From Wikipedia, the free encyclopedia
Apigenin[ 1]
Names
IUPAC name
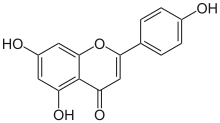
5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H -1-benzopyran-4-one
Other names
Apigenine; Chamomile; Apigenol; Spigenin; Versulin; 4′,5,7-Trihydroxyflavone; C.I. Natural Yellow 1
Identifiers
ChEBI
ChEMBL
ChemSpider
DrugBank
ECHA InfoCard 100.007.540
KEGG
UNII
InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H
Y Key: KZNIFHPLKGYRTM-UHFFFAOYSA-N
Y InChI=1/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H
Key: KZNIFHPLKGYRTM-UHFFFAOYAX
O=C\1c3c(O/C(=C/1)c2ccc(O)cc2)cc(O)cc3O
Properties
C 15 H 10 O 5
Molar mass
−1
Appearance
Yellow crystalline solid
Melting point
345 to 350 °C (653 to 662 °F; 618 to 623 K)
UV-vis (λmax )
267, 296sh, 336 nm in methanol[ 2]
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Apigenin (4′,5,7-trihydroxyflavone), found in many plants, is a natural product belonging to the flavone class that is the aglycone of several naturally occurring glycosides . It is a yellow crystalline solid that has been used to dye wool.
Sources in nature
Apigenin is found in many fruits and vegetables, but parsley , celery , celeriac , and chamomile tea are the most common sources.[ 3] flavonoids .[ 4]
Biosynthesis
Apigenin is biosynthetically derived from the general phenylpropanoid pathway and the flavone synthesis pathway.[ 5] Shikimate pathway .[ 6] phenylalanine ammonia lyase (PAL) to make cinnamate, followed by oxidation at the para position by cinnamate 4-hydroxylase (C4H) to produce p -coumarate. As L-tyrosine is already oxidized at the para position, it skips this oxidation and is simply deaminated by tyrosine ammonia lyase (TAL) to arrive at p -coumarate.[ 7] 4-coumarate CoA ligase (4CL) substitutes coenzyme A (CoA) at the carboxy group of p -coumarate. Entering the flavone synthesis pathway, the type III polyketide synthase enzyme chalcone synthase (CHS) uses consecutive condensations of three equivalents of malonyl CoA followed by aromatization to convert p -coumaroyl-CoA to chalcone.[ 8] Chalcone isomerase (CHI) then isomerizes the product to close the pyrone ring to make naringenin. Finally, a flavanone synthase (FNS) enzyme oxidizes naringenin to apigenin.[ 9] 2+ , and ascorbate as cofactors and FNS II, a membrane bound, NADPH dependent cytochrome p450 monooxygenase.[ 10]
Glycosides
The naturally occurring glycosides formed by the combination of apigenin with sugars include:
See also
References
^ Merck Index 763 .^ The Systematic Identification of Flavonoids. Mabry et al, 1970, page 81
^ The compound in the Mediterranean diet that makes cancer cells 'mortal' Emily Caldwell, Medical Express, May 20, 2013.^ Venigalla M, Gyengesi E, Münch G (August 2015). "Curcumin and Apigenin - novel and promising therapeutics against chronic neuroinflammation in Alzheimer's disease" . Neural Regeneration Research . 10 (8): 1181–5. doi :10.4103/1673-5374.162686 . PMC 4590215 PMID 26487830 . {{cite journal }}: CS1 maint: unflagged free DOI (link )^ Forkmann, G. (January 1991). "Flavonoids as Flower Pigments: The Formation of the Natural Spectrum and its Extension by Genetic Engineering". Plant Breeding . 106 (1): 1–26. doi :10.1111/j.1439-0523.1991.tb00474.x . ISSN 0179-9541 . ^ Herrmann KM (January 1995). "The shikimate pathway as an entry to aromatic secondary metabolism" . Plant Physiology . 107 (1): 7–12. doi :10.1104/pp.107.1.7 . PMC 161158 PMID 7870841 . ^ Lee H, Kim BG, Kim M, Ahn JH (September 2015). "Biosynthesis of Two Flavones, Apigenin and Genkwanin, in Escherichia coli". Journal of Microbiology and Biotechnology . 25 (9): 1442–8. doi :10.4014/jmb.1503.03011 . PMID 25975614 . ^ Austin MB, Noel JP (February 2003). "The chalcone synthase superfamily of type III polyketide synthases". Natural Product Reports . 20 (1): 79–110. CiteSeerX 10.1.1.131.8158 doi :10.1039/b100917f . PMID 12636085 . ^ Martens S, Forkmann G, Matern U, Lukacin R (September 2001). "Cloning of parsley flavone synthase I". Phytochemistry . 58 (1): 43–6. doi :10.1016/S0031-9422(01)00191-1 . PMID 11524111 . ^ Leonard E, Yan Y, Lim KH, Koffas MA (December 2005). "Investigation of two distinct flavone synthases for plant-specific flavone biosynthesis in Saccharomyces cerevisiae" . Applied and Environmental Microbiology . 71 (12): 8241–8. doi :10.1128/AEM.71.12.8241-8248.2005 . PMC 1317445 PMID 16332809 . ^ Meyer H, Bolarinwa A, Wolfram G, Linseisen J (2006). "Bioavailability of apigenin from apiin-rich parsley in humans". Annals of Nutrition & Metabolism . 50 (3): 167–72. doi :10.1159/000090736 . PMID 16407641 .
