Toluene
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Methylbenzene
| |||
| Other names
toluene
phenylmethane toluol Anisen | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.297 | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C7H8 or C6H5CH3 | |||
| Molar mass | 92.14 g/mol | ||
| Appearance | colorless liquid | ||
| Density | 0.8669 g/mL (20 °C) | ||
| Melting point | −93 °C | ||
| Boiling point | 110.6 °C | ||
| 0.47 g/l (20–25°C) | |||
Refractive index (nD)
|
1.497 (20 °C) | ||
| Viscosity | 0.590 cP at 20°C | ||
| Structure | |||
| 0.36 D | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
highly flammable | ||
| NFPA 704 (fire diamond) | |||
| Flash point | 4 °C (39 °F) | ||
Threshold limit value (TLV)
|
50 ml·m−3, 190 mg·m−3 | ||
| Related compounds | |||
| Supplementary data page | |||
| Toluene (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. Chemically it is a mono-substituted benzene derivative, i.e. one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.
It is an aromatic hydrocarbon that is widely used as an industrial feedstock and as a solvent. Like other solvents, toluene is sometimes also used as an inhalant drug for its intoxicating properties; however, this can potentially cause severe neurological harm.[1][2]
History
The name toluene was derived from the older name toluol, which refers to tolu balsam, an aromatic extract from the tropical Colombian tree Myroxylon balsamum, from which it was first isolated. It was originally named by Jöns Jakob Berzelius.
Chemical properties
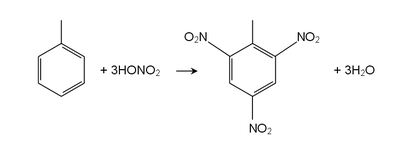
Toluene reacts as a normal aromatic hydrocarbon towards electrophilic aromatic substitution.[3][4][5] The methyl group makes it around 25 times more reactive than benzene in such reactions. It undergoes smooth sulfonation to give p-toluenesulfonic acid, and chlorination by Cl2 in the presence of FeCl3 to give ortho and para isomers of chlorotoluene. It undergoes nitration to give ortho and para nitrotoluene isomers, but if heated it can give dinitrotoluene and ultimately the explosive trinitrotoluene (TNT).
With other reagents the methyl side chain in toluene may react, undergoing oxidation. Reaction with basify potassium permanganate and diluted acid (e.g. sulfuric acid) or potassium permanganate with concentrated sulfuric acid, leads to benzoic acid, whereas reaction with chromyl chloride leads to benzaldehyde (Étard reaction). Halogenation can be performed under free radical conditions. For example, N-bromosuccinimide (NBS) heated with toluene in the presence of AIBN leads to benzyl bromide. Toluene can also be treated with elemental bromine in the presence of UV light (direct sunlight) to yield benzyl bromide.
Catalytic hydrogenation of toluene to methylcyclohexane requires a high pressure of hydrogen to go to completion, because of the stability of the aromatic system. pKa is approximately 45.
Production
Toluene occurs naturally at low levels in crude oil and is usually produced in the processes of making gasoline via a catalytic reformer, in an ethylene cracker or making coke from coal. Final separation (either via distillation or solvent extraction) takes place in a BTX plant.
Preparation
1. From benzene (Friedel–Crafts reaction)
Benzene reacts with methyl chloride in presence of anhydrous aluminium chloride to form toluene. The formation follows an electrophilic substitution reaction mechanism:
CH3Cl + AlCl3 → CH3+ + AlCl4-
C6H5H + CH3+ + AlCl4- → C6H5CH3 + HCl + AlCl3
The following catalysts can be used in place of AlCl3:
AlCl3 > SbCl3 > SnCl4 > BF3 > ZnCl2 > HgCl2
Note that the reaction is not very useful as the mono-alkyl derivative formed readily undergoes further alkylation at a still greater speed to produce polysubstituted products.
2. From bromobenzene (Wurtz-Fittig reaction)
The Wurtz-Fittig reaction is the reaction of an aryl halide and alkyl halide in presence of sodium metal to give substituted aromatic compounds.
When bromobenzene and methyl bromide react with sodium metal in dry ether solution, toluene is obtained.
C6H5Br + CH3Br + 2Na → C6H5CH3 + 2NaBr
3. From toluic acid (decarboxylation)
When sodium salt of toluic acid (o-, m-, p-) is heated with soda lime, toluene is obtained.
C6H4CH3COONa (sodium toluate) + NaOH → C6H5CH3 (toluene) + Na2CO3
4. From cresol
When cresol (o-, m-, p-) is distilled with zinc dust, toluene is obtained.
C6H4CH3OH (cresol) + Zn → C6H5CH3 (toluene) + ZnO
5. From toluenesulfonic acid
When toluenesulfonic acid is treated with superheated steam or boiled with HCl, toluene is obtained.
CH3C6H4SO3H (toluenesulfonic acid) + HOH (steam) → C6H5CH3 (toluene) + H2SO4 (sulfuric acid)
6. From toluidine
Toluidine is first diazotized with sodium nitrite (NaNO2) and HCl at low temperature. The diazonium compound thus obtained is heated with alkaline stannous chloride (SnCl2). This reaction gives toluene.
7. From Grignard reagent
When phenyl magnesium bromide (C6H5)MgBr is reacted with methyl bromide, toluene is obtained.
Using a Friedel–Crafts reaction, when an acid chloride is reacted with an aromatic hydrocarbon in the presence of anhydrous aluminium chloride (AlCl3), a mixed aliphatic/aromatic ketone is obtained. The ketone is then reduced with amalgamated zinc and concentrated HCl.
