Dehydroepiandrosterone

| |

| |
| Names | |
|---|---|
| IUPAC name
3β-Hydroxyandrost-5-en-17-one
| |
| Preferred IUPAC name
(3aS,3bR,7S,9aR,9bS,11aS)-7-Hydroxy-9a,11a-dimethyl-2,3,3a,3b,4,6,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-cyclopenta[a]phenanthren-1-one | |
| Other names
Androstenolone; Prasterone; Androst-5-en-3β-ol-17-one; 5,6-Didehydroepiandrosterone;[1] Dehydroisoepiandrosterone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.160 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H28O2 | |
| Molar mass | 288.424 g/mol |
| Melting point | 148.5 |
| Pharmacology | |
| QA14AA07 (WHO) G03EA03 (WHO) (combination with estrogen) | |
| By mouth, vaginal (insert), intramuscular injection (as prasterone enanthate), injection (as prasterone sodium sulfate) | |
| Pharmacokinetics: | |
| 50%[2] | |
| Hepatic[2] | |
| DHEA: 25 minutes[3] DHEA-S: 11 hours[3] | |
| Urine | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dehydroepiandrosterone (DHEA), also known as androstenolone, is an endogenous steroid hormone precursor.[4] It is one of the most abundant circulating steroids in humans.[5] DHEA is produced in the adrenal glands,[6] the gonads, and the brain.[7] It functions as a metabolic intermediate in the biosynthesis of the androgen and estrogen sex steroids both in the gonads and in various other tissues.[4][8][9] However, DHEA also has a variety of potential biological effects in its own right, binding to an array of nuclear and cell surface receptors,[10] and acting as a neurosteroid and modulator of neurotrophic factor receptors.[11]
In the United States, DHEA is sold as an over-the-counter supplement, and medication called prasterone.
Biological function
As an androgen
DHEA and other adrenal androgens such as androstenedione, although relatively weak androgens, are responsible for the androgenic effects of adrenarche, such as early pubic and axillary hair growth, adult-type body odor, increased oiliness of hair and skin, and mild acne.[12][13][14] DHEA is potentiated locally via conversion into testosterone and dihydrotestosterone (DHT) in the skin and hair follicles.[4] Women with complete androgen insensitivity syndrome (CAIS), who have a non-functional androgen receptor (AR) and are immune to the androgenic effects of DHEA and other androgens, have absent or only sparse/scanty pubic and axillary hair and body hair in general, demonstrating the role of DHEA and other androgens in body hair development at both adrenarche and pubarche.[15][16][17][18]
As an estrogen
DHEA is a weak estrogen.[4][10][19] In addition, it is transformed into potent estrogens such as estradiol in certain tissues such as the vagina, and thereby produces estrogenic effects in such tissues.[4]
As a neurosteroid
As a neurosteroid and neurotrophin, DHEA has important effects in the central nervous system.[20][21][22]
Biological activity
Hormonal activity
Androgen receptor
Although it functions as an endogenous precursor to more potent androgens such as testosterone and DHT, DHEA has been found to possess some degree of androgenic activity in its own right, acting as a low affinity (Ki = 1 μM), weak partial agonist of the androgen receptor (AR). However, its intrinsic activity at the receptor is quite weak, and on account of that, due to competition for binding with full agonists like testosterone, it can actually behave more like an antagonist depending on circulating testosterone and dihydrotestosterone (DHT) levels, and hence, like an antiandrogen. However, its affinity for the receptor is very low, and for that reason, is unlikely to be of much significance under normal circumstances.[19][23]