Aglycones
Monohydroxyflavone Dihydroxyflavones Trihydroxyflavones Tetrahydroxyflavones Pentahydroxyflavones O-methylated flavones
Glycosides
of apigenin of baicalein of hypolaetin of luteolin
Acetylated Sulfated glycosides Polymers Drugs
ER Tooltip Estrogen receptor
Agonists
Steroidal: 2-Hydroxyestradiol 2-Hydroxyestrone 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol 3α-Androstanediol 3α,5α-Dihydrolevonorgestrel 3β,5α-Dihydrolevonorgestrel 3α-Hydroxytibolone 3β-Hydroxytibolone 3β-Androstanediol 4-Androstenediol 4-Androstenedione 4-Fluoroestradiol 4-Hydroxyestradiol 4-Hydroxyestrone 4-Methoxyestradiol 4-Methoxyestrone 5-Androstenediol 7-Oxo-DHEA 7α-Hydroxy-DHEA 7α-Methylestradiol 7β-Hydroxyepiandrosterone 8,9-Dehydroestradiol 8,9-Dehydroestrone 8β-VE2 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED) 11β-Chloromethylestradiol 11β-Methoxyestradiol 15α-Hydroxyestradiol 16-Ketoestradiol 16-Ketoestrone 16α-Fluoroestradiol 16α-Hydroxy-DHEA 16α-Hydroxyestrone 16α-Iodoestradiol 16α-LE2 16β-Hydroxyestrone 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol) 17α-Estradiol (alfatradiol )17α-Dihydroequilenin 17α-Dihydroequilin 17α-Epiestriol (16α-hydroxy-17α-estradiol) 17α-Ethynyl-3α-androstanediol 17α-Ethynyl-3β-androstanediol 17β-Dihydroequilenin 17β-Dihydroequilin 17β-Methyl-17α-dihydroequilenin Abiraterone Abiraterone acetate Alestramustine Almestrone Anabolic steroids (e.g., testosterone and esters , methyltestosterone , metandienone (methandrostenolone) , nandrolone and esters , many others; via estrogenic metabolites)Atrimustine Bolandiol Bolandiol dipropionate Butolame Clomestrone Cloxestradiol
Conjugated estriol Conjugated estrogens Cyclodiol Cyclotriol DHEA DHEA-S ent -EstradiolEpiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol) Epimestrol Equilenin Equilin ERA-63 (ORG-37663) Esterified estrogens Estetrol Estradiol
Estramustine Estramustine phosphate Estrapronicate Estrazinol Estriol
Estrofurate Estrogenic substances Estromustine Estrone
Etamestrol (eptamestrol) Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol Ethylestradiol Etynodiol Etynodiol diacetate Hexolame Hippulin Hydroxyestrone diacetate Lynestrenol Lynestrenol phenylpropionate Mestranol Methylestradiol Moxestrol Mytatrienediol Nilestriol Norethisterone Noretynodrel Orestrate Pentolame Prodiame Prolame Promestriene RU-16117 Quinestradol Quinestrol Tibolone Xenoestrogens: Anise -related (e.g., anethole , anol , dianethole , dianol , photoanethole )Chalconoids (e.g., isoliquiritigenin , phloretin , phlorizin (phloridzin) , wedelolactone )Coumestans (e.g., coumestrol , psoralidin )Flavonoids (incl. 7,8-DHF , 8-prenylnaringenin , apigenin , baicalein , baicalin , biochanin A , calycosin , catechin , daidzein , daidzin , ECG , EGCG , epicatechin , equol , formononetin , glabrene , glabridin , genistein , genistin , glycitein , kaempferol , liquiritigenin , mirificin , myricetin , naringenin , penduletin , pinocembrin , prunetin , puerarin , quercetin , tectoridin , tectorigenin )Lavender oil Lignans (e.g., enterodiol , enterolactone , nyasol (cis -hinokiresinol) )Metalloestrogens (e.g., cadmium )Pesticides (e.g., alternariol , dieldrin , endosulfan , fenarimol , HPTE , methiocarb , methoxychlor , triclocarban , triclosan )Phytosteroids (e.g., digitoxin (digitalis ), diosgenin , guggulsterone )Phytosterols (e.g., β-sitosterol , campesterol , stigmasterol )Resorcylic acid lactones (e.g., zearalanone , α-zearalenol , β-zearalenol , zearalenone , zeranol (α-zearalanol) , taleranol (teranol, β-zearalanol) )Steroid -like (e.g., deoxymiroestrol , miroestrol )Stilbenoids (e.g., resveratrol , rhaponticin )Synthetic xenoestrogens (e.g., alkylphenols , bisphenols (e.g., BPA , BPF , BPS ), DDT , parabens , PBBs , PHBA , phthalates , PCBs )Others (e.g., agnuside , rotundifuran ) MixedSERMs Tooltip Selective estrogen receptor modulators ) Antagonists
