Mannose receptor: Difference between revisions
Major edit |
|||
| Line 37: | Line 37: | ||
|LocusSupplementaryData= |
|LocusSupplementaryData= |
||
}} |
}} |
||
The '''mannose receptor''' is a [[C-type lectin |
The '''mannose receptor''' is a [[C-type lectin]] primarily present on the surface of [[macrophages]] and [[dendritic cells]], but is also expressed on the surface of skin cells such as human [[dermal fibroblasts]] and [[keratinocytes]]<ref name="pmid11511295">{{cite journal | author = Szolnoky G, Bata-Csörgö Z, Kenderessy AS, Kiss M, Pivarcsi A, Novák Z, Nagy Newman K, Michel G, Ruzicka T, Maródi L, Dobozy A, Kemény L | title = A mannose-binding receptor is expressed on human keratinocytes and mediates killing of Candida albicans | journal = J. Invest. Dermatol. | volume = 117 | issue = 2 | pages = 205–13 |date=August 2001 | pmid = 11511295 | doi = 10.1046/j.1523-1747.2001.14071.x }}</ref><ref name="pmid10683150">{{cite journal | author = Sheikh H, Yarwood H, Ashworth A, Isacke CM | title = Endo180, an endocytic recycling glycoprotein related to the macrophage mannose receptor is expressed on fibroblasts, endothelial cells and macrophages and functions as a lectin receptor | journal = J. Cell. Sci. | volume = 113 ( Pt 6) | issue = | pages = 1021–32 |date=March 2000 | pmid = 10683150 | doi = }}</ref>. It is the first member of a family of endocytic receptors that includes [[UPARAP|Endo180 (CD280)]], [[PLA2R1|M-type PLA2R]], and [[LY75|DEC-205 (CD205)]]<ref name="East2002"> |
||
{{Cite journal | author=East L, Isacke C M | title=The mannose receptor family | journal=Biochimica et biophysica acta (BBA)- General subjects | volume=1572 | year=2001 | pages=364-386 | doi=10.1016/S0304-4165(02)00319-7}} |
|||
</ref> |
|||
. |
|||
The receptor recognises terminal [[mannose]], [[N-Acetylglucosamine|''N''-Acetylglucosamine]] and [[fucose]] residues of [[glycan]] moieties<ref name="Schlesinger1978"> |
|||
The function of this [[receptor (biochemistry)|receptor]] is to recognize complex [[carbohydrate]]s that are located on [[glycoprotein]]s that are a part of many different biological processes. Some of those processes include cell–cell recognition, serum glycoprotein turnover, and neutralization of [[pathogen]]s.<ref name=EntrezGene>{{cite web |url=http://www.ncbi.nlm.nih.gov/gene/4360 |title=MRC1 mannose receptor, C type 1 [Homo sapiens] |work=Entrez Gene |publisher=National Center for Biotechnology Information}}</ref> The protein also functions as a type 1 membrane [[immune receptor]] that mediates the [[endocytosis]] of glycoproteins by [[macrophage]]s.<ref name=EntrezGene/> The structure of these [[protein]]s allows it to bind to high mannose structures on the surface of potentially pathogenic [[virus]]es, [[bacteria]], and [[fungi]] so that they can be engulfed by the cell.<ref name=EntrezGene/> |
|||
{{Cite journal | author=Schlesinger P H, Doebber T W, Mandell B F, White R, DeSchryver C, Rodman J S, Miller M J, Stahl P | title=Plasma clearance of glycoproteins with terminal mannose and N-acetylglucosamine by liver non-parenchymal cells. Studies with beta-glucuronidase, N-acetyl-beta-D-glucosaminidase, ribonuclease B and agalacto-orosomucoid. | journal=Biochem J. | volume=176 | year=1978 | pages=103-109 }} |
|||
</ref> found on the surface of some [[microorganism|microorganisms]], playing a role in both the [[innate immune system|innate]] and [[adaptive immune system|adaptive immune systems]]. Additional functions include clearance of glycoproteins from the circulation, including sulphated [[glycoprotein]] [[hormone|hormones]] and glycoproteins released in response to [[pathology|pathological]] events<ref name="Lee2002"> |
|||
{{Cite journal | author=Lee S J, Evers S, Roeder D, Parlow A F, Risteli J, Risteli L, Lee Y C, Feizi T, Langen H, Nussenzweig M C | title=Mannose receptor-mediated regulation of serum glycoprotein homeostasis. | journal=Science | volume=295 | year=2002 | pages=1898-1901 }}</ref>. The mannose receptor recycles continuously between the [[plasma membrane]] and [[endosomal]] compartments in a [[clathrin]]-dependent manner.<ref name="Gazi2009"> |
|||
{{Cite journal | author=Gazi U, Martinez-Pomares L | title=Influence of the mannose receptor in host immune responses. | journal=Immunobiology | volume=214 | year=2009 | pages=554-561 }}</ref> |
|||
==Structure== |
|||
Mannose receptors have been researched as a target for [[vaccine]]s.<ref>{{cite journal |author=Keler T, Ramakrishna V, Fanger M |title=Mannose receptor-targeted vaccines |journal=Expert Opin Biol Ther |volume=4 |issue=12 |pages=1953–62 |year=2004 |pmid=15571457 |doi=10.1517/14712598.4.12.1953}}</ref> |
|||
===Domain organisation=== |
|||
[[File:mannose receptor domain organisation.jpg|thumb|upright=1.5|right|alt=The extracellular portion of the mannose receptor contains an N-terminal cystein-rich domain, a fibronectin type II domain and 8 C-type carbohydrate recogntion domains. This is followed by a transmembrane region and a short cytoplasmic C-terminal tail|Domain organisation of the mannose receptor, adapted from ''Introduction to Glycobiology''<ref name="IntroToGlycobiology"> {{Cite journal | author=Taylor M, Drickamer K | title=Introduction to glycobiology | journal=Oxford University Press | year=2011 | ISBN=978-0-19-956911-3}} |
|||
</ref> |
|||
]] |
|||
The mannose receptor is a type I [[transmembrane protein]], with an extracellular [[N-terminus]] and an intracellular [[C-terminus]]. It is first synthesised as an inactive precursor, but is [[proteolysis|proteolytically cleaved]] to its active form in the [[Golgi apparatus]]<ref name="Stahl1998"></ref>. The extracellular portion of the receptor is comprised of 8 consecutive C-type carbohydrate recognition domains (CRDs) closest to the plasma membrane, followed by a single [[fibronectin type II domain|fibronectin type II repeat domain]] and an N-terminal [[cysteine]]-rich domain. The cytoplasmic tail is not capable of [[signal transduction]] in isolation, since it lacks the appropriate signalling motifs<ref name="Martinez2012"> |
