Tramadol: Difference between revisions
+ img. (Ultracet tablets) |
No edit summary Tags: Mobile edit Mobile web edit |
||
| Line 16: | Line 16: | ||
<!-- Clinical data --> |
<!-- Clinical data --> |
||
| pronounce = tra' ma |
| pronounce = tra' ma doll |
||
| tradename = Ultram, Zytram, Qdolo, others<ref name=generics/> |
| tradename = Ultram, Zytram, Qdolo, others<ref name=generics/> |
||
| Drugs.com = {{drugs.com|monograph|tramadol-hydrochloride}} |
| Drugs.com = {{drugs.com|monograph|tramadol-hydrochloride}} |
||
Revision as of 00:55, 28 April 2022
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | tra' ma doll |
| Trade names | Ultram, Zytram, Qdolo, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a695011 |
| License data | |
| Pregnancy category |
|
| Dependence liability | Present[3] |
| Routes of administration | By mouth, intravenous (IV), intramuscular (IM), rectal |
| Drug class | Opioid analgesic[4] |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 70–75% (by mouth), 77% (rectal), 100% (IM)[6] |
| Protein binding | 20%[3] |
| Metabolism | Liver-mediated demethylation and glucuronidation via CYP2D6 & CYP3A4[6][7] |
| Metabolites | O-desmethyltramadol, N-desmethyltramadol |
| Onset of action | Less than 1 hour (by mouth)[3] |
| Elimination half-life | 6.3 ± 1.4 h[7] |
| Duration of action | 6 hours[8] |
| Excretion | Urine (95%)[9] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.043.912 |
| Chemical and physical data | |
| Formula | C16H25NO2 |
| Molar mass | 263.381 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 180 to 181 °C (356 to 358 °F) |
| |
| |
| | |
Tramadol, sold under the brand name Ultram among others,[1] is an opioid pain medication used to treat moderate to moderately severe pain.[3] When taken by mouth in an immediate-release formulation, the onset of pain relief usually begins within an hour.[3] It is also available by injection.[10] It may be sold in combination with paracetamol (acetaminophen) or as longer-acting formulations, such as ULTRACET (37.5 mg tramadol hydrochloride/ 325 mg acetaminophen tablets)[3][10][11]
As is typical of opioids, common side effects include constipation, itchiness, and nausea.[3] Serious side effects may include hallucinations, seizures, increased risk of serotonin syndrome, decreased alertness, and drug addiction.[3] A change in dosage may be recommended in those with kidney or liver problems.[3] It is not recommended in those who are at risk of suicide or in those who are pregnant.[3][10] While not recommended in women who are breastfeeding, those who take a single dose should not generally stop breastfeeding.[12] Tramadol is converted in the liver to O-desmethyltramadol (desmetramadol), an opioid with a stronger affinity to the μ-opioid receptor.[3][13] Tramadol is also a serotonin–norepinephrine reuptake inhibitor (SNRI).[3][14]
Tramadol was patented in 1963 and launched under the name "Tramal" in 1977 by the West German pharmaceutical company Grünenthal GmbH.[14][15] In the mid-1990s, it was approved in the United Kingdom and the United States.[14] It is available as a generic medication and marketed under many brand names worldwide.[1][3] In 2019, it was the 35th most commonly prescribed medication in the United States, with more than 19 million prescriptions.[16][17]
Medical uses



Tramadol[18] (a schedule IV drug in the US) is used primarily to treat mild to severe pain, both acute and chronic.[19][20] There is moderate evidence for use as a second-line treatment for fibromyalgia but is not FDA approved for this use;[21] however, its use is approved for treatment of fibromyalgia as a secondary painkiller by the NHS.[22]
Its analgesic effects take about one hour to come into effect and 2 to 4 h to peak after oral administration with an immediate-release formulation.[19][20] On a dose-by-dose basis, tramadol has about one-tenth the potency of morphine (thus 100 mg is commensurate with 10 mg morphine but may vary) and is practically equally potent when compared with pethidine and codeine.[23] For pain moderate in severity, its effectiveness is equivalent to that of codeine at low doses, and hydrocodone at very high doses; for severe pain it is less effective than morphine.[19]
These painkilling effects last about 6 h.[20] The potency of analgesia varies considerably as it depends on an individual's genetics. People with specific variants of CYP2D6 enzymes may not produce adequate amounts of the active metabolite (desmetramadol) for effective pain control.[9][19]
Sleep medicine physicians sometimes prescribe tramadol (or other opiate medications) for refractory restless legs syndrome (RLS);[24][25] that is, RLS that does not respond adequately to treatment with first-line medications such as dopamine agonists (like pramipexole) or alpha-2-delta (α2δ) ligands (gabapentinoids), often due to augmentation.[26]
Contraindications
Tramadol may not provide adequate pain control for individuals with certain genetic variants of CYP2D6 enzymes as they metabolize tramadol to the inactive molecule.[9][19] These genetic polymorphisms are not currently routinely tested for in clinical practice.[27]
Pregnancy and lactation
Tramadol's use in pregnancy is generally avoided, as it may cause some reversible withdrawal effects in the newborn.[28] A small prospective study in France found, while an increased risk of miscarriages existed, no major malformations were reported in the newborn.[28] Its use during lactation is also generally advised against, but a small trial found that infants breastfed by mothers taking tramadol were exposed to about 2.88% of the dose the mothers were taking. No evidence of this dose harming the newborn was seen.[28]
Labor and delivery
Its use as an analgesic during labor is not advised due to its long onset of action (1 hour).[28] The ratio of the mean concentration of the drug in the fetus compared to that of the mother when it is given intramuscularly for labor pains has been estimated to be 1:94.[28]
Children
Its use in children is generally advised against, although it may be done under the supervision of a specialist.[19] On 21 September 2015, the FDA started investigating the safety of tramadol in use in persons under the age of 17. The investigation was initiated because some of these people have experienced slowed or difficult breathing.[29] The FDA lists age under 12 years old as a contraindication.[30][31]
Elderly
The risk of opioid-related adverse effects such as respiratory depression, falls, cognitive impairment and sedation is increased.[19] Tramadol may interact with other medications and increase the risk for adverse events.[27]
Liver and kidney failure
The drug should be used with caution in those with liver or kidney failure, due to metabolism in the liver (to the active molecule desmetramadol) and elimination by the kidneys.[19]
Side effects
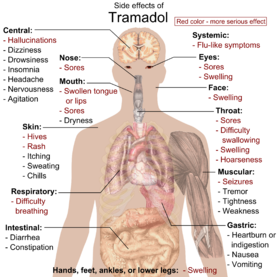
The most common adverse effects of tramadol include nausea, dizziness, dry mouth, indigestion, abdominal pain, vertigo, vomiting, constipation, drowsiness, and headache.[32][33] Other side effects may result from interactions with other medications. Tramadol has the same dose-dependent adverse effects as morphine including respiratory depression.[34]
Dependence and withdrawal
Long-term use of high doses of tramadol causes physical dependence and withdrawal syndrome.[35] These include both symptoms typical of opioid withdrawal and those associated with serotonin–norepinephrine reuptake inhibitor (SNRI) withdrawal; symptoms include numbness, tingling, paresthesia, and tinnitus.[36] Psychiatric symptoms may include hallucinations, paranoia, extreme anxiety, panic attacks, and confusion.[37] In most cases, tramadol withdrawal will set in 12–20 hours after the last dose, but this can vary.[36] Tramadol withdrawal typically lasts longer than that of other opioids. Seven days or more of acute withdrawal symptoms can occur as opposed to typically 3 or 4 days for other codeine analogues.[36]
Overdose
Recognised risk factors for tramadol overdose include respiratory depression, addiction, and seizures.[38] Naloxone only partially reverses the toxic effects of tramadol overdose and may increase the risk of seizures.[19]
Deaths with tramadol overdose have been reported and are increasing in frequency in Northern Ireland; the majority of these overdoses involve other drugs including alcohol.[38] There were 254 tramadol-related deaths in England and Wales in 2013, and 379 in Florida in 2011.[39][40] In 2011, 21,649 emergency room visits in the United States were related to tramadol.[41]
Interactions
Tramadol can interact with other medications with similar mechanisms of action.