Coregulator-binding modulators: ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown
Alcohols Barbiturates Benzodiazepines Carbamates Flavonoids Imidazoles Kava constituentsMonoureides Neuroactive steroids Nonbenzodiazepines Phenols Piperidinediones Pyrazolopyridines Quinazolinones Volatiles /gases Others/unsorted
3-Hydroxybutanal α-EMTBL AA-29504 Alogabat Avermectins (e.g., ivermectin )Bromide compounds (e.g., lithium bromide , potassium bromide , sodium bromide )Carbamazepine Chloralose Chlormezanone Clomethiazole Darigabat DEABL Deuterated etifoxine Dihydroergolines (e.g., dihydroergocryptine , dihydroergosine , dihydroergotamine , ergoloid (dihydroergotoxine) )DS2 Efavirenz Etazepine Etifoxine Fenamates (e.g., flufenamic acid , mefenamic acid , niflumic acid , tolfenamic acid )Fluoxetine Flupirtine Hopantenic acid KRM-II-81 Lanthanum Lavender oil Lignans (e.g., 4-O-methylhonokiol , honokiol , magnolol , obovatol )Loreclezole Menthyl isovalerate (validolum) Monastrol Niacin Niacinamide Org 25,435 Phenytoin Propanidid Retigabine (ezogabine) Safranal Seproxetine Stiripentol Sulfonylalkanes (e.g., sulfonmethane (sulfonal) , tetronal , trional )Terpenoids (e.g., borneol )Topiramate Valerian constituents (e.g., isovaleric acid , isovaleramide , valerenic acid , valerenol )
AMPAR Tooltip α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor KAR Tooltip Kainate receptor NMDAR Tooltip N-Methyl-D-aspartate receptor
Group I
mGluR1 Tooltip Metabotropic glutamate receptor 1 mGluR5 Tooltip Metabotropic glutamate receptor 5
Group II
mGluR2 Tooltip Metabotropic glutamate receptor 2 mGluR3 Tooltip Metabotropic glutamate receptor 3
Group III
mGluR4 Tooltip Metabotropic glutamate receptor 4 mGluR6 Tooltip Metabotropic glutamate receptor 6 mGluR7 Tooltip Metabotropic glutamate receptor 7 mGluR8 Tooltip Metabotropic glutamate receptor 8
PR Tooltip Progesterone receptor
Agonists
Testosterone derivatives: Progestins: 6,6-Difluoronorethisterone 6,6-Difluoronorethisterone acetate 17α-Allyl-19-nortestosterone Allylestrenol Altrenogest Chloroethynylnorgestrel Cingestol Danazol Desogestrel Dienogest Ethinylandrostenediol
Ethisterone Ethynerone Etonogestrel Etynodiol Etynodiol diacetate Gestodene Gestrinone Levonorgestrel Levonorgestrel esters (e.g., levonorgestrel butanoate )Lynestrenol Lynestrenol phenylpropionate Metynodiol Metynodiol diacetate Norelgestromin Norethisterone (norethindrone) Norethisterone esters (e.g., norethisterone acetate , norethisterone enanthate )Noretynodrel Norgesterone Norgestimate Norgestrel Norgestrienone Norvinisterone Oxendolone Quingestanol Quingestanol acetate Tibolone Tigestol Tosagestin ; Anabolic–androgenic steroids: 11β-Methyl-19-nortestosterone 11β-Methyl-19-nortestosterone dodecylcarbonate 19-Nor-5-androstenediol 19-Nor-5-androstenedione 19-Nordehydroepiandrosterone Bolandiol Bolandiol dipropionate Bolandione Dimethisterone Dienedione Dienolone Dimethandrolone Dimethandrolone buciclate Dimethandrolone dodecylcarbonate Dimethandrolone undecanoate Dimethyldienolone Dimethyltrienolone Ethyldienolone Ethylestrenol (ethylnandrol) Methyldienolone Metribolone (R-1881) Methoxydienone (methoxygonadiene) Mibolerone Nandrolone Nandrolone esters (e.g., nandrolone decanoate , nandrolone phenylpropionate )Norethandrolone Normethandrone (methylestrenolone, normethandrolone, normethisterone) RU-2309 Tetrahydrogestrinone Trenbolone (trienolone) Trenbolone esters (e.g., trenbolone acetate , trenbolone enanthate )Trendione Trestolone Trestolone acetate MixedSPRMs Tooltip Selective progesterone receptor modulators ) Antagonists
mPR Tooltip Membrane progesterone receptor PAQR Tooltip Progestin and adipoQ receptor )