|||
{{Cite journal | author=Martinex-Pomares L | title=The mannose receptor | journal=Journal of Leukocyte Biology | volume=92 | year=2012 | pages=1177-1186 | doi=10.1189/jlb.0512231}} |
|||
</ref>. |
|||
===N-terminal cysteine-rich domain=== |
|||
== Types == |
|||
The N-terminal cysteine-rich domain is [[homology|homologous]] to the [[ricin]] B chain and binds to sulphated sugar moieties, with particularly high affinity for [[N-Acetylgalactosamine|''N''-Acetylgalactosamine]] and [[galactose]] residues sulphated at positions 3 and 4 of their [[pyranose]] rings<ref name="Fiete1998"> |
|||
{{Cite journal | author=Fiete D J, Beranek M C, Baenziger J U | title=A cysteine-rich domain of the “mannose” receptor mediates GalNAc-4-SO4 binding | journal=PNAS] | volume=95 | year=1998 | pages=2089-2093 }} |
|||
</ref> |
|||
. Other ligands include [[chondroitin sulfate|chondroitin sulfates]] A and B, as well as sulphated [[sialyl-LewisX|Lewis<sup>x</sup>]] and [[sialyl-Lewis A|Lewis<sup>a</sup>]] structures<ref name="Gazi2009"></ref>. The mannose receptor is the only member of the family in which this domain is functional<ref name="Lee2002"></ref>. |
|||
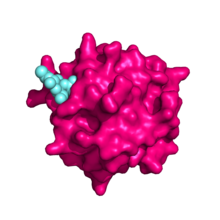
[[File:Mannose_receptor_Cys-rich_domain.png|thumb|left|alt=Pymol image of the mannose receptor N-terminal cystein-rich domain bound to its sulphated N-Acetylgalactosamine ligand. The sulphated ligand fits snugly into a pocket on the surface of the cysteine-rich domain|The mannose receptor N-terminal cysteine-rich domain (pink) bound to its sulphated ''N''-Acetylgalactosamine ligand (cyan). PBD ID: 1DQO]] |
|||
===Fibronectin type II repeat domain=== |
|||
The fibronectin type II repeat domain is conserved amongst all members of the mannose receptor family. [[collagen|Collagens]] I-IV bind this region with high affinity, while collagen V binds only weakly. Through this domain, the mannose receptor internalises collagen in macrophages and [[liver sinusoid|liver sinusoidal cells]], independent of the lectin activity of the receptor<ref name="Martinez2012"></ref>. Along with the N-terminal cysteine-rich domain, this domain is the most highly conserved between mice and humans (92%)<ref name="Stahl1998"> |
|||
{{Cite journal | author=Stahl P D, Ezekowitzb A B, | title=The mannose receptor is a pattern recognition receptor involved in host defense | journal=Current Opinion in Immunology | volume=10 | year=1998 | pages=50-55 | doi=10.1016/S0952-7915(98)80031-9 }} |
|||
</ref>. |
|||
===C-type CRDs=== |
|||
The 8 tandem CRDs in the extracellular region of the mannose receptor share only 30% homology with each other. They each contain at least some of the [[amino acid]] residues necessary for Ca<sup>2+</sup> and ligand binding, common to functional C-type CRDs. Only CRDs 4 and 5 contain all of the residues required for sugar binding, forming a [[protease]]-resistant ligand-binding core. The most common ligand is terminal mannose residues, but ''N''-acetylglucosamine and fucose also bind<ref name="Stahl1998"></ref>. |
|||
The main interaction between CRD-4 and its sugar ligand is through direct ligation to the conserved Ca<sup>2+</sup> in the sugar-binding site, in a similar way to the binding mechanism of [[mannan-binding lectin]] (MBL). However, a quarter of the [[free energy]] of sugar-binding is associated with the hydrophobic stacking interactions formed between one face of the sugar ring and the side chain of a conserved [[tyrosine]] residue in the binding site, which is not seen in MBL. Despite the similarities in mannose-binding between the mannose receptor and MBL, these differences suggest that mannose-binding by the mannose receptor [[evolution|evolved]] separately to that of other C-type lectins<ref name="Taylor1997"> |
|||
{{Cite journal | author=Mullin N P, Hitchen P G, Taylor M E | title=Mechanism of Ca2+ and Monosaccharide Binding to a C-type Carbohydrate-recognition Domain of the Macrophage Mannose Receptor | journal=Journal of Biological Chemistry | volume=272 | year=1997 | pages=5668-5681 }} |
|||
</ref>. |
|||
Individually, the CRDs bind mannose with only weak affinity. High affinity binding is thought to result from the clustering of multiple CRDs. This clustering allows for binding of [[multivalent]], branched ligands such as high-mannose [[N-linked glycosylation|N-linked]] [[oligosaccharide|oligosaccharides]]<ref name="Drickamer1996"> |
|||
{{Cite journal | author=Drickamer K, Weis W I | title=Structural Basis of Lectin-Carbohydrate Recognition | journal=Annual Review of Biochemistry | volume=65 | year=1996 | pages=441-473 | doi=10.1146/annurev.bi.65.070196.002301 }} |
|||
</ref>. |
|||
===Conformation=== |
|||
It has been suggested that the mannose receptor can exist in atleast two different structural [[conformation|conformations]]. The C-type CRDs are each separated by linker regions of 10-20 amino acids containing a number of [[proline]] residues, whose cyclic side chain is fairly rigid and favours a conformation in which the N-terminal cysteine-rich domain is extended as far away from the plasma membrane as possible<ref name="Llorca2008"> |
|||
{{Cite journal | author=Llorca O | title=Extended and bent conformations of the mannose receptor family. | journal=Cell Mol Life Sci. | volume=65 | year=2008 | pages=1302-1310 | doi=10.1007/s00018-007-7497-9 }} |
|||
</ref>. |
|||