Estrogen receptors
In addition to its affinity for the androgen receptor, DHEA has also been found to bind to (and activate) the ERα and ERβ estrogen receptors with Ki values of 1.1 μM and 0.5 μM, respectively, and EC50 values of >1 μM and 200 nM, respectively. Though it was found to be a partial agonist of the ERα with a maximal efficacy of 30–70%, the concentrations required for this degree of activation make it unlikely that the activity of DHEA at this receptor is physiologically meaningful. Remarkably however, DHEA acts as a full agonist of the ERβ with a maximal response similar to or actually slightly greater than that of estradiol, and its levels in circulation and local tissues in the human body are high enough to activate the receptor to the same degree as that seen with circulating estradiol levels at somewhat higher than their maximal, non-ovulatory concentrations; indeed, when combined with estradiol with both at levels equivalent to those of their physiological concentrations, overall activation of the ERβ was doubled.[10][19]
Other nuclear receptors
DHEA does not bind to or activate the progesterone, glucocorticoid, or mineralocorticoid receptors.[19][24] Other nuclear receptor targets of DHEA besides the androgen and estrogen receptors include the PPARα, PXR, and CAR.[25] However, whereas DHEA is a ligand of the PPARα and PXR in rodents, it is not in humans.[26] In addition to direct interactions, DHEA is thought to regulate a handful of other proteins via indirect, genomic mechanisms, including the enzymes CYP2C11 and 11β-HSD1 – the latter of which is essential for the biosynthesis of the glucocorticoids such as cortisol and has been suggested to be involved in the antiglucocorticoid effects of DHEA – and the carrier protein IGFBP1.[19][27]
Neurosteroid activity
Neurotransmitter receptors
DHEA has been found to directly act on several neurotransmitter receptors, including acting as a positive allosteric modulator of the NMDA receptor, as a negative allosteric modulator of the GABAA receptor, and as an agonist of the σ1 receptor.[28][25]
Neurotrophin receptors
In 2011, the surprising discovery was made that DHEA, as well as its sulfate ester, DHEA-S, directly bind to and activate TrkA and p75NTR, receptors of neurotrophins like nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), with high affinity.[25][29] DHEA was subsequently also found to bind to TrkB and TrkC with high affinity, though it only activated TrkC not TrkB.[25][30] DHEA and DHEA-S bound to these receptors with affinities in the low nanomolar range (around 5 nM), which were nonetheless approximately two orders of magnitude lower relative to highly potent polypeptide neurotrophins like NGF (0.01–0.1 nM).[25][29][30] In any case, DHEA and DHEA-S both circulate at requisite concentrations to activate these receptors and were thus identified as important endogenous neurotrophic factors.[25][29] They have since been labeled "steroidal microneurotrophins", due to their small-molecule and steroidal nature relative to their polypeptide neurotrophin counterparts.[31] Subsequent research has suggested that DHEA and/or DHEA-S may in fact be phylogenetically ancient "ancestral" ligands of the neurotrophin receptors from early on in the evolution of the nervous system.[25][30] The findings that DHEA binds to and potently activates neurotrophin receptors may explain the positive association between decreased circulating DHEA levels with age and age-related neurodegenerative diseases.[25][29]
Microtubule-associated protein 2