Tramadol acts as a serotonin-norephinephrine reuptake inhibitor and thus can interact with other serotonergic medications (selective serotonin reuptake inhibitors, serotonin–norepinephrine reuptake inhibitors, tricyclic antidepressants, triptans, cough and cold medications containing dextromethorphan, herbal products containing St. John’s wort, and medications that inhibit the metabolism of serotonin, such as monoamine oxidase inhibitors) and, in combination, may lead to serotonin syndrome. It may also make some serotonergic antagonist anti-emetic medications (ondansetron) less effective.[42]
Tramadol also acts as an opioid agonist and thus can increase the risk for side effects when used with other opioid analgesics (such as morphine, pethidine, tapentadol, oxycodone, and fentanyl).[43]
Tramadol is metabolized by CYP2D6 enzymes which contribute to the metabolism of approximately 25% of all medications. Any medications with the ability to inhibit or induce these enzymes may interact with tramadol.[42]
Tramadol increases the risk for seizures by lowering the seizure threshold. Using other medications that lower seizure threshold (such as antipsychotic medications or amphetamines), further increases this risk.[42]
Pharmacology
Mechanism of action
Tramadol induces analgesic effects through a variety of different targets on the noradrenergic system, serotoninergic system and opioid receptors system.[44] Tramadol exists as a racemic mixture, the positive enantiomer inhibits serotonin reuptake while the negative enantiomer inhibits noradrenaline re-uptake, by binding to and blocking the transporters.[45][8] Tramadol has also been shown to act as a serotonin releasing agent. Both enantiomers of tramadol are agonists of the μ-opioid receptor and its M1 metabolite, O-desmetramadol, is also a μ-opioid receptor agonist but is 6 times more potent than tramadol itself.[46] All these effects work synergistically to induce analgesia.
| Site | Tramadol | DSMT | Species | Ref |
|---|---|---|---|---|
| MOR | 1,600–12,486 2,120–8,300 ≥1,000 (EC50) |
5.4–18.6 17 ((+)) ≥240 (EC50) |
Human Rat Human |
[50][51][52] [53][54] [55][13] |
| DOR | >10,000 57,600–100,000 |
≥2,900 690 (+)) |
Human Rat |
[50][51][56] [54][53] |
| KOR | >10,000 42,700–81,000 |
≥450 1,800 (+)) |
Human Rat |
[50][51][56] [54][53] |
| SERT | ~900 (IC50) 992–1,190 |
>20,000 (IC50) 2,980 ((−)) (IC50) |
Human Rat |
[57] [54][13] |
| NET | 14,600 785 |
1,080 (−) (IC50) >860 (IC50) |
Human Rat |
[13] [54][13] |
| DAT | >100,000 | >20,000 | Rat | [58][56] |
| 5-HT1A | >20,000 | >20,000 | Rat | [56] |
| 5-HT2A | >20,000 | >20,000 | Rat | [56] |
| 5-HT2C | 1,000 (IC50) | 1,300 (IC50) | Rat | [59][60] |
| 5-HT3 | >20,000 | >20,000 | Rat | [56] |
| NK1 | IA | ? | Rat | [61][62] |
| M1 | >20,000 3,400 (IC50) |
>20,000 2,000 (IC50) |
Rat Multiple |
[56] [63][64] |
| M2 | ND | ND | ND | ND |
| M3 | 1,000 (IC50) | IA | Human | [64][65] |
| M4 | ND | ND | ND | ND |
| M5 | ND | ND | ND | ND |
| α7 | 7,400 | ND | Chicken | [66] |
| σ1 | >10,000 | ND | Rat | [47][67] |
| σ2 | >10,000 | ND | Rat | [47] |
| NMDAR | 16,400 (IC50) | 16,500 (IC50) | Human | [68] |
| NMDAR (MK-801) |
>20,000 | >20,000 | Rat | [56] |
| GABAA | >100,000 (IC50) | >100,000 (IC50) | Human | [68] |
| GlyR | >100,000 (IC50) | >100,000 (IC50) | Human | [68] |
| TRPA1 | 100– 10,000 (SI) |
1,000– 10,000 (SI) |
Human | [69] |
| TRPV1 | >10,000 (IC50) | >10,000 (IC50) | Human | [69][70] |
| Values are Ki (nM), unless otherwise noted. The smaller the value, the more strongly the drug binds to the site. | ||||
| Action | Value |
|---|---|
| 5-HT reuptake | 1,820 |
| 5-HT release | >10,000 |
| NE reuptake | 2,770 |
| NE release | >10,000 |
| DA reuptake | >10,000 |
| DA release | >10,000 |
| Values for reuptake inhibition are Ki (nM) and for release induction are EC50 (nM). | |
Tramadol has been found to possess these actions:[48][49][45]
- Agonist of the μ-opioid receptor (MOR) and to a far lesser extent of the δ-opioid receptor (DOR) and κ-opioid receptor (KOR)
- Serotonin reuptake inhibitor (SRI) and norepinephrine reuptake inhibitor; hence, an SNRI
- Serotonin 5-HT2C receptor antagonist
- M1 and M3 muscarinic acetylcholine receptor antagonist
- α7 nicotinic acetylcholine receptor antagonist
- NMDA receptor antagonist (very weak)
- TRPA1 inhibitor
Tramadol acts on the opioid receptors through its major active metabolite desmetramadol, which has as much as 700-fold higher affinity for the MOR relative to tramadol.[13] Moreover, tramadol itself has been found to possess no efficacy in activating the MOR in functional activity assays, whereas desmetramadol activates the receptor with high intrinsic activity (Emax equal to that of morphine).[55][13][72] As such, desmetramadol is exclusively responsible for the opioid effects of tramadol.[73] Both tramadol and desmetramadol have pronounced selectivity for the MOR over the DOR and KOR in terms of binding affinity.[56][51][53]
Tramadol is well-established as an SRI.[48][49] In addition, a few studies have found that it also acts as a serotonin releasing agent (1–10 μM), similar in effect to fenfluramine.[74][75][76][77] The serotonin releasing effects of tramadol could be blocked by sufficiently high concentrations of the serotonin reuptake inhibitor 6-nitroquipazine, which is in accordance with other serotonin releasing agents such as fenfluramine and MDMA.[74][76][77] However, two more recent studies failed to find a releasing effect of tramadol at respective concentrations up to 10 and 30 μM.[78][77][71] In addition to serotonergic activity, tramadol is also a norepinephrine reuptake inhibitor.[48][49] It is not a norepinephrine releasing agent.[79][80][81][71] Tramadol does not inhibit the reuptake or induce the release of dopamine.[79][71]
A positron emission tomography imaging study found that single oral 50-mg and 100-mg doses of tramadol to human volunteers resulted in 34.7% and 50.2% respective mean occupation of the serotonin transporter (SERT) in the thalamus.[82] The estimated median effective dose (ED50) for SERT occupancy hence was 98.1 mg, which was associated with a plasma tramadol level of about 330 ng/ml (1,300 nM).[82] The estimated maximum daily dosage of tramadol of 400 mg (100 mg q.i.d.) would result in as much as 78.7% occupancy of the SERT (in association with a plasma concentration of 1,220 ng/ml or 4,632 nM).[82] This is close to that of SSRIs, which occupy the SERT by 80% or more.[82]
Peak plasma concentrations during treatment with clinical dosages of tramadol have generally been found to be in the range of 70 to 592 ng/ml (266–2,250 nM) for tramadol and 55 to 143 ng/ml (221–573 nM) for desmetramadol.[20] The highest levels of tramadol were observed with the maximum oral daily dosage of 400 mg per day divided into one 100-mg dose every 6 hours (i.e., four 100-mg doses evenly spaced out per day).[20][83] Some accumulation of tramadol occurs with chronic administration; peak plasma levels with the maximum oral daily dosage (100 mg q.i.d.) are about 16% higher and the area-under-the-curve levels 36% higher than following a single oral 100-mg dose.[20] Positron emission tomography imaging studies have reportedly found that tramadol levels are at least four-fold higher in the brain than in plasma.[79][84] Conversely, brain levels of desmetramadol "only slowly approach those in plasma".[79] The plasma protein binding of tramadol is only 4 to 20%; hence, almost all tramadol in circulation is free, thus bioactive.[85][86][87]