Alternatively, interactions between neighbouring CRDs may hold them in close proximity to one another and cause the extracellular region of the receptor to bend, bringing the N-terminal cysteine-rich domain into close contact with the CRDs. This would position CRDs 4 and 5 furthest from the membrane to maximise their interaction with potential ligands. The resistance to proteolysis shown by CRDs 4 and 5 suggests physical interactions between the two domains does occur, thereby supporting the existence of this U-shaped conformation<ref name="Llorca2008"></ref>. |
|||
It is thought that transitions between these two conformations occur in a pH-dependent manner, regulating ligand selectivity and release during endocytosis. The lower, more acidic pH of early endosomes is thought to be responsible for ligand release<ref name="Llorca2008"></ref>. |
|||
===Proteolytic processing=== |
|||
A functional, soluble form of the mannose receptor is produced upon proteolytic cleavage of the membrane-bound form by [[metalloproteases]] found in the extracellular environment<ref name="Jordens1999">{{Cite journal | author=Jordens R, Thompson A, Amons R, Koning F | title=Human dendritic cells shed a functional, soluble form of the mannose receptor | journal=International Immunology| volume=11 | year=1999 | pages=1775–1780 }}</ref><ref name="Martinez1998"> |
|||
{{Cite journal | author=Martinez-Pomares L, Mahoney J A, Kaposzta R, Linehan S A, Stahl P D, Gordon S| title=A functional soluble form of the murine mannose receptor is produced by macrophages in vitro and is present in mouse serum. | journal=Journal of Biological Chemistry | volume=273 | year=1998 | pages=23376–23380 }}</ref>. The soluble protein consists of the entire extracellular region of the receptor and it may be involved in transport of mannosylated proteins away from sites of [[inflammation]]<ref name="Martinez2012"></ref>. Shedding of the mannose receptor from macrophages has been shown to be enhanced upon recognition of [[fungal]] [[pathogens]] such as [[Candida albicans|''Candida albicans'']] and [[Aspergillus fumigatus|''Aspergillus fumigatus'']], which suggests the soluble form may play a role in fungal pathogen recognition. In this way, the balance between membrane-bound and soluble mannose receptor could affect targeting of fungal pathogens during the course of infection<ref name="Gazi2011">{{Cite journal | author=Gazi U, Rosas M, Singh S, Heinsbroek S, Haq I, Johnson S, Brown G D, Williams D L, Taylor P R, Martinez-Pomares L | title=Fungal recognition enhances mannose receptor shedding through dectin-1 engagement | journal=Journal of Biological Chemistry | volume=286 | year=2011 | pages=7822–7829 }}</ref>. |
|||
===Glycosylation=== |
|||
The mannose receptor is heavily glycosylated and its N-linked glycosylation sites are highly conserved between mice and humans, indicating an important role for this [[post-translational modification]]. The presence of [[sialic acid]] residues on N-linked glycans of the mannose receptor is important for its role in binding both sulphated and mannosylated glycoproteins. Sialylation regulates multimerisation of the receptor, which is known to influence binding to sulphated glycoproteins. Terminal sialic acid residues are also known to be required for binding to mannosylated glycans. The absence of sialic acid reduces the receptors ability to bind and internalise mannosylated glycans, but does not affect its localisation to the plasma membrane or its endocytic activity<ref name="Martinez2012"></ref><ref name="Su2005"> |
|||
{{Cite journal | author=Su Y, Bakker T, Harris J, Tsang C, Brown G D, Wormald M R, Gordon S, Dwek R A, Rudd P M, Martinez-Pomares L | title=Glycosylation influences the lectin activities of the macrophage mannose receptor | journal=Journal of Biological Chemistry | volume=280 | year=2005 | pages=32811-32820 | doi=10.1074/jbc.M503457200 }} |
|||
</ref>. |
|||
==Function== |
|||
===Phagocytosis of pathogens=== |
|||
A number of pathogenic microorganisms, including ''C. albicans''<ref name="Martinez1998"></ref><ref name="Marodi1991">{{Cite journal | author=Marodi L, Korchak H M, Johnston Jr. R B | title=Mechanisms of host defence against Candida species I. Phagocytosis by monocytes and monocyte-derived macrophages | journal=Journal of Immunology | volume=146 | year=1991 | pages=2783–2789 }}</ref>, [[Pneumocystis carinii|''Pneumocystis carinii'']]<ref name="Ezekowitz1991">{{Cite journal | author=Ezekowitz R A, Williams D J, Koziel H, Armstrong M Y, Warner A, Richards F F, Rose R M | title=Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor | journal=Nature | volume=351 | year=1991 | pages=155-158 }}</ref><ref name="O'Riordan1995">{{Cite journal | author=O’Riordan D M, Standing J E, Limper A H | title=Pneumocystis carinii glycoprotein A binds macrophage mannose receptors | journal=Infection and Immunity | volume=63 | year=1995 | pages=779–784 }}</ref> and [[Leishmania donovani|''Leishmania donovani'']]<ref name="Chakraborty1998">{{Cite journal | author=Chakraborty R, Chakraborty P, Basu M K | title=Macrophage mannosyl fucosyl receptor: its role in invasion of virulent and avirulent L. donovani promastigotes | journal=Bioscience Reports | volume=18 | year=1998 | pages=129-142 }}</ref><ref name="Chakraborty2001">{{Cite journal | author=Chakraborty P, Ghosh D, Basu M K | title=Modulation of macrophage mannose receptor affects the uptake of virulent and avirulent Leishmania donovani promastigotes | journal=Journal of Parasitology | volume=87 | year=2001 | pages=1023-1027 }}</ref> display glycans on their surfaces with terminal mannose residues that are recognised by the C-type CRDs of the mannose receptor, thereby acting as a marker of non-self. Upon recognition, the receptor internalises the bound pathogen and transports it to lysosomes for degradation via the [[endocytosis|phagocytic pathway]]. In this way, the mannose receptor acts as a [[pattern recognition receptor]]. The presence of a di-aromatic FENTLY (Phe-Glu-Asn-Thr-Leu-Tyr) sequence motif in the cytoplasmic tail of the receptor is vital for its clathrin-mediated internalisation<ref name="Gazi2009"></ref>. This is supported by the evidence that [[COS cells|Cos-1 cells]] transfected with the mannose receptor lacking its C-terminal tail are unable to endocytose ''C. albicans'' and ''P. carinii''<ref name="Gazi2009"></ref>. |