Similarly to pregnenolone, its synthetic derivative 3β-methoxypregnenolone (MAP-4343), and progesterone, DHEA has been found to bind to microtubule-associated protein 2 (MAP2), specifically the MAP2C subtype (Kd = 27 μM).[25] However, it is unclear whether DHEA increases binding of MAP2 to tubulin like pregnenolone.[25]
ADHD
Some research has shown that DHEA levels are too low in people with ADHD, and treatment with methylphenidate or bupropion (stimulant type of medications) normalizes DHEA levels. [32]
Other activity
G6PDH inhibitor
DHEA is an uncompetitive inhibitor of G6PDH (Ki = 17 μM; IC50 = 18.7 μM), and is able to lower NADPH levels and reduce NADPH-dependent free radical production.[33][34] It is thought that this action may possibly be responsible for much of the antiinflammatory, antihyperplastic, chemopreventative, antihyperlipidemic, antidiabetic, and antiobesic, as well as certain immunomodulating activities of DHEA (with some experimental evidence to support this notion available).[33][34][35][36] However, it has also been said that inhibition of G6PDH activity by DHEA in vivo has not been observed and that the concentrations required for DHEA to inhibit G6PDH in vitro are very high, thus making the possible contribution of G6PDH inhibition to the effects of DHEA uncertain.[34]
Cancer
DHEA supplements have been promoted as chemopreventative,[33][34][35][36] for their claimed cancer prevention properties. There is scientific evidence to support these claims.[33][34][35][36]
Miscellaneous
DHEA has been found to competitively inhibit TRPV1.[28]
Biochemistry
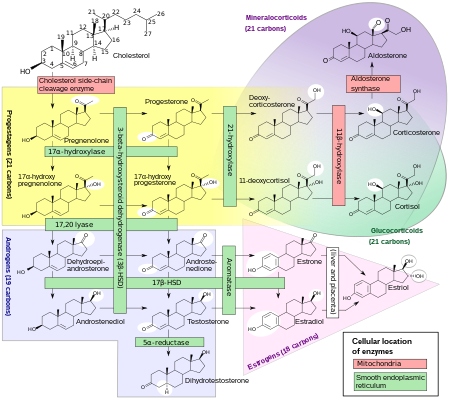
Biosynthesis
DHEA is produced in the zona reticularis of the adrenal cortex under the control of adrenocorticotropic hormone (ACTH) and by the gonads under the control of gonadotropin-releasing hormone (GnRH).[38][39] It is also produced in the brain.[40] DHEA is synthesized from cholesterol via the enzymes cholesterol side-chain cleavage enzyme (CYP11A1; P450scc) and 17α-hydroxylase/17,20-lyase (CYP17A1), with pregnenolone and 17α-hydroxypregnenolone as intermediates.[41] It is derived mostly from the adrenal cortex, with only about 10% being secreted from the gonads.[42][43][44] Approximately 50 to 70% of circulating DHEA originates from desulfation of DHEA-S in peripheral tissues.[42] DHEA-S itself originates almost exclusively from the adrenal cortex, with 95 to 100% being secreted from the adrenal cortex in women.[38][44]
Increasing endogenous production
Regular exercise is known to increase DHEA production in the body.[45][46] Calorie restriction has also been shown to increase DHEA in primates.[47] Some theorize that the increase in endogenous DHEA brought about by calorie restriction is partially responsible for the longer life expectancy known to be associated with calorie restriction.[48]
Distribution
In the circulation, DHEA is mainly bound to albumin, with a small amount bound to sex hormone-binding globulin (SHBG).[49][50] The small remainder of DHEA not associated with albumin or SHBG is unbound and free in the circulation.[49]
DHEA easily crosses the blood–brain barrier into the central nervous system.[40]
Metabolism
DHEA is transformed into DHEA-S by sulfation at the C3β position via the sulfotransferase enzymes SULT2A1 and to a lesser extent SULT1E1.[41][51][52] This occurs naturally in the adrenal cortex and during first-pass metabolism in the liver and intestines when exogenous DHEA is administered orally.[51] Levels of DHEA-S in circulation are approximately 250 to 300 times those of DHEA.[20] DHEA-S in turn can be converted back into DHEA in peripheral tissues via steroid sulfatase (STS).[53][54]