Correspondence to effects
Co-administration of quinidine, a potent CYP2D6 enzyme inhibitor, with tramadol, a combination which results in markedly reduced levels of desmetramadol, was found not to significantly affect the analgesic effects of tramadol in human volunteers.[13][86] However, other studies have found that the analgesic effects of tramadol are significantly decreased or even absent in CYP2D6 poor metabolizers.[13][73] The analgesic effects of tramadol are only partially reversed by naloxone in human volunteers,[13] hence indicating that its opioid action is unlikely the sole factor; tramadol's analgesic effects are also partially reversed by α2-adrenergic receptor antagonists such as yohimbine, the 5-HT3 receptor antagonist ondansetron, and the 5-HT7 receptor antagonists SB-269970 and SB-258719.[20][88] Pharmacologically, tramadol is similar to tapentadol and methadone in that it not only binds to the MOR, but also inhibits the reuptake of serotonin and norepinephrine[6] due to its action on the noradrenergic and serotonergic systems, such as its "atypical" opioid activity.[89]
Tramadol has inhibitory actions on the 5-HT2C receptor. Antagonism of 5-HT2C could be partially responsible for tramadol's reducing effect on depressive and obsessive–compulsive symptoms in patients with pain and co-morbid neurological illnesses.[59] 5-HT2C blockade may also account for its lowering of the seizure threshold, as 5-HT2C knockout mice display significantly increased vulnerability to epileptic seizures, sometimes resulting in spontaneous death. However, the reduction of seizure threshold could be attributed to tramadol's putative inhibition of GABAA receptors at high doses (significant inhibition at 100 μM).[68][45] In addition, desmetramadol is a high-affinity ligand of the DOR, and activation of this receptor could be involved in tramadol's ability to provoke seizures in some individuals, as DOR agonists are well known for inducing seizures.[53]
Nausea and vomiting caused by tramadol are thought to be due to activation of the 5-HT3 receptor via increased serotonin levels.[57] In accordance, the 5-HT3 receptor antagonist ondansetron can be used to treat tramadol-associated nausea and vomiting.[57] Tramadol and desmetramadol themselves do not bind to the 5-HT3 receptor.[57][49]
Pharmacokinetics

Tramadol undergoes hepatic metabolism via the cytochrome P450 isozyme CYP2B6, CYP2D6, and CYP3A4, being O- and N-demethylated to five different metabolites. Of these, desmetramadol (O-desmethyltramadol) is the most significant, since it has 200 times the μ-affinity of (+)-tramadol, and furthermore has an elimination half-life of 9 hours, compared with 6 hours for tramadol itself. As with codeine, in the 6% of the population who have reduced CYP2D6 activity (hence reducing metabolism), a reduced analgesic effect is seen. Those with decreased CYP2D6 activity require a dose increase of 30% to achieve the same degree of pain relief as those with a normal level of CYP2D6 activity.[90][91]
Phase II hepatic metabolism renders the metabolites water-soluble, which are excreted by the kidneys. Thus, reduced doses may be used in renal and hepatic impairment.[20]
Its volume of distribution is around 306 l after oral administration and 203 l after parenteral administration.[20]
Chemistry
Tramadol is marketed as a racemic mixture of both R- and S-stereoisomers,[6] because the two isomers complement each other's analgesic activities.[6] The (+)-isomer is predominantly active as an opiate with a higher affinity for the µ-opiate receptor (20 times higher affinity than the (-)-isomer).[92]
Synthesis and stereoisomerism

|

|
| (1R,2R)-tramadol | (1S,2S)-tramadol |

|

|
| (1R,2S)-tramadol | (1S,2R)-tramadol |
The chemical synthesis of tramadol is described in the literature.[93] Tramadol [2-(dimethylaminomethyl)-1-(3-methoxyphenyl)cyclohexanol] has two stereogenic centers at the cyclohexane ring. Thus, 2-(dimethylaminomethyl)-1-(3-methoxyphenyl)cyclohexanol may exist in four different configurational forms:
- (1R,2R)-isomer
- (1S,2S)-isomer
- (1R,2S)-isomer
- (1S,2R)-isomer
The synthetic pathway leads to the racemate (1:1 mixture) of (1R,2R)-isomer and the (1S,2S)-isomer as the main products. Minor amounts of the racemic mixture of the (1R,2S)-isomer and the (1S,2R)-isomer are formed as well. The isolation of the (1R,2R)-isomer and the (1S,2S)-isomer from the diastereomeric minor racemate [(1R,2S)-isomer and (1S,2R)-isomer] is realized by the recrystallization of the hydrochlorides. The drug tramadol is a racemate of the hydrochlorides of the (1R,2R)-(+)- and the (1S,2S)-(−)-enantiomers. The resolution of the racemate [(1R,2R)-(+)-isomer / (1S,2S)-(−)-isomer] was described[94] employing (R)-(−)- or (S)-(+)-mandelic acid. This process does not find industrial application, since tramadol is used as a racemate, despite known different physiological effects[95] of the (1R,2R)- and (1S,2S)-isomers, because the racemate showed higher analgesic activity than either enantiomer in animals[96] and in humans.[97]
Detection in biological fluids
Tramadol and desmetramadol may be quantified in blood, plasma or serum to monitor for abuse, confirm a diagnosis of poisoning or assist in the forensic investigation of a sudden death. Most commercial opiate immunoassay screening tests do not cross-react significantly with tramadol or its major metabolites, so chromatographic techniques must be used to detect and quantitate these substances. The concentration of desmetramadol in the blood or plasma of a person who has taken tramadol is generally 10–20% those of the parent drug.[98][99][100]
Society and culture
Formulations
Available dosage forms include liquids, syrups, drops, elixirs, effervescent tablets and powders for mixing with water, capsules, tablets including extended-release formulations, suppositories, compounding powder, and injections.[19]
Patent history
The U.S. Food and Drug Administration (FDA) approved tramadol in March 1995, and an extended-release (ER) formulation in September 2005.[101] ER Tramadol was protected by US patents nos. 6,254,887[102] and 7,074,430.[103][104] The FDA listed the patents' expiration as 10 May 2014.[103] However, in August 2009, US District Court for the District of Delaware ruled the patents invalid, a decision upheld the following year by the Court of Appeals for the Federal Circuit. Manufacture and distribution of generic equivalents of Ultram ER in the United States was therefore permitted prior to the expiration of the patents.[105]
Legal status
Effective 18 August 2014, tramadol has been placed into Schedule IV of the federal Controlled Substances Act in the United States.[106][107] Before that, some US states had already classified tramadol as a Schedule IV controlled substance under their respective state laws.[108][109][110]