|||
Surprisingly, mannose receptor [[knockout mouse|knockout mice]] do not show increased susceptibility to infection, which suggests that the receptor is not essential for phagocytosis. However, its involvement cannot be rejected since other mechanisms may compensate. For example, infection of knockout mice with ''P. carinii'' resulted in increased recruitment of macrophages to the site of infection. Furthermore, other receptors present on the surface of phagocytic cells, such as [[DC-SIGN]], SIGNR1 and Endo180, exhibit similar ligand binding ability to the mannose receptor and so it is likely that in its absence, these proteins are able to compensate and induce phagocytosis<ref name="Gazi2009"></ref>. |
|||
The ability of the mannose receptor to aid in pathogen internalisation is also thought to facilitate infection by [[Mycobacterium tuberculosis|''Mycobacterium tuberculosis'']] and [[Mycobacterium leprae|''Mycobacterium leprae'']]. These [[bacteria]] reside and multiply in macrophages, preventing formation of the phagolysosome to avoid degradation. Hence, by mediating their entrance into the macrophage, the mannose receptor helps these pathogens to infect and grow in their target cell<ref name="Gazi2009"></ref><ref name="Kang2005">{{Cite journal | author=Kang P B, Azad A K, Torrelles J B, Kaufman T M, Beharka A, Tibesar E, DesJardin L E, Schlesinger L S | title=The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis | journal=Journal of Experimental Medicine | volume=202 | year=2005 | pages=987-999 }}</ref>. |
|||
===Antigen presentation=== |
|||
The mannose receptor may also play a role in antigen uptake and presentation by immature dendritic cells in the adaptive immune system. Upon binding to the receptor, mannosylated antigens are internalised and transported to endocytic compartments within the cell for loading onto [[Major Histocompatibility Complex]] (MHC) molecules or other related antigen-presentation molecules. An indirect example of this is the processing of the [[glycolipid]] antigen [[lipoarabinomannan]], derived from [[Mycobacteria]]. Lipoarabinomannan (LAM) is presented to T cells in complex with CD1b, but is also able to bind to the mannose receptor. Since the presence of [[mannan]], an alternative ligand, inhibits LAM-dependent T cell proliferation, it is suggested that the receptor binds extracellular LAM, internalises it and then transports it to endocytic vesicles to be loaded onto CD1b<ref name="Stahl1998"></ref>. |
|||
Mature dendritic cells and macrophages use the mannose receptor for antigen presentation in a different way. The cleaved, soluble receptor binds to circulating antigens and directs them to effector cells in [[lymphatic system|lymphoid organs]] via its cysteine-rich domain, thus activating the adaptive immune system<ref name="Stahl1998"></ref>. |
|||
===Intracellular signalling=== |
|||
The cytoplasmic tail of the mannose receptor does not contain any signalling motifs, yet the receptor has proven to be essential for production of both pro- and anti-inflammatory [[cytokines]], indicating a more passive role for the receptor in phagocytosis of pathogens<ref name="Stahl1998"></ref><ref name="Gazi2009"></ref>. This suggests that the mannose receptor is assisted by other cell surface receptors in order to trigger a signalling cascade. For example, it has been shown that [[HEK 293 cells]] co-transfected with human mannose receptor and human [[TLR 2|Toll-like receptor 2]] [[cDNA]] are able to secrete [[IL-8]] in response to ''P. carinii'' infection, whereas those transfected with either receptor alone did not<ref name="Tachado2007"> |
|||
{{Cite journal | author=Tachado S D, Zhang J, Zhu J, Patel N, Cushion M, Koziel H | title=Pneumocystis-mediated IL-8 release by macrophages requires coexpression of mannose receptors and TLR2 | journal=Journal of Leukocyte Biology| volume=81 | year=2007 | pages=205-211 }}</ref>. It is possible that the two receptors form a complex on the cell surface that facilitates signal transduction upon pathogenic challenge. |
|||
===Resolution of inflammation=== |
|||
Another key role of the mannose receptor is to regulate the levels of molecules released into the circulation during the inflammatory response. In response to pathological events, glycoproteins including lysosomal [[hydrolases]], [[tissue plasminogen activator]] and neutrophil [[myeloperoxidase]] are released to help fight off any invading microorganisms. Once the threat has subsisded, these glycoproteins can be damaging to host tissues so their levels in the circulation must be strictly controlled<ref name="Gazi2009"></ref>. |
|||
High-mannose oligosaccharides present on the surface of these glycoproteins act to mark their transient nature, since they are eventually recognised by the mannose receptor and removed from the circulation. Mannose receptor knockout mice are less able to clear these proteins, and show increased concentrations of a number of lysosomal hydrolases in the blood<ref name="Lee2002"></ref>. |
|||
Consistent with this function, the mannose receptor is expressed at low levels during inflammation and at high levels during the resolution of inflammation, to ensure inflammatory agents are removed from the circulation only at the appropriate time<ref name="Lee2002"></ref>. |
|||
===Clearance of glycoprotein hormones=== |
|||
The N-terminal cysteine-rich domain of the mannose receptor plays an important role in the recognition of sulphated glycoprotein hormones and their clearance from the circulation<ref name="Stahl1998"></ref>. |
|||