The terminal half-life of DHEA is short at only 15 to 30 minutes.[55] In contrast, the terminal half-life of DHEA-S is far longer, at 7 to 10 hours.[55] As DHEA-S can be converted back into DHEA, it serves as a circulating reservoir for DHEA, thereby extending the duration of DHEA.[56][20]
Metabolites of DHEA include DHEA-S, 7α-hydroxy-DHEA, 7β-hydroxy-DHEA, 7-keto-DHEA, 7α-hydroxyepiandrosterone, and 7β-hydroxyepiandrosterone, as well as androstenediol and androstenedione.[8]
Pregnancy
During pregnancy, DHEA-S is metabolized into the sulfates of 16α-hydroxy-DHEA and 15α-hydroxy-DHEA in the fetal liver as intermediates in the production of the estrogens estriol and estetrol, respectively.[57]
Levels
Prior to puberty, DHEA and DHEA-S levels elevate upon differentiation of the zona reticularis of the adrenal cortex.[25] Peak levels of DHEA and DHEA-S are observed around age 20, which is followed by an age-dependent decline throughout life eventually back to prepubertal concentrations.[25] Plasma levels of DHEA in adult men are 10 to 25 nM, in premenopausal women are 5 to 30 nM, and in postmenopausal women are 2 to 20 nM.[25] Conversely, DHEA-S levels are an order of magnitude higher at 1–10 μM.[25] Levels of DHEA and DHEA-S decline to the lower nanomolar and micromolar ranges in men and women aged 60 to 80 years.[25]
DHEA levels are as follows:[58]
- Adult men: 180–1250 ng/dL
- Adult women: 130–980 ng/dL
- Pregnant women: 135–810 ng/dL
- Prepubertal children (<1 year): 26–585 ng/dL
- Prepubertal children (1–5 years): 9–68 ng/dL
- Prepubertal children (6–12 years): 11–186 ng/dL
- Adolescent boys (Tanner II–III): 25–300 ng/dL
- Adolescent girls (Tanner II–III): 69–605 ng/dL
- Adolescent boys (Tanner IV–V): 100–400 ng/dL
- Adolescent girls (Tanner IV–V): 165–690 ng/dL
Measurement
As almost all DHEA is derived from the adrenal glands, blood measurements of DHEA-S/DHEA are useful to detect excess adrenal activity as seen in adrenal cancer or hyperplasia, including certain forms of congenital adrenal hyperplasia. Women with polycystic ovary syndrome tend to have elevated levels of DHEA-S.[59]
Chemistry
DHEA, also known as androst-5-en-3β-ol-17-one, is a naturally occurring androstane steroid and a 17-ketosteroid.[60] It is closely related structurally to androstenediol (androst-5-ene-3β,17β-diol), androstenedione (androst-4-ene-3,17-dione), and testosterone (androst-4-en-17β-ol-3-one).[60] DHEA is the 5-dehydro analogue of epiandrosterone (5α-androstan-3β-ol-17-one) and is also known as 5-dehydroepiandrosterone or as δ5-epiandrosterone.[60]
Isomers
The term "dehydroepiandrosterone" is ambiguous chemically because it does not include the specific positions within epiandrosterone at which hydrogen atoms are missing. DHEA itself is 5,6-didehydroepiandrosterone or 5-dehydroepiandrosterone. A number of naturally occurring isomers also exist and may have similar activities. Some isomers of DHEA are 1-dehydroepiandrosterone (1-androsterone) and 4-dehydroepiandrosterone.[61] These isomers are also technically "DHEA", since they are dehydroepiandrosterones in which hydrogens are removed from the epiandrosterone skeleton.
Dehydroandrosterone (DHA) is the 3α-epimer of DHEA and is also an endogenous androgen.
History
DHEA was first isolated from human urine in 1934 by Adolf Butenandt and Kurt Tscherning.[62]
See also
References
- ^ Devillers J (27 April 2009). Endocrine Disruption Modeling. CRC Press. pp. 339–. ISBN 978-1-4200-7636-3.
- ^ a b Cupp MJ, Tracy TS (10 December 2002). Dietary Supplements: Toxicology and Clinical Pharmacology. Springer Science & Business Media. pp. 135–. ISBN 978-1-59259-303-3.
- ^ a b Oddens BJ, Vermeulen A (15 November 1996). Androgens and the Aging Male. CRC Press. pp. 5–. ISBN 978-1-85070-763-9.
