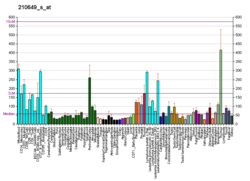
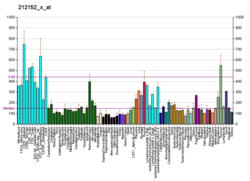
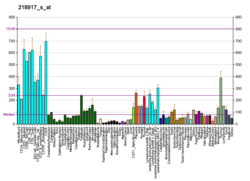
ARID1A
AT-rich interactive domain-containing protein 1A is a protein that in humans is encoded by the ARID1A gene.[5][6][7]
Function
[edit]ARID1A is a member of the SWI/SNF family, whose members have helicase and ATPase activities and are thought to regulate transcription of certain genes by altering the chromatin structure around those genes. The encoded protein is part of the large ATP-dependent chromatin remodelling complex SWI/SNF, which is required for transcriptional activation of genes normally repressed by chromatin. It possesses at least two conserved domains that could be important for its function. First, it has an ARID domain, which is a DNA-binding domain that can specifically bind an AT-rich DNA sequence known to be recognized by a SWI/SNF complex at the beta-globin locus. Second, the C-terminus of the protein can stimulate glucocorticoid receptor-dependent transcriptional activation. The protein encoded by this gene confers specificity to the SWI/SNF complex and recruits the complex to its targets through either protein-DNA or protein-protein interactions.[8] Two transcript variants encoding different isoforms have been found for this gene.[7]
Clinical significance
[edit]Gene encoding for ARID1A is the most frequently mutated SWI/SNF subunit across cancers.[9] This gene has been commonly found mutated in different cancers leading to loss of function, including gastric cancers,[10] colon cancer,[11] ovarian clear cell carcinoma,[12] liver cancer,[13] lymphoma[14] and pancreatic cancer.[15] In breast cancer distant metastases acquire inactivation mutations in ARID1A not seen in the primary tumor, and reduced ARID1A expression confers resistance to different drugs such as trastuzumab and mTOR inhibitors. These findings provide a rationale for why tumors accumulate ARID1A mutations.[16][17]
Research
[edit]Lack of this gene/protein seems to protect rats from some types of liver damage.[18]
Interactions
[edit]ARID1A has been shown to interact with SMARCB1[19][20] and SMARCA4.[20][21]
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000117713 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000007880 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Takeuchi T, Furihata M, Heng HH, Sonobe H, Ohtsuki Y (June 1998). "Chromosomal mapping and expression of the human B120 gene". Gene. 213 (1–2): 189–193. doi:10.1016/S0378-1119(98)00194-2. PMID 9630625.
- ^ Takeuchi T, Chen BK, Qiu Y, Sonobe H, Ohtsuki Y (December 1997). "Molecular cloning and expression of a novel human cDNA containing CAG repeats". Gene. 204 (1–2): 71–77. doi:10.1016/S0378-1119(97)00525-8. PMID 9434167.
- ^ a b "Entrez Gene: ARID1A AT rich interactive domain 1A (SWI-like)".
- ^ Patil A, Strom AR, Paulo JA, Collings CK, Ruff KM, Shinn MK, et al. (October 2023). "A disordered region controls cBAF activity via condensation and partner recruitment". Cell. 186 (22): 4936–4955.e26. doi:10.1016/j.cell.2023.08.032. PMC 10792396. PMID 37788668.
- ^ Gourisankar S, Krokhotin A, Wenderski W, Crabtree GR (November 2023). "Context-specific functions of chromatin remodellers in development and disease". Nature Reviews. Genetics. 25 (5): 340–361. doi:10.1038/s41576-023-00666-x. PMID 38001317.
- ^ Wang K, Kan J, Yuen ST, Shi ST, Chu KM, Law S, et al. (October 2011). "Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer". Nature Genetics. 43 (12): 1219–1223. doi:10.1038/ng.982. PMID 22037554. S2CID 8884065.
- ^ Mathur R, Alver BH, San Roman AK, Wilson BG, Wang X, Agoston AT, et al. (February 2017). "ARID1A loss impairs enhancer-mediated gene regulation and drives colon cancer in mice". Nature Genetics. 49 (2): 296–302. doi:10.1038/ng.3744. PMC 5285448. PMID 27941798.