Tramadol is classified in Schedule 4 (prescription only) in Australia, rather than as a Schedule 8 Controlled Drug (Possession without authority illegal) like most other opioids.[19]
Effective May 2008, Sweden classified tramadol as a controlled substance in the same category as codeine and dextropropoxyphene, but allows a normal prescription to be used.[111]
The UK classified tramadol as a Class C, Schedule 3 controlled drug on 10 June 2014, but exempted it from the safe custody requirement.[citation needed]
Misuse
Illicit use of the drug is thought to be a major factor in the success of the Boko Haram terrorist organization.[112][113][114] When used at higher doses, the drug "can produce similar effects to heroin."[112] Said one former member, "whenever we took tramadol, nothing mattered to us anymore except what we were sent to do because it made us very high and very bold, it was impossible to go on a mission without taking it."[112] Tramadol is also used as a coping mechanism in the Gaza Strip.[115] It is also abused in the United Kingdom, inspiring the title of the TV show Frankie Boyle's Tramadol Nights (2010).[116][117]
Research
Investigational uses
- Diabetic neuropathy [118][119]
- Antidepressant[120]
- Postherpetic neuralgia [121][122]
- Premature ejaculation[123][124]
- Adjunct to local anaesthesia[125]
False findings about sources in nature
In 2013, researchers reported that tramadol was found in relatively high concentrations (1%+) in the roots of the African pin cushion tree (Nauclea latifolia).[126] In 2014, however, it was reported that the presence of tramadol in the tree roots was the result of tramadol having been administered to cattle by farmers in the region:[127] tramadol and its metabolites were present in the animals' excreta, which contaminated the soil around the trees. Therefore, tramadol and its mammalian metabolites were found in tree roots in the far north of Cameroon, but not in the south where it is not administered to farm animals.[127]
A 2014 editorial in Lab Times online contested the notion that tramadol in tree roots was the result of anthropogenic contamination, stating that samples were taken from trees that grew in national parks, where livestock were forbidden; it also quoted researcher Michel de Waard, who stated that "thousands and thousands of tramadol-treated cattle sitting around a single tree and urinating there" would be required to produce the concentrations discovered.[128]
In 2015, radiocarbon analysis confirmed that the tramadol found in N.latifolia roots could not be plant-derived and was of synthetic origin.[129]
Veterinary medicine
Tramadol may be used to treat post-operative, injury-related, and chronic (e.g., cancer-related) pain in dogs and cats as well as rabbits, coatis, many small mammals including rats and flying squirrels, guinea pigs, ferrets, and raccoons.[130]
| Species | Half-life (h) for parent drug | Half-life (h) for desmetramadol | Maximum plasma concentration (ng/mL) for parent drug | Maximum plasma concentration (ng/mL) for desmetramadol |
|---|---|---|---|---|
| Camel | 3.2 (IM), 1.3 (IV) | – | 0.44 (IV) | – |
| Cat | 3.40 (oral), 2.23 (IV) | 4.82 (oral), 4.35 (IV) | 914 (oral), 1323 (IV) | 655 (oral), 366 (IV) |
| Dog | 1.71 (oral), 1.80 (IV), 2.24 (rectal) | 2.18 (oral), 90-5000 (IV) | 1402.75 (oral) | 449.13 (oral), 90–350 (IV) |
| Donkey | 4.2 (oral), 1.5 (IV) | – | 2817 (oral) | – |
| Goat | 2.67 (oral), 0.94 (IV) | – | 542.9 (oral) | – |
| Horses | 1.29–1.53 (IV), 10.1 (oral) | 4 (oral) | 637 (IV), 256 (oral) | 47 (oral) |
| Llama | 2.54 (IM), 2.12 (IV) | 7.73 (IM), 10.4 (IV) | 4036 (IV), 1360 (IM) | 158 (IV), 158 (IM) |
References
- ^ a b c "Tramadol". Drugs.com. Retrieved 22 December 2018.
- ^ "Tramadol Use During Pregnancy". Drugs.com. 14 October 2019. Retrieved 7 February 2020.
- ^ a b c d e f g h i j k l m "Tramadol Hydrochloride". The American Society of Health-System Pharmacists. Retrieved 1 December 2014.
- ^ a b "Tramadol". MedlinePlus. American Society of Health-System Pharmacists. 1 September 2008. Retrieved 29 September 2009.
- ^ "List of nationally authorised medicinal products" (PDF). European Medicines Agency. 28 January 2021. Retrieved 21 July 2021.
{{cite web}}: CS1 maint: url-status (link) - ^ a b c d e Brayfield, A, ed. (13 December 2013). "Tramadol Hydrochloride". Martindale: The Complete Drug Reference. Pharmaceutical Press. Retrieved 5 April 2014.
- ^ a b "Ultram, Ultram ER (tramadol) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 28 November 2013.
- ^ a b Dayer P, Desmeules J, Collart L (1997). "[Pharmacology of tramadol]". Drugs. 53 (Suppl 2): 18–24. doi:10.2165/00003495-199700532-00006. PMID 9190321. S2CID 46970093.
- ^ a b c "Australian Label: Tramadol Sandoz 50 mg capsules" (PDF). TGA eBusiness Services. 4 November 2011. Retrieved 6 April 2014.
- ^ a b c British national formulary : BNF 74 (74 ed.). British Medical Association. 2017. pp. 447–448. ISBN 978-0857112989.
- ^ "ULTRACET (tramadol hydrochloride/ acetaminophen tablets)" (PDF). FDA. US Government. Retrieved 14 April 2022.
- ^ "Tramadol Pregnancy and Breastfeeding Warnings". Drugs.com. Retrieved 5 September 2016.
- ^ a b c d e f g h i j Raffa RB, Buschmann H, Christoph T, Eichenbaum G, Englberger W, Flores CM, et al. (July 2012). "Mechanistic and functional differentiation of tapentadol and tramadol". Expert Opinion on Pharmacotherapy. 13 (10): 1437–1449. doi:10.1517/14656566.2012.696097. PMID 22698264. S2CID 24226747.
- ^ a b c Leppert W (November–December 2009). "Tramadol as an analgesic for mild to moderate cancer pain". Pharmacological Reports. 61 (6): 978–992. doi:10.1016/s1734-1140(09)70159-8. PMID 20081232.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 528. ISBN 9783527607495.
- ^ "The Top 300 of 2019". ClinCalc. Retrieved 16 October 2021.
- ^ "Tramadol – Drug Usage Statistics". ClinCalc. Retrieved 16 October 2021.
- ^ "Tramadol Hydrochloride Tablet Uses, Dosage, Benefits, Side Effects, Warnings, Free Advice About Tramadol 2021". 19 November 2021. Retrieved 9 December 2021.
- ^ a b c d e f g h i j k Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ^ a b c d e f g h i Grond S, Sablotzki A (2004). "Clinical pharmacology of tramadol". Clinical Pharmacokinetics. 43 (13): 879–923. doi:10.2165/00003088-200443130-00004. PMID 15509185. S2CID 32347667.
- ^ MacLean AJ, Schwartz TL (May 2015). "Tramadol for the treatment of fibromyalgia". Expert Review of Neurotherapeutics. 15 (5): 469–475. doi:10.1586/14737175.2015.1034693. PMID 25896486. S2CID 26613022.
- ^ "Treatment – Fibromyalgia (NHS)". NHS. 20 February 2019. Archived from the original on 4 September 2020. Retrieved 4 September 2020.
- ^ Lee CR, McTavish D, Sorkin EM (August 1993). "Tramadol. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in acute and chronic pain states". Drugs. 46 (2): 313–340. doi:10.2165/00003495-199346020-00008. PMID 7691519.
- ^ Silber MH, Becker PM, Buchfuhrer MJ, Earley CJ, Ondo WG, Walters AS, Winkelman JW (January 2018). "The Appropriate Use of Opioids in the Treatment of Refractory Restless Legs Syndrome". Mayo Clinic Proceedings. 93 (1): 59–67. doi:10.1016/j.mayocp.2017.11.007. PMID 29304922.
