}</ref> |
||
== References == |
== References == |
||
Revision as of 01:37, 5 May 2012
Template:PBB E-selectin, also known as CD62 antigen-like family member E (CD62E), endothelial-leukocyte adhesion molecule 1 (ELAM-1), or leukocyte-endothelial cell adhesion molecule 2 (LECAM2), is a cell adhesion molecule expressed only on endothelial cells activated by cytokines. Like other selectins, it plays an important part in inflammation. In humans, E-selectin is encoded by the SELE gene.[1]
Structure
E selectin has a cassette structure: an N-terminal, C-type lectin domain, an epidermal-growth-factor (EGF)-like domain, 6 consensus repeat units, a transmembrane domain (TM) and an intracellular cytoplasmic tail (cyto). The three-dimensional structure of the ligand-binding region of human E-selectin has been determined at 2.0 Å resolution in 1994.[2] The structure reveals limited contact between the two domains and a coordination of Ca2+ not predicted from other C-type lectins. Structure/function analysis indicates a defined region and specific amino-acid side chains that may be involved in ligand binding. The E-selectin bind weakly to sialyl LewisX sialyl LewisX (SLeX, NeuNAcα2,3Galβ1,4[Fucα1,3]GlcNAc) tetrasaccharide are solved in 2000.[3]
Gene and Regulation
In humans, E-selectin is encoded by the SELE gene. Its CRD, EGF-like, CR repeat, and transmembrane domains are each encoded by separate exons, whereas the E-selectin cytosolic domain derives from two exons. The E-selectin locus flanks the L-selectin locus on chromosome 1.[4]
Different from P-selectin, which is stored in vesicles called Weibel-Palade bodies, E-selectin is not stored in the cell and has to be transcribed, translated, and transported to the cell surface. The production of E-selectin is stimulated by the expression of P-selectin which is stimulated by tumor necrosis factor α (TNFα), and it can also be stimulated by interleukin-1 (IL-1) and lipopolysaccharide (LPS).[5][6] It takes about two hours, after cytokine recognition, for E-selectin to be expressed on the endothelial cell's surface, with maximal expression of E-selectin occurring around 6-12 hours after cytokine recognition by the cell.[6] E-selectin production levels-out 24 hours after the cytokine signal, and continues to be expressed for three days.
Shear forces are also found to affect E-selectin expression. A high laminar shear enhances acute endothelial cell response to interleukin-1ß in naïve or shear-conditioned endothelial cells as may be found in the pathological setting of ischemia/reperfusion injury while conferring rapid E-selectin downregulation to protect against chronic inflammation.[7]
Phytoestrogens, plant compounds with estrogen-like biological activity, such as genistein, formononetin, biochanin A and daidzein, as well as the mix were found able to reduce E-selectin as well as VCAM-1and ICAM-1 on cell surface and in culture supernatant.[8]
Ligands
E-selectin recognizes and binds to sialylated carbohydrates present on the surface proteins of certain leukocytes. These carbohydrates include members of the Lewis X and Lewis A families found on monocytes, granulocytes, and T-lymphocytes.[9]
E-selectin ligands are expressed by neutrophils, monocytes, eosinophils, memory-effector T-like lymphocytes, and natural killer cells. Each of these cell types is found in acute and chronic inflammatory sites in association with expression of E-selectin, thus implicating E-selectin in the recruitment of these cells to such inflammatory sites. Efforts to assign a causal relationship to this association have included the generation and analysis of mice homozygous for null alleles at the E-selectin locus. These mice maintain virtually normal leukocyte recruitment in a peritoneal exudate model and in a delayed-type hypersensitivity model, both of which measure recruitment of neutrophils and other leukocytes in the context of acute inflammation. However, a leukocyte-trafficking defect in these mice is unmasked by administration of a blocking monoclonal antibody against P-selectin, a treatment that does not yield a leukocyte-trafficking defect in wild-type animals. These observations imply that both E- and P-selectin maintain an apparent functional redundancy, at least in mice, in the limited circumstances where inflammation has been carefully examined. Nevertheless, other animal studies using blocking anti-E-selectin antibodies have shown that E-selectin can provide an essential, nonredundant contribution to leukocyte trafficking in acute inflammation.
Function
During inflammation, E-selectin plays an important part in recruiting leukocytes to the site of injury. The local release of cytokines IL-1 and TNF-α by damaged cells induces the over-expression of E-selectin on endothelial cells of nearby blood vessels.[9] Leukocytes in the blood expressing the correct ligand will bind with low affinity to E-selectin, also under the shear stress of blood flow, causing the leukocytes to "roll" along the internal surface of the blood vessel as temporary interactions are made and broken.