- ^ Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. (October 2010). "ARID1A mutations in endometriosis-associated ovarian carcinomas". The New England Journal of Medicine. 363 (16): 1532–1543. doi:10.1056/NEJMoa1008433. PMC 2976679. PMID 20942669.
- ^ Sun X, Wang SC, Wei Y, Luo X, Jia Y, Li L, et al. (November 2017). "Arid1a Has Context-Dependent Oncogenic and Tumor Suppressor Functions in Liver Cancer". Cancer Cell. 32 (5): 574–589.e6. doi:10.1016/j.ccell.2017.10.007. PMC 5728182. PMID 29136504.
- ^ Barisic D, Chin CR, Meydan C, Teater M, Tsialta I, Mlynarczyk C, et al. (March 2024). "ARID1A orchestrates SWI/SNF-mediated sequential binding of transcription factors with ARID1A loss driving pre-memory B cell fate and lymphomagenesis". Cancer Cell. 42 (4): 583–604.e11. doi:10.1016/j.ccell.2024.02.010. PMC 11407687. PMID 38458187.
- ^ Shain AH, Giacomini CP, Matsukuma K, Karikari CA, Bashyam MD, Hidalgo M, et al. (January 2012). "Convergent structural alterations define SWItch/Sucrose NonFermentable (SWI/SNF) chromatin remodeler as a central tumor suppressive complex in pancreatic cancer". Proceedings of the National Academy of Sciences of the United States of America. 109 (5): E252–E259. Bibcode:2012PNAS..109E.252S. doi:10.1073/pnas.1114817109. PMC 3277150. PMID 22233809.
- ^ Berns K, Sonnenblick A, Gennissen A, Brohée S, Hijmans EM, Evers B, et al. (November 2016). "Loss of ARID1A Activates ANXA1, which Serves as a Predictive Biomarker for Trastuzumab Resistance". Clinical Cancer Research. 22 (21): 5238–5248. doi:10.1158/1078-0432.CCR-15-2996. PMID 27172896.
- ^ Yates LR, Knappskog S, Wedge D, Farmery JH, Gonzalez S, Martincorena I, et al. (August 2017). "Genomic Evolution of Breast Cancer Metastasis and Relapse". Cancer Cell. 32 (2): 169–184.e7. doi:10.1016/j.ccell.2017.07.005. PMC 5559645. PMID 28810143.
- ^ "Tissue Regeneration Promoted through Gene Suppression". Genetic Engineering & Biotechnology News. March 2016.
- ^ Kato H, Tjernberg A, Zhang W, Krutchinsky AN, An W, Takeuchi T, et al. (February 2002). "SYT associates with human SNF/SWI complexes and the C-terminal region of its fusion partner SSX1 targets histones". The Journal of Biological Chemistry. 277 (7): 5498–5505. doi:10.1074/jbc.M108702200. hdl:2066/170683. PMID 11734557.
- ^ a b Wang W, Côté J, Xue Y, Zhou S, Khavari PA, Biggar SR, et al. (October 1996). "Purification and biochemical heterogeneity of the mammalian SWI-SNF complex". The EMBO Journal. 15 (19): 5370–5382. doi:10.1002/j.1460-2075.1996.tb00921.x. PMC 452280. PMID 8895581.
- ^ Zhao K, Wang W, Rando OJ, Xue Y, Swiderek K, Kuo A, et al. (November 1998). "Rapid and phosphoinositol-dependent binding of the SWI/SNF-like BAF complex to chromatin after T lymphocyte receptor signaling". Cell. 95 (5): 625–636. doi:10.1016/S0092-8674(00)81633-5. PMID 9845365. S2CID 3184211.
Further reading
[edit]- Martens JA, Winston F (April 2003). "Recent advances in understanding chromatin remodeling by Swi/Snf complexes". Current Opinion in Genetics & Development. 13 (2): 136–142. doi:10.1016/S0959-437X(03)00022-4. PMID 12672490.
- Maruyama K, Sugano S (January 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–174. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Wang W, Xue Y, Zhou S, Kuo A, Cairns BR, Crabtree GR (September 1996). "Diversity and specialization of mammalian SWI/SNF complexes". Genes & Development. 10 (17): 2117–2130. doi:10.1101/gad.10.17.2117. PMID 8804307.