Glycoprotein hormones such as [[lutropin]], which triggers release of the egg during [[ovulation]], must stimulate their receptors in pulses to avoid [[homologous desensitization|receptor desensitisation]]. Glycans on their surface are capped with sulphated ''N''-Acetylgalactosamine (GalNAc), making them ligands for the cysteine-rich ricin homology domain of the mannose receptor. This tag ensures a cycle of release, stimulation, and removal from the circulation<ref name="IntroToGlycobiology"></ref>. |
|||
Knockout mice lacking the enzyme required to add the sulphated GalNAc capping structure show longer half-lives for lutropin, which results in increased receptor activation and [[oestrogen]] production. Female knockout mice reach sexual maturity faster than their wild-type counterparts, have a longer [[oestrus cycle]] and produce more litters. Thus, the sulphated GalNAc tag is very important in regulating serum concentrations of certain glycoprotein hormones <ref name="IntroToGlycobiology"></ref>. |
|||
==Types== |
|||
Humans express two types of mannose receptors, each encoded by its own gene: |
Humans express two types of mannose receptors, each encoded by its own gene: |
||
| Line 49: | Line 135: | ||
{| class="wikitable" |
{| class="wikitable" |
||
|- |
|- |
||
! Gene !! Protein !! |
! Gene !! Protein !! Alternative names |
||
|- |
|- |
||
| MRC1 || Macrophage mannose receptor 1 || C-type mannose receptor 1,<br />C-type lectin domain family 13 member D (CLEC13D),<br />CD206, MMR |
| MRC1 || Macrophage mannose receptor 1 || C-type mannose receptor 1,<br />C-type lectin domain family 13 member D (CLEC13D),<br />CD206, MMR |
||
| Line 56: | Line 142: | ||
|} |
|} |
||
==Applications in health and disease== |
|||
The MRC1 gene is in close proximity to MRC1L1 and has a gene loci that includes the gene, MRC1L1, as well as LOC340843 and LOC340893.<ref name=EntrezGene/> The gene also consist of nearly two identical regions, some think that they are duplicated regions.<ref name=EntrezGene/> Recombinant proteins produced in yeast may be subject to increased addition of mannose, in patterns different from those of a human cell.<ref>{{cite journal |author=Vlahopoulos S, Gritzapis AD, Perez SA, Cacoullos N, Papamichail M, Baxevanis CN |title=Mannose addition by yeast Pichia Pastoris on recombinant HER-2 protein inhibits recognition by the monoclonal antibody herceptin |journal=Vaccine |volume=27 |issue=34 |pages=4704–8 |date=July 2009 |pmid=19520203 |doi=10.1016/j.vaccine.2009.05.063 }}</ref> Dendritic cells of the immune system possess a mannose receptor that enables them to take up mannosylated proteins, and utilize fragments of them for antigen presentation.<ref>{{cite journal |author=Shimizu K, Fujii S |title=An adjuvant role of in situ dendritic cells (DCs) in linking innate and adaptive immunity |journal=Front. Biosci. |volume=13 |issue= |pages=6193–201 |year=2008 |pmid=18508653 |doi= |url=http://www.bioscience.org/2008/v13/af/3147/fulltext.htm}}</ref> |
|||
The selective internalisation properties of the mannose receptor indicate a number of potential applications in health and disease. By manipulating the glycosylation of important [[bioactive]] proteins to a highly mannosylated state, their serum levels could be tightly regulated and they could be targeted specifically to cells expressing the mannose receptor. There is also potential for use of the mannose receptor as a target for improved macrophage activation and antigen presentation<ref name="Lee2002"></ref><ref name="Stahl1998"></ref>. |
|||
==Function== |
|||
The macrophage mannose receptor also interacts with [[HIV]] gp120 envelope protein, allowing uptake of the virus.<ref name="pmid19224860">{{cite journal | author = Lai J, Bernhard OK, Turville SG, Harman AN, Wilkinson J, Cunningham AL | title = Oligomerization of the macrophage mannose receptor enhances gp120-mediated binding of HIV-1 | journal = J. Biol. Chem. | volume = 284 | issue = 17 | pages = 11027–38 |date=April 2009 | pmid = 19224860 | pmc = 2670108 | doi = 10.1074/jbc.M809698200 }}</ref> These interactions affect [[gp120]] and its ability to bind to the [[B cells]] through MRC1 receptors and an increase production of matrix mannose receptors.<ref name=EntrezGene/> The HIV protein [[Nef (protein)|Nef]] interacts with MRC1 by down regulating the receptors on the surface of [[macrophage]]s and [[dendritic cell]]s. The [[Tat (HIV)|tat]] protein represses the [[Transcription (genetics)|transcription]] of MRC1 promoters by down regulating the expression of MRC1.<ref name=EntrezGene/> All of these proteins interact with MRC1 so that the virus can continue to spread and more research is being conducted trying to understand how stop these interactions from occurring. |
|||
==See also== |
|||
* [[mannose]] |
|||
==References== |
==References== |
||
Revision as of 15:51, 27 March 2014
| mannose receptor, C type 1 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | MRC1 | ||||||
| Alt. symbols | CD206 | ||||||
| NCBI gene | 4360 | ||||||
| HGNC | 7228 | ||||||
| OMIM | 153618 | ||||||
| RefSeq | NM_002438 | ||||||
| UniProt | P22897 | ||||||
| Other data | |||||||
| Locus | Chr. 10 p13 | ||||||
| |||||||
| mannose receptor, C type 2 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | MRC2 | ||||||
| Alt. symbols | CD280 | ||||||
| NCBI gene | 9902 | ||||||
| HGNC | 16875 | ||||||
| RefSeq | NM_006039 | ||||||
| UniProt | Q9UBG0 | ||||||
| Other data | |||||||
| Locus | Chr. 17 q23 | ||||||
| |||||||
The mannose receptor is a C-type lectin primarily present on the surface of macrophages and dendritic cells, but is also expressed on the surface of skin cells such as human dermal fibroblasts and keratinocytes[1][2]. It is the first member of a family of endocytic receptors that includes Endo180 (CD280), M-type PLA2R, and DEC-205 (CD205)[3] .