In summary, a number of opioid medications in low dose appear effective in refractory RLS. The risks of opioid use are relatively low, taking into account the much lower doses used for RLS compared with those in patients with pain syndromes. As long as reasonable precautions are taken, the risk-benefit ratio is acceptable and opioids should not be unreasonably withheld from such patients.
- ^ Winkelmann J, Allen RP, Högl B, Inoue Y, Oertel W, Salminen AV, et al. (July 2018). "Treatment of restless legs syndrome: Evidence-based review and implications for clinical practice (Revised 2017)§". Movement Disorders. 33 (7): 1077–1091. doi:10.1002/mds.27260. PMID 29756335. S2CID 21669996.
- ^ Lipford MC, Silber MH (December 2012). "Long-term use of pramipexole in the management of restless legs syndrome". Sleep Medicine. 13 (10): 1280–1285. doi:10.1016/j.sleep.2012.08.004. PMID 23036265.
For the purposes of this study, augmentation was defined as earlier onset, increased severity, [increased] duration, or new anatomic distribution of RLS symptoms during treatment.
- ^ a b Miotto K, Cho AK, Khalil MA, Blanco K, Sasaki JD, Rawson R (January 2017). "Trends in Tramadol: Pharmacology, Metabolism, and Misuse". Anesthesia and Analgesia. 124 (1): 44–51. doi:10.1213/ANE.0000000000001683. PMID 27861439. S2CID 24224625.
- ^ a b c d e Bloor M, Paech MJ, Kaye R (April 2012). "Tramadol in pregnancy and lactation". International Journal of Obstetric Anesthesia. 21 (2): 163–167. doi:10.1016/j.ijoa.2011.10.008. PMID 22317891.
- ^ "FDA Drug Safety Communication: FDA evaluating the risks of using the pain medicine tramadol in children aged 17 and younger". FDA. FDA Drug Safety and Availability. Retrieved 21 September 2015.
- ^ Commissioner, Office of the. "Press Announcements – FDA statement from Douglas Throckmorton, M.D., deputy center director for regulatory programs, Center for Drug Evaluation and Research, on new warnings about the use of codeine and tramadol in children & nursing mothers". www.fda.gov. Retrieved 21 April 2017.
- ^ "FDA Drug Safety Communication: FDA restricts use of prescription codeine pain and cough medicines and tramadol pain medicines in children; recommends against use in breastfeeding women". Food and Drug Administration. 9 February 2019.
- ^ Langley PC, Patkar AD, Boswell KA, Benson CJ, Schein JR (January 2010). "Adverse event profile of tramadol in recent clinical studies of chronic osteoarthritis pain". Current Medical Research and Opinion. 26 (1): 239–251. doi:10.1185/03007990903426787. PMID 19929615. S2CID 20703694.
- ^ Keating GM (2006). "Tramadol sustained-release capsules". Drugs. 66 (2): 223–230. doi:10.2165/00003495-200666020-00006. PMID 16451094. S2CID 22620947.
- ^ ""Weak" opioid analgesics. Codeine, dihydrocodeine and tramadol: no less risky than morphine". Prescrire International. 25 (168): 45–50. February 2016. PMID 27042732.
- ^ "Withdrawal syndrome and dependence: tramadol too". Prescrire International. 12 (65): 99–100. June 2003. PMID 12825576.
- ^ a b c Epstein DH, Preston KL, Jasinski DR (July 2006). "Abuse liability, behavioral pharmacology, and physical-dependence potential of opioids in humans and laboratory animals: lessons from tramadol". Biological Psychology. 73 (1): 90–99. doi:10.1016/j.biopsycho.2006.01.010. PMC 2943845. PMID 16497429.
- ^ Senay EC, Adams EH, Geller A, Inciardi JA, Muñoz A, Schnoll SH, et al. (April 2003). "Physical dependence on Ultram (tramadol hydrochloride): both opioid-like and atypical withdrawal symptoms occur". Drug and Alcohol Dependence. 69 (3): 233–241. CiteSeerX 10.1.1.524.5426. doi:10.1016/S0376-8716(02)00321-6. PMID 12633909.
- ^ a b Randall C, Crane J (March 2014). "Tramadol deaths in Northern Ireland: a review of cases from 1996 to 2012". Journal of Forensic and Legal Medicine. 23: 32–36. doi:10.1016/j.jflm.2014.01.006. PMID 24661703.
- ^ White M. "Tramadol Deaths in the United Kingdom" (pdf_e). Public Health England.
- ^ Fauber J (22 December 2013). "Killing Pain: Tramadol the 'Safe' Drug of Abuse".
- ^ Scheck J (19 October 2016). "Tramadol: The Opioid Crisis for the rest of the World". The Wall Street Journal. Dow Jones & Co. Retrieved 4 January 2019.
- ^ a b c Miotto K, Cho AK, Khalil MA, Blanco K, Sasaki JD, Rawson R (January 2017). "Trends in Tramadol: Pharmacology, Metabolism, and Misuse". Anesthesia and Analgesia. 124 (1): 44–51. doi:10.1213/ANE.0000000000001683. PMID 27861439. S2CID 24224625.
- ^ Pasternak, G.W. MD, PhD (March 2012). "Preclinical Pharmacology and Opioid Combinations". Pain Medicine. 13 (1): 4–11. doi:10.1111/j.1526-4637.2012.01335.x. PMID 22420604.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hitchings A, Lonsdale D, Burrage D, Baker E (2015). Top 100 drugs : clinical pharmacology and practical prescribing. Churchill Livingstone Elsevier. pp. 168–169. ISBN 978-0-7020-5516-4.
- ^ a b c Vazzana M, Andreani T, Fangueiro J, Faggio C, Silva C, Santini A, et al. (March 2015). "Tramadol hydrochloride: pharmacokinetics, pharmacodynamics, adverse side effects, co-administration of drugs and new drug delivery systems". Biomedicine & Pharmacotherapy. 70: 234–238. doi:10.1016/j.biopha.2015.01.022. PMID 25776506.
- ^ "Tramadol". www.drugbank.ca.
- ^ a b c Roth BL, Driscol J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- ^ a b c d Minami K, Uezono Y, Ueta Y (March 2007). "Pharmacological aspects of the effects of tramadol on G-protein coupled receptors". Journal of Pharmacological Sciences. 103 (3): 253–260. doi:10.1254/jphs.cr0060032. PMID 17380034.
- ^ a b c d e Minami K, Ogata J, Uezono Y (October 2015). "What is the main mechanism of tramadol?". Naunyn-Schmiedeberg's Archives of Pharmacology. 388 (10): 999–1007. doi:10.1007/s00210-015-1167-5. PMID 26292636. S2CID 9066672.
- ^ a b c Wentland MP, Lou R, Lu Q, Bu Y, VanAlstine MA, Cohen DJ, Bidlack JM (January 2009). "Syntheses and opioid receptor binding properties of carboxamido-substituted opioids". Bioorganic & Medicinal Chemistry Letters. 19 (1): 203–208. doi:10.1016/j.bmcl.2008.10.134. PMID 19027293.
- ^ a b c d Shen Q, Qian Y, Huang X, Xu X, Li W, Liu J, Fu W (April 2016). "Discovery of Potent and Selective Agonists of δ Opioid Receptor by Revisiting the "Message-Address" Concept". ACS Medicinal Chemistry Letters. 7 (4): 391–396. doi:10.1021/acsmedchemlett.5b00423. PMC 4834657. PMID 27096047.