- ^ a b c d e Labrie F, Luu-The V, Bélanger A, Lin SX, Simard J, Pelletier G, Labrie C (November 2005). "Is dehydroepiandrosterone a hormone?". J. Endocrinol. 187 (2): 169–96. doi:10.1677/joe.1.06264. PMID 16293766.
- ^ William F Ganong MD, 'Review of Medical Physiology', 22nd Ed, McGraw Hill, 2005, p. 362.
- ^ The Merck Index, 13th Edition, 7798
- ^ Schulman RA, Dean C (2007). Solve It With Supplements. New York City: Rodale, Inc. p. 100. ISBN 978-1-57954-942-8.
DHEA (Dehydroepiandrosterone) is a common hormone produced in the adrenal glands, the gonads, and the brain.
- ^ a b Mo Q, Lu SF, Simon NG (April 2006). "Dehydroepiandrosterone and its metabolites: differential effects on androgen receptor trafficking and transcriptional activity". The Journal of Steroid Biochemistry and Molecular Biology. 99 (1): 50–8. doi:10.1016/j.jsbmb.2005.11.011. PMID 16524719. S2CID 30489004.
- ^ Scott T (1996). Concise Encyclopedia Biology. Walter de Gruyter. p. 49. ISBN 978-3-11-010661-9. Retrieved 25 May 2012.
- ^ a b c Webb SJ, Geoghegan TE, Prough RA, Michael Miller KK (2006). "The biological actions of dehydroepiandrosterone involves multiple receptors". Drug Metabolism Reviews. 38 (1–2): 89–116. doi:10.1080/03602530600569877. PMC 2423429. PMID 16684650.
- ^ Friess E, Schiffelholz T, Steckler T, Steiger A (December 2000). "Dehydroepiandrosterone--a neurosteroid". European Journal of Clinical Investigation. 30 Suppl 3: 46–50. doi:10.1046/j.1365-2362.2000.0300s3046.x. PMID 11281367. S2CID 30733847.
- ^ Pescovitz OH, Eugster EA (2004). Pediatric Endocrinology: Mechanisms, Manifestations, and Management. Lippincott Williams & Wilkins. pp. 362–. ISBN 978-0-7817-4059-3.
- ^ Fima Lifshitz (26 December 2006). Pediatric Endocrinology: Growth, Adrenal, Sexual, Thyroid, Calcium, and Fluid Balance Disorders. CRC Press. pp. 289–. ISBN 978-1-4200-4272-6.
- ^ Salhan S (1 August 2011). Textbook of Gynecology. JP Medical Ltd. pp. 94–. ISBN 978-93-5025-369-4.
- ^ Lavery JP, Sanfilippo JS (6 December 2012). Pediatric and Adolescent Obstetrics and Gynecology. Springer Science & Business Media. pp. 45–. ISBN 978-1-4612-5064-7.
- ^ Nussbaum RL, McInnes RR, Willard HF (28 April 2015). Thompson & Thompson Genetics in Medicine. Elsevier Health Sciences. pp. 102–. ISBN 978-0-323-39206-8.
- ^ Setchell ME, Hudson CN (4 April 2013). Shaw's Textbook of Operative Gynaecology. Elsevier Health Sciences. pp. 129–. ISBN 978-81-312-3481-5.
- ^ Bissonnette B, Dalens B (20 July 2006). Syndromes: Rapid Recognition and Perioperative Implications. McGraw Hill Professional. p. 184. ISBN 978-0-07-135455-4.
- ^ a b c d e Chen F, Knecht K, Birzin E, Fisher J, Wilkinson H, Mojena M, Moreno CT, Schmidt A, Harada S, Freedman LP, Reszka AA (November 2005). "Direct agonist/antagonist functions of dehydroepiandrosterone". Endocrinology. 146 (11): 4568–76. doi:10.1210/en.2005-0368. PMID 15994348.