As the inflammatory response progresses, chemokines released by injured tissue enter the blood vessels and activate the rolling leukocytes, which are now able to tightly bind to the endothelial surface and begin making their way into the tissue.[9]
P-selectin has a similar function, but is expressed on the endothelial cell surface within minutes as it is stored within the cell rather than produced on demand.[9]
Pathological relevance
1. In cases of elevated blood glucose levels, such as in sepsis, E-selectin expression is higher than normal, resulting in greater microvascular permeability. The greater permeability leads to edema of the skeletal endothelium, resulting in skeletal muscle ischemia and eventually necrosis. This underlying pathology is the cause of the symptomatic disease critical illness polyneuromyopathy (CIPNM).[10] Traditional Chinese herbal medicines, like berberine downregulate E-selectin.[11]
2. E-selectin also plays a role in pathogen attachment. Study shows the adherence of Porphyromonas gingivalis to human umbilical vein endothelial cells increases with the induction of E-selectin expression by TNF-α. An antibody to E-selectin and sialyl Lewis X suppressed P. gingivalis adherence to stimulated HUVECs. P. gingivalis mutants lacking OmpA-like proteins Pgm6/7 had reduced adherence to stimulated HUVECs, but fimbriae-deficient mutants were not affected. E-selecin-mediated P. gingivalis adherence activated endothelial exocytosis. These results suggest that the interaction between host E-selectin and pathogen Pgm6/7 mediates P. gingivalis adherence to endothelial cells and may trigger vascular inflammation.[12]
3.Acute coronary syndrome The immunohistochemical expressions of E-selectin and PECAM-1 were significantly increased at intima in vulnerable plaques of ACS group, especially in neovascular endothelial cells, and positively correlated with inflammatory cell density, suggesting that PECAM-1 and E-selectin might play an important role in inflammatory reaction and development of vulnerable plaque. E-selectin Ser128Arg polymorphism is associated with ACS, and it might be a risk factor for ACS.[13]
4. Nicotine-mediated induction of E-selectin in aortic endothelial cells requires Src kinase and E2F1 transcriptional activity. Smoking is highly correlated with enhanced likelihood of atherosclerosis by inducing endothelial dysfunction. In endothelial cells, various cell-adhesion molecules including E-selectin, are shown to be upregulated upon exposure to nicotine, the addictive component of tobacco smoke. Nicotine-stimulated adhesion of monocytes to endothelial cells is dependent on the activation of α7-nAChRs, β-Arr1 and cSrc regulated increase in E2F1-mediated transcription of E-selectin gene. Therefore, agents such as RRD-251 that can target activity of E2F1 may have potential therapeutic benefit against cigarette smoke induced atherosclerosis.[14]
5. E-selectin is also an emerging biomarker for the metastatic potential of some cancers including colorectal cancer and recurrences.[15]
It's also found that E-selectin expression increased in human ruptured cerebral aneurysm tissues. E-selectin might be an important factor involved in the process of cerebral aneurysm formation and rupture, by promoting inflammation and weakening cerebral artery walls.[16]
References
- ^ Collins T, Williams A, Johnston GI, Kim J, Eddy R, Shows T, Gimbrone MA, Bevilacqua MP (1991). "Structure and chromosomal location of the gene for endothelial-leukocyte adhesion molecule 1". J. Biol. Chem. 266 (4): 2466–73. PMID 1703529.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Graves, B.J. (10). "Insight into E-selectin/ligand interaction from the crystal structure and mutagenesis of the lec/EGF domains". Nature: 532–538.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Somers, WS (29). "Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1". Cell. 103 (3): 467–479.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Varki A, Richard D Cummings, Jeffrey D Esko, Hudson H Freeze, Pamela Stanley, Carolyn R Bertozzi, Gerald W Hart, and Marilynn E Etzler., ed. (2009). "26". Essentials of Glycobiology (2nd ed.). Cold Spring Harbor Laboratory Press. ISBN 9780879697709.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ Janeway C (2005). Immunobiology: the immune system in health and disease. New York: Garland Science. ISBN 0-8153-4101-6.
- ^ a b Leeuwenberg JF, Smeets EF, Neefjes JJ, Shaffer MA, Cinek T, Jeunhomme TM, Ahern TJ, Buurman WA (1992). "E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro". Immunology. 77 (4): 543–9. PMC 1421640. PMID 1283598.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Huang,, Ryan B. (24). "Shear stress modulation of IL-1β-induced E-selectin expression in human endothelial cells". PLoS One. 7 (2). doi:10.1371/journal.pone.0031874.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help)CS1 maint: extra punctuation (link) CS1 maint: unflagged free DOI (link) - ^ Andrade, CM (2012). "Effects of phytoestrogens derived from soy bean on expression of adhesion molecules on HUVEC". Climacteric. 15 (2).
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ a b c d Robbins SL, Cotran RS, Kumar V, Collins T (1999). Robbins pathologic basis of disease. Philadelphia: WB Saunders. ISBN 0-7216-7335-X.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Visser LH (2006). "Critical illness polyneuropathy and myopathy: clinical features, risk factors and prognosis". Eur. J. Neurol. 13 (11): 1203–12. doi:10.1111/j.1468-1331.2006.01498.x. PMID 17038033.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Hu Y, Chen X, Duan H, Hu Y, Mu X (2009). "Chinese herbal medicinal ingredients inhibit secretion of IL-6, IL-8, E-selectin and TXB2 in LPS-induced rat intestinal microvascular endothelial cells". Immunopharmacol Immunotoxicol. 31 (4): 550–5. doi:10.3109/08923970902814129. PMID 19874221.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Komatsu, T (16). "E-selectin Mediates Porphyromonas gingivalis Adherence to Human Endothelial Cells". Infection and Immunity.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Fang, F (39). "PECAM-1 and E-selectin expression in vulnerable plague and their relationships to myocardial Leu125Val polymorphism of PECAM-1 and Ser128Arg polymorphism of E-selectin in patients with acute coronary syndrome". Zhonghua Xin Xue Guan Bing Za Zhi.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Alamanda, V (3). "Nicotine-mediated induction of E-selectin in aortic endothelial cells requires Src kinase and E2F1 transcriptional activity". Biochemical and Biophysical Research Communication.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Sato H, Usuda N, Kuroda M, Hashimoto S, Maruta M, Maeda K (2010). "Significance of serum concentrations of E-selectin and CA19-9 in the prognosis of colorectal cancer". Jpn. J. Clin. Oncol. 40 (11): 1073–80. doi:10.1093/jjco/hyq095. PMID 20576794.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Jia, W (2011). "E-selectin expression increased in human ruptured cerebral aneurysm tissues". Canadian Journal Neurological Sciences.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help)
Further reading
External links
- E-Selectin at the U.S. National Library of Medicine Medical Subject Headings (MeSH)