- ^ Volpe DA, McMahon Tobin GA, Mellon RD, Katki AG, Parker RJ, Colatsky T, et al. (April 2011). "Uniform assessment and ranking of opioid μ receptor binding constants for selected opioid drugs". Regulatory Toxicology and Pharmacology. 59 (3): 385–390. doi:10.1016/j.yrtph.2010.12.007. PMID 21215785.
- ^ a b c d e Potschka H, Friderichs E, Löscher W (September 2000). "Anticonvulsant and proconvulsant effects of tramadol, its enantiomers and its M1 metabolite in the rat kindling model of epilepsy". British Journal of Pharmacology. 131 (2): 203–212. doi:10.1038/sj.bjp.0703562. PMC 1572317. PMID 10991912.
- ^ a b c d e Codd EE, Shank RP, Schupsky JJ, Raffa RB (September 1995). "Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: structural determinants and role in antinociception". The Journal of Pharmacology and Experimental Therapeutics. 274 (3): 1263–1270. PMID 7562497.
- ^ a b Gillen C, Haurand M, Kobelt DJ, Wnendt S (August 2000). "Affinity, potency and efficacy of tramadol and its metabolites at the cloned human mu-opioid receptor". Naunyn-Schmiedeberg's Archives of Pharmacology. 362 (2): 116–121. doi:10.1007/s002100000266. PMID 10961373. S2CID 10459734.
- ^ a b c d e f g h i Frink MC, Hennies HH, Englberger W, Haurand M, Wilffert B (November 1996). "Influence of tramadol on neurotransmitter systems of the rat brain". Arzneimittel-Forschung. 46 (11): 1029–1036. PMID 8955860.
- ^ a b c d Barann M, Urban B, Stamer U, Dorner Z, Bönisch H, Brüss M (February 2006). "Effects of tramadol and O-demethyl-tramadol on human 5-HT reuptake carriers and human 5-HT3A receptors: a possible mechanism for tramadol-induced early emesis". European Journal of Pharmacology. 531 (1–3): 54–58. doi:10.1016/j.ejphar.2005.11.054. PMID 16427041.
- ^ Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL (January 1992). "Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an 'atypical' opioid analgesic". The Journal of Pharmacology and Experimental Therapeutics. 260 (1): 275–285. PMID 1309873.
- ^ a b Ogata J, Minami K, Uezono Y, Okamoto T, Shiraishi M, Shigematsu A, Ueta Y (May 2004). "The inhibitory effects of tramadol on 5-hydroxytryptamine type 2C receptors expressed in Xenopus oocytes". Anesthesia and Analgesia. 98 (5): 1401–6, table of contents. doi:10.1213/01.ANE.0000108963.77623.A4. PMID 15105221. S2CID 41718739.
- ^ Horishita T, Minami K, Uezono Y, Shiraishi M, Ogata J, Okamoto T, Shigematsu A (2006). "The tramadol metabolite, O-desmethyl tramadol, inhibits 5-hydroxytryptamine type 2C receptors expressed in Xenopus Oocytes". Pharmacology. 77 (2): 93–99. doi:10.1159/000093179. PMID 16679816. S2CID 23775035.
- ^ Okamoto T, Minami K, Uezono Y, Ogata J, Shiraishi M, Shigematsu A, Ueta Y (July 2003). "The inhibitory effects of ketamine and pentobarbital on substance p receptors expressed in Xenopus oocytes". Anesthesia and Analgesia. 97 (1): 104–10, table of contents. doi:10.1213/01.ANE.0000066260.99680.11. PMID 12818951. S2CID 35809206.
- ^ Minami K, Yokoyama T, Ogata J, Uezono Y (2011). "The tramadol metabolite O-desmethyl tramadol inhibits substance P-receptor functions expressed in Xenopus oocytes". Journal of Pharmacological Sciences. 115 (3): 421–424. doi:10.1254/jphs.10313sc. PMID 21372504.
- ^ Shiraishi M, Minami K, Uezono Y, Yanagihara N, Shigematsu A (October 2001). "Inhibition by tramadol of muscarinic receptor-induced responses in cultured adrenal medullary cells and in Xenopus laevis oocytes expressing cloned M1 receptors". The Journal of Pharmacology and Experimental Therapeutics. 299 (1): 255–260. PMID 11561087.
- ^ a b Nakamura M, Minami K, Uezono Y, Horishita T, Ogata J, Shiraishi M, et al. (July 2005). "The effects of the tramadol metabolite O-desmethyl tramadol on muscarinic receptor-induced responses in Xenopus oocytes expressing cloned M1 or M3 receptors". Anesthesia and Analgesia. 101 (1): 180–6, table of contents. doi:10.1213/01.ANE.0000154303.93909.A3. PMID 15976229. S2CID 23861688.
- ^ Shiga Y, Minami K, Shiraishi M, Uezono Y, Murasaki O, Kaibara M, Shigematsu A (November 2002). "The inhibitory effects of tramadol on muscarinic receptor-induced responses in Xenopus oocytes expressing cloned M(3) receptors". Anesthesia and Analgesia. 95 (5): 1269–73, table of contents. doi:10.1097/00000539-200211000-00031. PMID 12401609. S2CID 39621215.
- ^ Shiraishi M, Minami K, Uezono Y, Yanagihara N, Shigematsu A, Shibuya I (May 2002). "Inhibitory effects of tramadol on nicotinic acetylcholine receptors in adrenal chromaffin cells and in Xenopus oocytes expressing alpha 7 receptors". British Journal of Pharmacology. 136 (2): 207–216. doi:10.1038/sj.bjp.0704703. PMC 1573343. PMID 12010769.
- ^ Sánchez-Fernández C, Montilla-García Á, González-Cano R, Nieto FR, Romero L, Artacho-Cordón A, et al. (January 2014). "Modulation of peripheral μ-opioid analgesia by σ1 receptors". The Journal of Pharmacology and Experimental Therapeutics. 348 (1): 32–45. doi:10.1124/jpet.113.208272. PMID 24155346. S2CID 6884854.
- ^ a b c d Hara K, Minami K, Sata T (May 2005). "The effects of tramadol and its metabolite on glycine, gamma-aminobutyric acidA, and N-methyl-D-aspartate receptors expressed in Xenopus oocytes". Anesthesia and Analgesia. 100 (5): 1400–1405. doi:10.1213/01.ANE.0000150961.24747.98. PMID 15845694. S2CID 35342038.
- ^ a b Miyano K, Minami K, Yokoyama T, Ohbuchi K, Yamaguchi T, Murakami S, et al. (April 2015). "Tramadol and its metabolite m1 selectively suppress transient receptor potential ankyrin 1 activity, but not transient receptor potential vanilloid 1 activity". Anesthesia and Analgesia. 120 (4): 790–798. doi:10.1213/ANE.0000000000000625. PMID 25642661. S2CID 8082654.
- ^ Marincsák R, Tóth BI, Czifra G, Szabó T, Kovács L, Bíró T (June 2008). "The analgesic drug, tramadol, acts as an agonist of the transient receptor potential vanilloid-1". Anesthesia and Analgesia. 106 (6): 1890–1896. doi:10.1213/ane.0b013e318172fefc. PMID 18499628. S2CID 45854233.
- ^ a b c d Rothman RB, Baumann MH (2006). "Therapeutic potential of monoamine transporter substrates". Current Topics in Medicinal Chemistry. 6 (17): 1845–1859. doi:10.2174/156802606778249766. PMID 17017961.
- ^ Minami K, Sudo Y, Miyano K, Murphy RS, Uezono Y (June 2015). "µ-Opioid receptor activation by tramadol and O-desmethyltramadol (M1)". Journal of Anesthesia. 29 (3): 475–479. doi:10.1007/s00540-014-1946-z. PMID 25394761. S2CID 7091648.