- Wang W, Côté J, Xue Y, Zhou S, Khavari PA, Biggar SR, et al. (October 1996). "Purification and biochemical heterogeneity of the mammalian SWI-SNF complex". The EMBO Journal. 15 (19): 5370–5382. doi:10.1002/j.1460-2075.1996.tb00921.x. PMC 452280. PMID 8895581.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (October 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–156. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Dallas PB, Cheney IW, Liao DW, Bowrin V, Byam W, Pacchione S, et al. (June 1998). "p300/CREB binding protein-related protein p270 is a component of mammalian SWI/SNF complexes". Molecular and Cellular Biology. 18 (6): 3596–3603. doi:10.1128/MCB.18.6.3596. PMC 108941. PMID 9584200.
- Dallas PB, Pacchione S, Wilsker D, Bowrin V, Kobayashi R, Moran E (May 2000). "The human SWI-SNF complex protein p270 is an ARID family member with non-sequence-specific DNA binding activity". Molecular and Cellular Biology. 20 (9): 3137–3146. doi:10.1128/MCB.20.9.3137-3146.2000. PMC 85608. PMID 10757798.
- Nie Z, Xue Y, Yang D, Zhou S, Deroo BJ, Archer TK, et al. (December 2000). "A specificity and targeting subunit of a human SWI/SNF family-related chromatin-remodeling complex". Molecular and Cellular Biology. 20 (23): 8879–8888. doi:10.1128/MCB.20.23.8879-8888.2000. PMC 86543. PMID 11073988.
- Takeuchi T, Nicole S, Misaki A, Furihata M, Iwata J, Sonobe H, et al. (February 2001). "Expression of SMARCF1, a truncated form of SWI1, in neuroblastoma". The American Journal of Pathology. 158 (2): 663–672. doi:10.1016/S0002-9440(10)64008-4. PMC 1850330. PMID 11159203.
- Kozmik Z, Machon O, Králová J, Kreslová J, Paces J, Vlcek C (April 2001). "Characterization of mammalian orthologues of the Drosophila osa gene: cDNA cloning, expression, chromosomal localization, and direct physical interaction with Brahma chromatin-remodeling complex". Genomics. 73 (2): 140–148. doi:10.1006/geno.2001.6477. PMID 11318604.
- Kato H, Tjernberg A, Zhang W, Krutchinsky AN, An W, Takeuchi T, et al. (February 2002). "SYT associates with human SNF/SWI complexes and the C-terminal region of its fusion partner SSX1 targets histones". The Journal of Biological Chemistry. 277 (7): 5498–5505. doi:10.1074/jbc.M108702200. hdl:2066/170683. PMID 11734557.
- Lemon B, Inouye C, King DS, Tjian R (2002). "Selectivity of chromatin-remodelling cofactors for ligand-activated transcription". Nature. 414 (6866): 924–928. doi:10.1038/414924a. PMID 11780067. S2CID 4391100.
- Hurlstone AF, Olave IA, Barker N, van Noort M, Clevers H (May 2002). "Cloning and characterization of hELD/OSA1, a novel BRG1 interacting protein". The Biochemical Journal. 364 (Pt 1): 255–264. doi:10.1042/bj3640255. PMC 1222568. PMID 11988099.
- Inoue H, Furukawa T, Giannakopoulos S, Zhou S, King DS, Tanese N (November 2002). "Largest subunits of the human SWI/SNF chromatin-remodeling complex promote transcriptional activation by steroid hormone receptors". The Journal of Biological Chemistry. 277 (44): 41674–41685. doi:10.1074/jbc.M205961200. PMID 12200431.
- Nie Z, Yan Z, Chen EH, Sechi S, Ling C, Zhou S, et al. (April 2003). "Novel SWI/SNF chromatin-remodeling complexes contain a mixed-lineage leukemia chromosomal translocation partner". Molecular and Cellular Biology. 23 (8): 2942–2952. doi:10.1128/MCB.23.8.2942-2952.2003. PMC 152562. PMID 12665591.
External links
[edit]- ARID1A+protein,+human at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Human ARID1A genome location and ARID1A gene details page in the UCSC Genome Browser.
- Overview of all the structural information available in the PDB for UniProt: O14497 (AT-rich interactive domain-containing protein 1A) at the PDBe-KB.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.