The receptor recognises terminal mannose, N-Acetylglucosamine and fucose residues of glycan moieties[4] found on the surface of some microorganisms, playing a role in both the innate and adaptive immune systems. Additional functions include clearance of glycoproteins from the circulation, including sulphated glycoprotein hormones and glycoproteins released in response to pathological events[5]. The mannose receptor recycles continuously between the plasma membrane and endosomal compartments in a clathrin-dependent manner.[6]
Structure
Domain organisation

The mannose receptor is a type I transmembrane protein, with an extracellular N-terminus and an intracellular C-terminus. It is first synthesised as an inactive precursor, but is proteolytically cleaved to its active form in the Golgi apparatus[8]. The extracellular portion of the receptor is comprised of 8 consecutive C-type carbohydrate recognition domains (CRDs) closest to the plasma membrane, followed by a single fibronectin type II repeat domain and an N-terminal cysteine-rich domain. The cytoplasmic tail is not capable of signal transduction in isolation, since it lacks the appropriate signalling motifs[9].
N-terminal cysteine-rich domain
The N-terminal cysteine-rich domain is homologous to the ricin B chain and binds to sulphated sugar moieties, with particularly high affinity for N-Acetylgalactosamine and galactose residues sulphated at positions 3 and 4 of their pyranose rings[10] . Other ligands include chondroitin sulfates A and B, as well as sulphated Lewisx and Lewisa structures[6]. The mannose receptor is the only member of the family in which this domain is functional[5].
Fibronectin type II repeat domain
The fibronectin type II repeat domain is conserved amongst all members of the mannose receptor family. Collagens I-IV bind this region with high affinity, while collagen V binds only weakly. Through this domain, the mannose receptor internalises collagen in macrophages and liver sinusoidal cells, independent of the lectin activity of the receptor[9]. Along with the N-terminal cysteine-rich domain, this domain is the most highly conserved between mice and humans (92%)[8].
C-type CRDs
The 8 tandem CRDs in the extracellular region of the mannose receptor share only 30% homology with each other. They each contain at least some of the amino acid residues necessary for Ca2+ and ligand binding, common to functional C-type CRDs. Only CRDs 4 and 5 contain all of the residues required for sugar binding, forming a protease-resistant ligand-binding core. The most common ligand is terminal mannose residues, but N-acetylglucosamine and fucose also bind[8].
The main interaction between CRD-4 and its sugar ligand is through direct ligation to the conserved Ca2+ in the sugar-binding site, in a similar way to the binding mechanism of mannan-binding lectin (MBL). However, a quarter of the free energy of sugar-binding is associated with the hydrophobic stacking interactions formed between one face of the sugar ring and the side chain of a conserved tyrosine residue in the binding site, which is not seen in MBL. Despite the similarities in mannose-binding between the mannose receptor and MBL, these differences suggest that mannose-binding by the mannose receptor evolved separately to that of other C-type lectins[11].
Individually, the CRDs bind mannose with only weak affinity. High affinity binding is thought to result from the clustering of multiple CRDs. This clustering allows for binding of multivalent, branched ligands such as high-mannose N-linked oligosaccharides[12].
Conformation
It has been suggested that the mannose receptor can exist in atleast two different structural conformations. The C-type CRDs are each separated by linker regions of 10-20 amino acids containing a number of proline residues, whose cyclic side chain is fairly rigid and favours a conformation in which the N-terminal cysteine-rich domain is extended as far away from the plasma membrane as possible[13].
Alternatively, interactions between neighbouring CRDs may hold them in close proximity to one another and cause the extracellular region of the receptor to bend, bringing the N-terminal cysteine-rich domain into close contact with the CRDs. This would position CRDs 4 and 5 furthest from the membrane to maximise their interaction with potential ligands. The resistance to proteolysis shown by CRDs 4 and 5 suggests physical interactions between the two domains does occur, thereby supporting the existence of this U-shaped conformation[13].
It is thought that transitions between these two conformations occur in a pH-dependent manner, regulating ligand selectivity and release during endocytosis. The lower, more acidic pH of early endosomes is thought to be responsible for ligand release[13].
Proteolytic processing
A functional, soluble form of the mannose receptor is produced upon proteolytic cleavage of the membrane-bound form by metalloproteases found in the extracellular environment[14][15]. The soluble protein consists of the entire extracellular region of the receptor and it may be involved in transport of mannosylated proteins away from sites of inflammation[9]. Shedding of the mannose receptor from macrophages has been shown to be enhanced upon recognition of fungal pathogens such as Candida albicans and Aspergillus fumigatus, which suggests the soluble form may play a role in fungal pathogen recognition. In this way, the balance between membrane-bound and soluble mannose receptor could affect targeting of fungal pathogens during the course of infection[16].
Glycosylation
The mannose receptor is heavily glycosylated and its N-linked glycosylation sites are highly conserved between mice and humans, indicating an important role for this post-translational modification. The presence of sialic acid residues on N-linked glycans of the mannose receptor is important for its role in binding both sulphated and mannosylated glycoproteins. Sialylation regulates multimerisation of the receptor, which is known to influence binding to sulphated glycoproteins. Terminal sialic acid residues are also known to be required for binding to mannosylated glycans. The absence of sialic acid reduces the receptors ability to bind and internalise mannosylated glycans, but does not affect its localisation to the plasma membrane or its endocytic activity[9][17].