- ^ a b Coller JK, Christrup LL, Somogyi AA (February 2009). "Role of active metabolites in the use of opioids". European Journal of Clinical Pharmacology. 65 (2): 121–139. doi:10.1007/s00228-008-0570-y. PMID 18958460. S2CID 9977741.
- ^ a b Driessen B, Reimann W (January 1992). "Interaction of the central analgesic, tramadol, with the uptake and release of 5-hydroxytryptamine in the rat brain in vitro". British Journal of Pharmacology. 105 (1): 147–151. doi:10.1111/j.1476-5381.1992.tb14226.x. PMC 1908625. PMID 1596676.
- ^ Bamigbade TA, Davidson C, Langford RM, Stamford JA (September 1997). "Actions of tramadol, its enantiomers and principal metabolite, O-desmethyltramadol, on serotonin (5-HT) efflux and uptake in the rat dorsal raphe nucleus". British Journal of Anaesthesia. 79 (3): 352–356. doi:10.1093/bja/79.3.352. PMID 9389855. S2CID 15630689.
- ^ a b Reimann W, Schneider F (May 1998). "Induction of 5-hydroxytryptamine release by tramadol, fenfluramine and reserpine". European Journal of Pharmacology. 349 (2–3): 199–203. doi:10.1016/S0014-2999(98)00195-2. PMID 9671098.
- ^ a b c Gobbi M, Moia M, Pirona L, Ceglia I, Reyes-Parada M, Scorza C, Mennini T (September 2002). "p-Methylthioamphetamine and 1-(m-chlorophenyl)piperazine, two non-neurotoxic 5-HT releasers in vivo, differ from neurotoxic amphetamine derivatives in their mode of action at 5-HT nerve endings in vitro". Journal of Neurochemistry. 82 (6): 1435–1443. doi:10.1046/j.1471-4159.2002.01073.x. hdl:10533/173421. PMID 12354291. S2CID 13397864.
- ^ Gobbi M, Mennini T (April 1999). "Release studies with rat brain cortical synaptosomes indicate that tramadol is a 5-hydroxytryptamine uptake blocker and not a 5-hydroxytryptamine releaser". European Journal of Pharmacology. 370 (1): 23–26. doi:10.1016/s0014-2999(99)00123-5. PMID 10323276.
- ^ a b c d Driessen B, Reimann W, Giertz H (March 1993). "Effects of the central analgesic tramadol on the uptake and release of noradrenaline and dopamine in vitro". British Journal of Pharmacology. 108 (3): 806–811. doi:10.1111/j.1476-5381.1993.tb12882.x. PMC 1908052. PMID 8467366.
- ^ Reimann W, Hennies HH (June 1994). "Inhibition of spinal noradrenaline uptake in rats by the centrally acting analgesic tramadol". Biochemical Pharmacology. 47 (12): 2289–2293. doi:10.1016/0006-2952(94)90267-4. PMID 8031323.
- ^ Halfpenny DM, Callado LF, Hopwood SE, Bamigbade TA, Langford RM, Stamford JA (December 1999). "Effects of tramadol stereoisomers on norepinephrine efflux and uptake in the rat locus coeruleus measured by real time voltammetry". British Journal of Anaesthesia. 83 (6): 909–915. doi:10.1093/bja/83.6.909. PMID 10700792. S2CID 17830312.
- ^ a b c d Ogawa K, Tateno A, Arakawa R, Sakayori T, Ikeda Y, Suzuki H, Okubo Y (June 2014). "Occupancy of serotonin transporter by tramadol: a positron emission tomography study with [11C]DASB". The International Journal of Neuropsychopharmacology. 17 (6): 845–850. doi:10.1017/S1461145713001764. PMID 24423243.
- ^ "Tramadol Dosage Guide with Precautions".
- ^ Tao Q, Stone DJ, Borenstein MR, Codd EE, Coogan TP, Desai-Krieger D, et al. (April 2002). "Differential tramadol and O-desmethyl metabolite levels in brain vs. plasma of mice and rats administered tramadol hydrochloride orally". Journal of Clinical Pharmacy and Therapeutics. 27 (2): 99–106. doi:10.1046/j.1365-2710.2002.00384.x. PMID 11975693. S2CID 42370985.
- ^ Gibson TP (July 1996). "Pharmacokinetics, efficacy, and safety of analgesia with a focus on tramadol HCl". The American Journal of Medicine. 101 (1A): 47S–53S. doi:10.1016/s0002-9343(96)90035-2. PMID 8764760.
- ^ a b Dayer P, Collart L, Desmeules J (1994). "The pharmacology of tramadol". Drugs. 47 (Suppl 1): 3–7. doi:10.2165/00003495-199400471-00003. PMID 7517823. S2CID 33474225.
- ^ Nobilis M, Kopecký J, Kvetina J, Chládek J, Svoboda Z, Vorísek V, et al. (March 2002). "High-performance liquid chromatographic determination of tramadol and its O-desmethylated metabolite in blood plasma. Application to a bioequivalence study in humans". Journal of Chromatography A. 949 (1–2): 11–22. doi:10.1016/S0021-9673(01)01567-9. PMID 11999728.
- ^ Yanarates O, Dogrul A, Yildirim V, Sahin A, Sizlan A, Seyrek M, et al. (March 2010). "Spinal 5-HT7 receptors play an important role in the antinociceptive and antihyperalgesic effects of tramadol and its metabolite, O-Desmethyltramadol, via activation of descending serotonergic pathways". Anesthesiology. 112 (3): 696–710. doi:10.1097/ALN.0b013e3181cd7920. PMID 20179508. S2CID 11913166.
- ^ Micó JA, Ardid D, Berrocoso E, Eschalier A (July 2006). "Antidepressants and pain". Trends in Pharmacological Sciences. 27 (7): 348–354. doi:10.1016/j.tips.2006.05.004. PMID 16762426.
- ^ Leppert W (2011). "CYP2D6 in the metabolism of opioids for mild to moderate pain". Pharmacology. 87 (5–6): 274–285. doi:10.1159/000326085. PMID 21494059.
- ^ Samer CF, Lorenzini KI, Rollason V, Daali Y, Desmeules JA (June 2013). "Applications of CYP450 testing in the clinical setting". Molecular Diagnosis & Therapy. 17 (3): 165–184. doi:10.1007/s40291-013-0028-5. PMC 3663206. PMID 23588782.
- ^ "Tramadol Hydrochloride 50mg Capsules". UK Electronic Medicines Compendium. January 2016. Retrieved 16 March 2017.
- ^ Pharmaceutical Substances, Axel Kleemann, Jürgen Engel, Bernd Kutscher and Dieter Reichert, 4. ed. (2000) 2 volumes, Thieme-Verlag Stuttgart (Germany), p. 2085 bis 2086, ISBN 978-1-58890-031-9; since 2003 online with biannual actualizations.
- ^ Zynovy Z, Meckler H (2000). "A Practical Procedure for the Resolution of (+)- and (−)-Tramadol". Organic Process Research & Development. 4 (4): 291–294. doi:10.1021/op000281v.
- ^ Burke D, Henderson DJ (April 2002). "Chirality: a blueprint for the future". British Journal of Anaesthesia. 88 (4): 563–576. doi:10.1093/bja/88.4.563. PMID 12066734.
- ^ Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL, et al. (October 1993). "Complementary and synergistic antinociceptive interaction between the enantiomers of tramadol". The Journal of Pharmacology and Experimental Therapeutics. 267 (1): 331–340. PMID 8229760.
- ^ Grond S, Meuser T, Zech D, Hennig U, Lehmann KA (September 1995). "Analgesic efficacy and safety of tramadol enantiomers in comparison with the racemate: a randomised, double-blind study with gynaecological patients using intravenous patient-controlled analgesia". Pain. 62 (3): 313–320. doi:10.1016/0304-3959(94)00274-I. PMID 8657431. S2CID 34150137.