C6H6 + ClCOCH3 → C6H5COCH3 (acetophenone) + HCl
C6H5COCH3 + 4H → C6H5CH2CH3 (ethyl benzene)
This method is used to produce alkyl benzenes other than toluene.
Uses
Toluene is a common solvent, able to dissolve paints, paint thinners, silicone sealants,[6] many chemical reactants, rubber, printing ink, adhesives (glues), lacquers, leather tanners, and disinfectants. It can also be used as a fullerene indicator, and is a raw material for toluene diisocyanate (used in the manufacture of polyurethane foam) and TNT. In addition, it is used as a solvent to create a solution of carbon nanotubes. It is also used as a cement for fine polystyrene kits (by dissolving and then fusing surfaces) as it can be applied very precisely by brush and contains none of the bulk of an adhesive.
Industrial uses of toluene include dealkylation to benzene, and the disproportionation to a mixture of benzene and xylene in the BTX process. When oxidized it yields benzaldehyde and benzoic acid, two important intermediates in chemistry. It is also used as a carbon source for making Multi-Wall Carbon Nanotubes. Toluene can be used to break open red blood cells in order to extract hemoglobin in biochemistry experiments.
Toluene can be used as an octane booster in gasoline fuels used in internal combustion engines. Toluene at 86% by volume fueled all the turbo Formula 1 teams in the 1980s, first pioneered by the Honda team. The remaining 14% was a "filler" of n-heptane, to reduce the octane to meet Formula 1 fuel restrictions. Toluene at 100% can be used as a fuel for both two-stroke and four-stroke engines; however, due to the density of the fuel and other factors, the fuel does not vaporize easily unless preheated to 70 degrees Celsius (Honda accomplished this in their Formula 1 cars by routing the fuel lines through the muffler system to heat the fuel). Toluene also poses similar problems as alcohol fuels, as it eats through standard rubber fuel lines and has no lubricating properties as standard gasoline does, which can break down fuel pumps and cause upper cylinder bore wear.
In Australia, toluene has been found to have been illegally combined with petrol in fuel outlets for sale as standard vehicular fuel. Toluene attracts no fuel excise, while other fuels are taxed at over 40%, so fuel suppliers are able to profit from substituting the cheaper toluene for petrol. This substitution is likely to affect engine performance and result in additional wear and tear. The extent of toluene substitution has not been determined.[7][8]
Toluene has also been used as a coolant for its good heat transfer capabilities in sodium cold traps used in nuclear reactor system loops.
Toluene has also been used in the process of removing the cocaine from coca leaves in the production of Coca-Cola syrup.[9]
Biology
Similarly to many other solvents such as 1,1,1-trichloroethane and some alkylbenzenes, toluene has been shown to act as a non-competitive NMDA receptor antagonist and GABAA receptor positive allosteric modulator.[10] It is abused as an inhalant likely on account of the euphoric and dissociative effects these actions produce.[10] Additionally, toluene has been shown to display antidepressant-like effects in rodents in the forced swim test (FST) and the tail suspension test (TST).[10]
Toxicology and metabolism
Toluene should not be inhaled due to its health effects. Low to moderate levels can cause tiredness, confusion, weakness, drunken-type actions, memory loss, nausea, loss of appetite, and hearing and color vision loss. These symptoms usually disappear when exposure is stopped. Inhaling high levels of toluene in a short time may cause light-headedness, nausea, or sleepiness. It can also cause unconsciousness, and even death.[11][12]
Toluene is, however, much less toxic than benzene, and has consequently largely replaced it as an aromatic solvent in chemical preparation.
See also
- Organic chemistry
- Water pollution
- Isotoluenes are toluene isomers
Footnotes
- ^ Streicher HZ, Gabow PA, Moss AH, Kono D, Kaehny WD (1981). "Syndromes of toluene sniffing in adults". Ann. Intern. Med. 94 (6): 758–62. PMID 7235417.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Devathasan G, Low D, Teoh PC, Wan SH, Wong PK (1984). "Complications of chronic glue (toluene) abuse in adolescents". Aust N Z J Med. 14 (1): 39–43. PMID 6087782.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ B. S. Furnell et al., Vogel's Textbook of Practical Organic Chemistry, 5th edition, Longman/Wiley, New York, 1989
- ^ L. G. Wade, Organic Chemistry, 5th ed., p. 871, Prentice Hall, Upper Saddle RIver, New Jersey, 2003
- ^ J. March, Advanced Organic Chemistry, 4th ed., p. 723, Wiley, New York, 1992
- ^ Dual cure, low-solvent silicone pressure sensitive adhesives - Patent 6387487
- ^ http://www.libertyoil.com.au/www/230/1001164/displayarticle/1001248.html
- ^ http://www.abc.net.au/worldtoday/stories/s106466.htm
- ^ Merory, Joseph (1968). Food Flavorings: Composition, Manufacture and Use (2nd ed.). Westport, CT: AVI Publishing Company, Inc..
- ^ a b c Cruz SL, Soberanes-Chávez P, Páez-Martinez N, López-Rubalcava C (2009). "Toluene has antidepressant-like actions in two animal models used for the screening of antidepressant drugs". Psychopharmacology. 204 (2): 279–86. doi:10.1007/s00213-009-1462-2. ISBN 0021300914622. PMID 19151967.
{{cite journal}}: Check|isbn=value: invalid prefix (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Health Effects of Toluene", Canadian Centre for Occupational Health and Safety
- ^ [http://www.atsdr.cdc.gov/csem/toluene/physiologic_effects.html "Toluene Toxicity Physiologic Effects"], Agency for Toxic Substances and Disease Registry
External links
- ATSDR - Case Studies in Environmental Medicine: Toluene Toxicity U.S. Department of Health and Human Services (public domain)
- External solubility data