Function
Phagocytosis of pathogens
A number of pathogenic microorganisms, including C. albicans[15][18], Pneumocystis carinii[19][20] and Leishmania donovani[21][22] display glycans on their surfaces with terminal mannose residues that are recognised by the C-type CRDs of the mannose receptor, thereby acting as a marker of non-self. Upon recognition, the receptor internalises the bound pathogen and transports it to lysosomes for degradation via the phagocytic pathway. In this way, the mannose receptor acts as a pattern recognition receptor. The presence of a di-aromatic FENTLY (Phe-Glu-Asn-Thr-Leu-Tyr) sequence motif in the cytoplasmic tail of the receptor is vital for its clathrin-mediated internalisation[6]. This is supported by the evidence that Cos-1 cells transfected with the mannose receptor lacking its C-terminal tail are unable to endocytose C. albicans and P. carinii[6].
Surprisingly, mannose receptor knockout mice do not show increased susceptibility to infection, which suggests that the receptor is not essential for phagocytosis. However, its involvement cannot be rejected since other mechanisms may compensate. For example, infection of knockout mice with P. carinii resulted in increased recruitment of macrophages to the site of infection. Furthermore, other receptors present on the surface of phagocytic cells, such as DC-SIGN, SIGNR1 and Endo180, exhibit similar ligand binding ability to the mannose receptor and so it is likely that in its absence, these proteins are able to compensate and induce phagocytosis[6].
The ability of the mannose receptor to aid in pathogen internalisation is also thought to facilitate infection by Mycobacterium tuberculosis and Mycobacterium leprae. These bacteria reside and multiply in macrophages, preventing formation of the phagolysosome to avoid degradation. Hence, by mediating their entrance into the macrophage, the mannose receptor helps these pathogens to infect and grow in their target cell[6][23].
Antigen presentation
The mannose receptor may also play a role in antigen uptake and presentation by immature dendritic cells in the adaptive immune system. Upon binding to the receptor, mannosylated antigens are internalised and transported to endocytic compartments within the cell for loading onto Major Histocompatibility Complex (MHC) molecules or other related antigen-presentation molecules. An indirect example of this is the processing of the glycolipid antigen lipoarabinomannan, derived from Mycobacteria. Lipoarabinomannan (LAM) is presented to T cells in complex with CD1b, but is also able to bind to the mannose receptor. Since the presence of mannan, an alternative ligand, inhibits LAM-dependent T cell proliferation, it is suggested that the receptor binds extracellular LAM, internalises it and then transports it to endocytic vesicles to be loaded onto CD1b[8].
Mature dendritic cells and macrophages use the mannose receptor for antigen presentation in a different way. The cleaved, soluble receptor binds to circulating antigens and directs them to effector cells in lymphoid organs via its cysteine-rich domain, thus activating the adaptive immune system[8].
Intracellular signalling
The cytoplasmic tail of the mannose receptor does not contain any signalling motifs, yet the receptor has proven to be essential for production of both pro- and anti-inflammatory cytokines, indicating a more passive role for the receptor in phagocytosis of pathogens[8][6]. This suggests that the mannose receptor is assisted by other cell surface receptors in order to trigger a signalling cascade. For example, it has been shown that HEK 293 cells co-transfected with human mannose receptor and human Toll-like receptor 2 cDNA are able to secrete IL-8 in response to P. carinii infection, whereas those transfected with either receptor alone did not[24]. It is possible that the two receptors form a complex on the cell surface that facilitates signal transduction upon pathogenic challenge.
Resolution of inflammation
Another key role of the mannose receptor is to regulate the levels of molecules released into the circulation during the inflammatory response. In response to pathological events, glycoproteins including lysosomal hydrolases, tissue plasminogen activator and neutrophil myeloperoxidase are released to help fight off any invading microorganisms. Once the threat has subsisded, these glycoproteins can be damaging to host tissues so their levels in the circulation must be strictly controlled[6].
High-mannose oligosaccharides present on the surface of these glycoproteins act to mark their transient nature, since they are eventually recognised by the mannose receptor and removed from the circulation. Mannose receptor knockout mice are less able to clear these proteins, and show increased concentrations of a number of lysosomal hydrolases in the blood[5].
Consistent with this function, the mannose receptor is expressed at low levels during inflammation and at high levels during the resolution of inflammation, to ensure inflammatory agents are removed from the circulation only at the appropriate time[5].
Clearance of glycoprotein hormones
The N-terminal cysteine-rich domain of the mannose receptor plays an important role in the recognition of sulphated glycoprotein hormones and their clearance from the circulation[8].
Glycoprotein hormones such as lutropin, which triggers release of the egg during ovulation, must stimulate their receptors in pulses to avoid receptor desensitisation. Glycans on their surface are capped with sulphated N-Acetylgalactosamine (GalNAc), making them ligands for the cysteine-rich ricin homology domain of the mannose receptor. This tag ensures a cycle of release, stimulation, and removal from the circulation[7].
Knockout mice lacking the enzyme required to add the sulphated GalNAc capping structure show longer half-lives for lutropin, which results in increased receptor activation and oestrogen production. Female knockout mice reach sexual maturity faster than their wild-type counterparts, have a longer oestrus cycle and produce more litters. Thus, the sulphated GalNAc tag is very important in regulating serum concentrations of certain glycoprotein hormones [7].
Types
Humans express two types of mannose receptors, each encoded by its own gene:
| Gene | Protein | Alternative names |
|---|---|---|
| MRC1 | Macrophage mannose receptor 1 | C-type mannose receptor 1, C-type lectin domain family 13 member D (CLEC13D), CD206, MMR |
| MRC2 | Macrophage mannose receptor 2 | C-type mannose receptor 2, Urokinase-type plasminogen activator receptor-associated protein, CD280 |
Applications in health and disease
The selective internalisation properties of the mannose receptor indicate a number of potential applications in health and disease. By manipulating the glycosylation of important bioactive proteins to a highly mannosylated state, their serum levels could be tightly regulated and they could be targeted specifically to cells expressing the mannose receptor. There is also potential for use of the mannose receptor as a target for improved macrophage activation and antigen presentation[5][8].