- ^ a b c Weizman A (1 February 2008). Neuroactive Steroids in Brain Function, Behavior and Neuropsychiatric Disorders: Novel Strategies for Research and Treatment. Springer Science & Business Media. pp. 229–. ISBN 978-1-4020-6854-6.
- ^ Gravanis AG, Mellon SH (24 June 2011). Hormones in Neurodegeneration, Neuroprotection, and Neurogenesis. John Wiley & Sons. pp. 349–. ISBN 978-3-527-63397-5.
- ^ Sex difference in the human brain, their underpinnings and implications. Elsevier. 3 December 2010. pp. 127–. ISBN 978-0-444-53631-0.
- ^ Gao W, Bohl CE, Dalton JT (September 2005). "Chemistry and structural biology of androgen receptor". Chemical Reviews. 105 (9): 3352–70. doi:10.1021/cr020456u. PMC 2096617. PMID 16159155.
- ^ Lindschau C, Kirsch T, Klinge U, Kolkhof P, Peters I, Fiebeler A (September 2011). "Dehydroepiandrosterone-induced phosphorylation and translocation of FoxO1 depend on the mineralocorticoid receptor". Hypertension. 58 (3): 471–8. doi:10.1161/HYPERTENSIONAHA.111.171280. PMID 21747041.
- ^ a b c d e f g h i j k l m n o Prough RA, Clark BJ, Klinge CM (April 2016). "Novel mechanisms for DHEA action". Journal of Molecular Endocrinology. 56 (3): R139–55. doi:10.1530/JME-16-0013. PMID 26908835.
- ^ Watson RR (22 July 2011). DHEA in Human Health and Aging. CRC Press. pp. 208–. ISBN 978-1-4398-3884-6.
- ^ Kalimi M, Shafagoj Y, Loria R, Padgett D, Regelson W (February 1994). "Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA)". Molecular and Cellular Biochemistry. 131 (2): 99–104. doi:10.1007/BF00925945. PMID 8035785. S2CID 26893297.
- ^ a b King SR (9 November 2012). Neurosteroids and the Nervous System. Springer Science & Business Media. pp. 15–16. ISBN 978-1-4614-5559-2.
- ^ a b c d Lazaridis I, Charalampopoulos I, Alexaki VI, Avlonitis N, Pediaditakis I, Efstathopoulos P, Calogeropoulou T, Castanas E, Gravanis A (April 2011). "Neurosteroid dehydroepiandrosterone interacts with nerve growth factor (NGF) receptors, preventing neuronal apoptosis". PLOS Biology. 9 (4): e1001051. doi:10.1371/journal.pbio.1001051. PMC 3082517. PMID 21541365.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c Pediaditakis I, Iliopoulos I, Theologidis I, Delivanoglou N, Margioris AN, Charalampopoulos I, Gravanis A (January 2015). "Dehydroepiandrosterone: an ancestral ligand of neurotrophin receptors". Endocrinology. 156 (1): 16–23. doi:10.1210/en.2014-1596. PMID 25330101.
- ^ Gravanis A, Calogeropoulou T, Panoutsakopoulou V, Thermos K, Neophytou C, Charalampopoulos I (October 2012). "Neurosteroids and microneurotrophins signal through NGF receptors to induce prosurvival signaling in neuronal cells". Science Signaling. 5 (246): pt8. doi:10.1126/scisignal.2003387. PMID 23074265. S2CID 26914550.
- ^ Lee, M. S.; Yang, J. W.; Ko, Y. H.; Han, C.; Kim, S. H.; Lee, M. S.; Joe, S. H.; Jung, I. K. (2008). "Effects of methylphenidate and bupropion on DHEA-S and cortisol plasma levels in attention-deficit hyperactivity disorder". Child Psychiatry and Human Development. 39 (2): 201–209. doi:10.1007/s10578-007-0081-6. PMID 17763937. S2CID 11041447.