- ^ Karhu D, El-Jammal A, Dupain T, Gaulin D, Bouchard S (September 2007). "Pharmacokinetics and dose proportionality of three Tramadol Contramid OAD tablet strengths". Biopharmaceutics & Drug Disposition. 28 (6): 323–330. doi:10.1002/bdd.561. PMID 17575561. S2CID 22720069.
- ^ Tjäderborn M, Jönsson AK, Hägg S, Ahlner J (December 2007). "Fatal unintentional intoxications with tramadol during 1995-2005". Forensic Science International. 173 (2–3): 107–111. doi:10.1016/j.forsciint.2007.02.007. PMID 17350197.
- ^ Baselt, R. (2017) Disposition of Toxic Drugs and Chemicals in Man, 11th edition, Biomedical Publications, Seal Beach, CA, pp. 2185-2188, ISBN 978-0-692-77499-1.
- ^ McCarberg B (June 2007). "Tramadol extended-release in the management of chronic pain". Therapeutics and Clinical Risk Management. 3 (3): 401–410. PMC 2386353. PMID 18488071.
- ^ US patent 6254887, Miller RB, Leslie ST, Malkowska ST, Smith KJ, Wimmer S, Winkler H, Hahn U, Prater DA, "Controlled Release Tramadol", issued 3 July 2001
- ^ a b FDA AccessData entry for Tramadol Hydrochloride. Retrieved 17 August 2009.
- ^ US patent 7074430, Miller RB, Malkowska ST, Wimmer S, Hahn U, Leslie ST, Smith KJ, Winkler H, Prater DA, "Controlled Release Tramadol Tramadol Formulation", issued 11 July 2006
- ^ Purdue Pharma Prods. L.P. v. Par Pharm., Inc., 377 Fed.Appx. 978 (Fed. Cir. 2010).
- ^ "DEA controls tramadol as a schedule IV controlled substance effective August 18, 2014". FDA Law Blog. 2 July 2014.
- ^ "Federal Registrar" (PDF). gpo.gov.
- ^ "TRAMADOL (Trade Names: Ultram, Ultracet)". Drug Enforcement Administration (February 2011)
- ^ "Tennessee News: Tramadol and Carisoprodol Now Classified Schedule IV". National Association of Boards of Pharmacy (8 June 2011). Retrieved on 26 December 2012.
- ^ "State of Ohio Board of Pharmacy" (PDF). Pharmacy.ohio.gov. 18 August 2014. Archived from the original (PDF) on 29 December 2016. Retrieved 8 November 2016.
- ^ "Substansen tramadol nu narkotikaklassad på samma sätt som kodein och dextropropoxifen" (in Swedish). Lakemedelsverket. 14 May 2008. Retrieved 12 August 2019.
- ^ a b c "If you take Tramadol away, you make Boko Haram weak". African Arguments. 15 March 2019. Retrieved 18 March 2019.
- ^ "Drugs for war: Opioid abuse in West Africa". BBC News. Retrieved 18 March 2019.
- ^ "The Dangerous Opioid from India". www.csis.org. Retrieved 18 March 2019.
- ^ Berger, Miriam (7 January 2019). "Gaza's Opioid Problem". The Nation. ISSN 0027-8378. Retrieved 18 March 2019.
- ^ Winstock AR, Borschmann R, Bell J (September 2014). "The non-medical use of tramadol in the UK: findings from a large community sample". International Journal of Clinical Practice. 68 (9): 1147–1151. doi:10.1111/ijcp.12429. PMID 24734958. S2CID 21883884.
- ^ "Tramadol Abuse & Addiction Causes". UK Addiction Treatment Centres. 8 August 2018.
- ^ Harati Y, Gooch C, Swenson M, Edelman S, Greene D, Raskin P, et al. (June 1998). "Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy". Neurology. 50 (6): 1842–1846. doi:10.1212/WNL.50.6.1842. PMID 9633738. S2CID 45709223.
- ^ Harati Y, Gooch C, Swenson M, Edelman SV, Greene D, Raskin P, et al. (2000). "Maintenance of the long-term effectiveness of tramadol in treatment of the pain of diabetic neuropathy". Journal of Diabetes and Its Complications. 14 (2): 65–70. doi:10.1016/S1056-8727(00)00060-X. PMID 10959067.
- ^ Barber J (April 2011). "Examining the use of tramadol hydrochloride as an antidepressant". Experimental and Clinical Psychopharmacology. 19 (2): 123–130. doi:10.1037/a0022721. PMID 21463069.
- ^ Göbel H, Stadler T (1997). "[Treatment of post-herpes zoster pain with tramadol. Results of an open pilot study versus clomipramine with or without levomepromazine]". Drugs (in French). 53 (Suppl 2): 34–39. doi:10.2165/00003495-199700532-00008. PMID 9190323. S2CID 46986791.
- ^ Boureau F, Legallicier P, Kabir-Ahmadi M (July 2003). "Tramadol in post-herpetic neuralgia: a randomized, double-blind, placebo-controlled trial". Pain. 104 (1–2): 323–331. doi:10.1016/S0304-3959(03)00020-4. PMID 12855342. S2CID 42979548.
- ^ Wu T, Yue X, Duan X, Luo D, Cheng Y, Tian Y, Wang K (September 2012). "Efficacy and safety of tramadol for premature ejaculation: a systematic review and meta-analysis". Urology. 80 (3): 618–624. doi:10.1016/j.urology.2012.05.035. PMID 22840860.
- ^ Wong BL, Malde S (January 2013). "The use of tramadol "on-demand" for premature ejaculation: a systematic review". Urology. 81 (1): 98–103. doi:10.1016/j.urology.2012.08.037. PMID 23102445.
- ^ Ryan T, Hodge A, Holyoak R, Vlok R, Melhuish T, Binks M, et al. (July 2019). "Tramadol as an adjunct to intra-articular local anaesthetic infiltration in knee arthroscopy: a systematic review and meta-analysis". ANZ Journal of Surgery. 89 (7–8): 827–832. doi:10.1111/ans.14920. PMID 30684306. S2CID 59275648.
- ^ Boumendjel A, Sotoing Taïwe G, Ngo Bum E, Chabrol T, Beney C, Sinniger V, et al. (November 2013). "Occurrence of the synthetic analgesic tramadol in an African medicinal plant". Angewandte Chemie. 52 (45): 11780–11784. doi:10.1002/anie.201305697. PMID 24014188.
- ^ a b Kusari S, Tatsimo SJ, Zühlke S, Talontsi FM, Kouam SF, Spiteller M (November 2014). "Tramadol--a true natural product?". Angewandte Chemie. 53 (45): 12073–12076. doi:10.1002/anie.201406639. PMID 25219922.
- ^ Who Really did it First? Nature or a Pharmacist?, in Lab Times online; by Nicola Hunt; published 22 September 2014; retrieved 21 November 2015
- ^ Kusari S, Tatsimo SJ, Zühlke S, Spiteller M (January 2016). "Synthetic Origin of Tramadol in the Environment". Angewandte Chemie. 55 (1): 240–243. doi:10.1002/anie.201508646. PMID 26473295.
- ^ a b Souza MJ, Cox SK (January 2011). "Tramadol use in zoologic medicine". The Veterinary Clinics of North America. Exotic Animal Practice. 14 (1): 117–130. doi:10.1016/j.cvex.2010.09.005. PMID 21074707.
Further reading
- Dean L (2015). "Tramadol Therapy and CYP2D6 Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 28520365. Bookshelf ID: NBK315950.
External links
- "Tramadol". Drug Information Portal. U.S. National Library of Medicine.
- "Tramadol hydrochloride". Drug Information Portal. U.S. National Library of Medicine.