References
- ^ Szolnoky G, Bata-Csörgö Z, Kenderessy AS, Kiss M, Pivarcsi A, Novák Z, Nagy Newman K, Michel G, Ruzicka T, Maródi L, Dobozy A, Kemény L (August 2001). "A mannose-binding receptor is expressed on human keratinocytes and mediates killing of Candida albicans". J. Invest. Dermatol. 117 (2): 205–13. doi:10.1046/j.1523-1747.2001.14071.x. PMID 11511295.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sheikh H, Yarwood H, Ashworth A, Isacke CM (March 2000). "Endo180, an endocytic recycling glycoprotein related to the macrophage mannose receptor is expressed on fibroblasts, endothelial cells and macrophages and functions as a lectin receptor". J. Cell. Sci. 113 ( Pt 6): 1021–32. PMID 10683150.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ East L, Isacke C M (2001). "The mannose receptor family". Biochimica et biophysica acta (BBA)- General subjects. 1572: 364–386. doi:10.1016/S0304-4165(02)00319-7.
- ^
Schlesinger P H, Doebber T W, Mandell B F, White R, DeSchryver C, Rodman J S, Miller M J, Stahl P (1978). "Plasma clearance of glycoproteins with terminal mannose and N-acetylglucosamine by liver non-parenchymal cells. Studies with beta-glucuronidase, N-acetyl-beta-D-glucosaminidase, ribonuclease B and agalacto-orosomucoid". Biochem J. 176: 103–109.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e
Lee S J, Evers S, Roeder D, Parlow A F, Risteli J, Risteli L, Lee Y C, Feizi T, Langen H, Nussenzweig M C (2002). "Mannose receptor-mediated regulation of serum glycoprotein homeostasis". Science. 295: 1898–1901.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h Gazi U, Martinez-Pomares L (2009). "Influence of the mannose receptor in host immune responses". Immunobiology. 214: 554–561.
- ^ a b c Taylor M, Drickamer K (2011). "Introduction to glycobiology". Oxford University Press. ISBN 978-0-19-956911-3.
- ^ a b c d e f g h
Stahl P D, Ezekowitzb A B, (1998). "The mannose receptor is a pattern recognition receptor involved in host defense". Current Opinion in Immunology. 10: 50–55. doi:10.1016/S0952-7915(98)80031-9.
{{cite journal}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ a b c d Martinex-Pomares L (2012). "The mannose receptor". Journal of Leukocyte Biology. 92: 1177–1186. doi:10.1189/jlb.0512231.
- ^
Fiete D J, Beranek M C, Baenziger J U (1998). "A cysteine-rich domain of the "mannose" receptor mediates GalNAc-4-SO4 binding". PNAS]. 95: 2089–2093.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
Mullin N P, Hitchen P G, Taylor M E (1997). "Mechanism of Ca2+ and Monosaccharide Binding to a C-type Carbohydrate-recognition Domain of the Macrophage Mannose Receptor". Journal of Biological Chemistry. 272: 5668–5681.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Drickamer K, Weis W I (1996). "Structural Basis of Lectin-Carbohydrate Recognition". Annual Review of Biochemistry. 65: 441–473. doi:10.1146/annurev.bi.65.070196.002301.
- ^ a b c Llorca O (2008). "Extended and bent conformations of the mannose receptor family". Cell Mol Life Sci. 65: 1302–1310. doi:10.1007/s00018-007-7497-9.
- ^ Jordens R, Thompson A, Amons R, Koning F (1999). "Human dendritic cells shed a functional, soluble form of the mannose receptor". International Immunology. 11: 1775–1780.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b
Martinez-Pomares L, Mahoney J A, Kaposzta R, Linehan S A, Stahl P D, Gordon S (1998). "A functional soluble form of the murine mannose receptor is produced by macrophages in vitro and is present in mouse serum". Journal of Biological Chemistry. 273: 23376–23380.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gazi U, Rosas M, Singh S, Heinsbroek S, Haq I, Johnson S, Brown G D, Williams D L, Taylor P R, Martinez-Pomares L (2011). "Fungal recognition enhances mannose receptor shedding through dectin-1 engagement". Journal of Biological Chemistry. 286: 7822–7829.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
Su Y, Bakker T, Harris J, Tsang C, Brown G D, Wormald M R, Gordon S, Dwek R A, Rudd P M, Martinez-Pomares L (2005). "Glycosylation influences the lectin activities of the macrophage mannose receptor". Journal of Biological Chemistry. 280: 32811–32820. doi:10.1074/jbc.M503457200.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Marodi L, Korchak H M, Johnston Jr. R B (1991). "Mechanisms of host defence against Candida species I. Phagocytosis by monocytes and monocyte-derived macrophages". Journal of Immunology. 146: 2783–2789.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ezekowitz R A, Williams D J, Koziel H, Armstrong M Y, Warner A, Richards F F, Rose R M (1991). "Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor". Nature. 351: 155–158.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ O’Riordan D M, Standing J E, Limper A H (1995). "Pneumocystis carinii glycoprotein A binds macrophage mannose receptors". Infection and Immunity. 63: 779–784.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Chakraborty R, Chakraborty P, Basu M K (1998). "Macrophage mannosyl fucosyl receptor: its role in invasion of virulent and avirulent L. donovani promastigotes". Bioscience Reports. 18: 129–142.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Chakraborty P, Ghosh D, Basu M K (2001). "Modulation of macrophage mannose receptor affects the uptake of virulent and avirulent Leishmania donovani promastigotes". Journal of Parasitology. 87: 1023–1027.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kang P B, Azad A K, Torrelles J B, Kaufman T M, Beharka A, Tibesar E, DesJardin L E, Schlesinger L S (2005). "The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis". Journal of Experimental Medicine. 202: 987–999.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
Tachado S D, Zhang J, Zhu J, Patel N, Cushion M, Koziel H (2007). "Pneumocystis-mediated IL-8 release by macrophages requires coexpression of mannose receptors and TLR2". Journal of Leukocyte Biology. 81: 205–211.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- mannose+receptor at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Macrophage Mannose Receptor