- ^ a b c d Schwartz AG, Pashko LL (April 2004). "Dehydroepiandrosterone, glucose-6-phosphate dehydrogenase, and longevity". Ageing Research Reviews. 3 (2): 171–87. doi:10.1016/j.arr.2003.05.001. PMID 15177053. S2CID 11871872.
- ^ a b c d e Ciolino HP, MacDonald CJ, Yeh GC (July 2002). "Inhibition of carcinogen-activating enzymes by 16alpha-fluoro-5-androsten-17-one". Cancer Research. 62 (13): 3685–90. PMID 12097275.
- ^ a b c McCormick DL, Johnson WD, Kozub NM, Rao KV, Lubet RA, Steele VE, Bosland MC (February 2007). "Chemoprevention of rat prostate carcinogenesis by dietary 16alpha-fluoro-5-androsten-17-one (fluasterone), a minimally androgenic analog of dehydroepiandrosterone". Carcinogenesis. 28 (2): 398–403. doi:10.1093/carcin/bgl141. PMID 16952912.
- ^ a b c Auci D, Kaler L, Subramanian S, Huang Y, Frincke J, Reading C, Offner H (September 2007). "A new orally bioavailable synthetic androstene inhibits collagen-induced arthritis in the mouse: androstene hormones as regulators of regulatory T cells". Annals of the New York Academy of Sciences. 1110 (1): 630–40. Bibcode:2007NYASA1110..630A. doi:10.1196/annals.1423.066. PMID 17911478. S2CID 32258529.
- ^ Häggström, Mikael; Richfield, David (2014). "Diagram of the pathways of human steroidogenesis". WikiJournal of Medicine. 1 (1). doi:10.15347/wjm/2014.005. ISSN 2002-4436.
- ^ a b Erkkola R (2006). The Menopause. Elsevier. pp. 5–. ISBN 978-0-444-51830-9.
- ^ Kleine B, Rossmanith WG (11 February 2016). Hormones and the Endocrine System: Textbook of Endocrinology. Springer. pp. 264–265. ISBN 978-3-319-15060-4.
- ^ a b Pizzorno JE (2013). Textbook of Natural Medicine. Elsevier Health Sciences. pp. 711–. ISBN 978-1-4377-2333-5.
- ^ a b Rainey WE, Nakamura Y (February 2008). "Regulation of the adrenal androgen biosynthesis". The Journal of Steroid Biochemistry and Molecular Biology. 108 (3–5): 281–6. doi:10.1016/j.jsbmb.2007.09.015. PMC 2699571. PMID 17945481.
- ^ a b Adler RA (14 December 2009). Osteoporosis: Pathophysiology and Clinical Management. Springer Science & Business Media. pp. 387–. ISBN 978-1-934115-19-0.
- ^ Schill WB, Comhaire FH, Hargreave TB (26 August 2006). Andrology for the Clinician. Springer Science & Business Media. pp. 243–. ISBN 978-3-540-33713-3.
- ^ a b Linos DA, van Heerden JA (5 December 2005). Adrenal Glands: Diagnostic Aspects and Surgical Therapy. Springer Science & Business Media. pp. 161–. ISBN 978-3-540-26861-1.
- ^ Filaire E, Duché P, Lac G (October 1998). "Effects of amount of training on the saliva concentrations of cortisol, dehydroepiandrosterone and on the dehydroepiandrosterone: cortisol concentration ratio in women over 16 weeks of training". European Journal of Applied Physiology and Occupational Physiology. 78 (5): 466–71. doi:10.1007/s004210050447. PMID 9809849. S2CID 20583279.
- ^ Copeland JL, Consitt LA, Tremblay MS (April 2002). "Hormonal responses to endurance and resistance exercise in females aged 19-69 years". The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 57 (4): B158–65. doi:10.1093/gerona/57.4.B158. PMID 11909881.
- ^ Mattison JA, Lane MA, Roth GS, Ingram DK (2003). "Calorie restriction in rhesus monkeys". Experimental Gerontology. 38 (1–2): 35–46. doi:10.1016/S0531-5565(02)00146-8. PMID 12543259. S2CID 41481691..
- ^ Roberts E (February 1999). "The importance of being dehydroepiandrosterone sulfate (in the blood of primates): a longer and healthier life?". Biochemical Pharmacology. 57 (4): 329–46. doi:10.1016/S0006-2952(98)00246-9. PMID 9933021..
- ^ a b Alesci S, Manoli I, Blackman MR (29 December 2004). "Dehydroepiandrosterone (DHEA)". In Coates PM, Blackman MR, Cragg GM, Levine M, Moss J, White JD (eds.). Encyclopedia of Dietary Supplements (Print). CRC Press. pp. 169–. ISBN 978-0-8247-5504-1.
- ^ Becker KL (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 712–. ISBN 978-0-7817-1750-2.
- ^ a b Mueller JW, Gilligan LC, Idkowiak J, Arlt W, Foster PA (October 2015). "The Regulation of Steroid Action by Sulfation and Desulfation". Endocr Rev. 36 (5): 526–63. doi:10.1210/er.2015-1036. PMC 4591525. PMID 26213785.
- ^ Lash LH (2005). Drug Metabolism and Transport: Molecular Methods and Mechanisms. Springer Science & Business Media. pp. 353–. ISBN 978-1-59259-832-8.
- ^ Morfin R (2 September 2003). DHEA and the Brain. CRC Press. pp. 28–. ISBN 978-0-203-30121-0.
- ^ Karasek M (2006). Aging and Age-related Diseases: The Basics. Nova Publishers. pp. 66–. ISBN 978-1-59454-426-2.
- ^ a b White BA, Porterfield SP (2013). Endocrine and Reproductive Physiology, Mosby Physiology Monograph Series (with Student Consult Online Access),4: Endocrine and Reproductive Physiology. Elsevier Health Sciences. pp. 164–. ISBN 978-0-323-08704-9.
- ^ Kalimi MY, Regelson W (2000). Dehydroepiandrosterone (DHEA): Biochemical, Physiological and Clinical Aspects. Walter de Gruyter. pp. 41–. ISBN 978-3-11-016111-3.
- ^ Zbella, E. A.; Ilekis, J.; Scommegna, A.; Benveniste, R. "Competitive studies with dehydroepiandrosterone sulfate and 16 alpha-hydroxydehydroepiandrosterone sulfate in cultured human choriocarcinoma JEG-3 cells: effect on estrone, 17 beta-estradiol, and estriol secretion". The Journal of Clinical Endocrinology and Metabolism. 63 (3): 751–757. doi:10.1210/jcem-63-3-751. ISSN 0021-972X. PMID 2942557.
- ^ https://www.questdiagnostics.com/hcp/intguide/EndoMetab/EndoManual_AtoZ_PDFs/DHEA.pdf [bare URL PDF]
- ^ Banaszewska B, Spaczyński RZ, Pelesz M, Pawelczyk L (2003). "Incidence of elevated LH/FSH ratio in polycystic ovary syndrome women with normo- and hyperinsulinemia". Roczniki Akademii Medycznej W Bialymstoku. 48: 131–4. PMID 14737959.
- ^ a b c Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 641–. ISBN 978-1-4757-2085-3.
- ^ Edith Josephy; F. Radt (1 December 2013). Elsevier's Encyclopaedia of Organic Chemistry: Series III: Carboisocyclic Condensed Compounds. Springer. pp. 2608–. ISBN 978-3-662-25863-7.
- ^ Schwartz AG, Pashko LL (2001). "Potential therapeutic use of dehydroepiandrosterone and structural analogs". Diabetes Technology & Therapeutics. 3 (2): 221–4. doi:10.1089/152091501300209589. PMID 11478328.
