Dextromethorphan: Difference between revisions
→Contraindications: Most people don't think about grapefruit when taking over the counter medications. |
|||
| Line 143: | Line 143: | ||
=== Drug interactions === |
=== Drug interactions === |
||
Dextromethorphan should not be taken with [[Monoamine oxidase inhibitor]]s (MAOIs)<ref name="nhtsa" /> or [[Serotonin reuptake inhibitor]]s (like SSRIs, SNRis, TCAs, etc.)<ref name="nhtsa" /> due to the potential for [[serotonin syndrome]], which is a potentially life-threatening condition that can occur rapidly, due to a buildup of an excessive amount of serotonin in the body.{{Citation needed|date=March 2011}} |
Dextromethorphan should not be taken with [[Monoamine oxidase inhibitor]]s (MAOIs)<ref name="nhtsa" /> or [[Serotonin reuptake inhibitor]]s (like SSRIs, SNRis, TCAs, etc.)<ref name="nhtsa" /> due to the potential for [[serotonin syndrome]], which is a potentially life-threatening condition that can occur rapidly, due to a buildup of an excessive amount of serotonin in the body.{{Citation needed|date=March 2011}} |
||
=== Food interactions === |
|||
Caution should be exercised when taking Dextromethorphan when drinking [[grapefruit]] juice or eating grapefruits, as [[List of drugs affected by grapefruit|compounds in grapefruit affect a number of drugs]]. |
|||
== See also == |
== See also == |
||
Revision as of 13:17, 4 April 2011
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 11%[1] |
| Metabolism | Hepatic (liver) enzymes: major CYP2D6, minor CYP3A4, and minor CYP3A5 |
| Elimination half-life | 1.4–3.9 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.321 |
| Chemical and physical data | |
| Formula | C18H25NO |
| Molar mass | 271.4 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 111 °C (232 °F) |
| |
| |
| (verify) | |
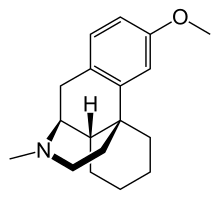
Dextromethorphan (DXM or DM) is an antitussive (cough suppressant) drug. It is one of the active ingredients in many over-the-counter cold and cough medicines, such as Robitussin, NyQuil, Dimetapp, Vicks, Coricidin, Tussin, Delsym, and others, including generic labels. Dextromethorphan has also found other uses in medicine, ranging from pain relief to psychological applications. It is sold in syrup, tablet, spray, and lozenge forms. In its pure form, dextromethorphan occurs as a white powder.[citation needed]
DXM is also used recreationally. When exceeding label-specified maximum dosages, dextromethorphan acts as a dissociative hallucinogen. Its mechanism of action is via multiple effects, including actions as a nonselective serotonin reuptake inhibitor[citation needed] and a sigma-1 receptor[citation needed] agonist and the action of its major metabolite dextrorphan as an NMDA receptor antagonist, producing effects similar to those of the controlled substances ketamine and phencyclidine (PCP),[2] as well as the active metabolite 3-methoxymorphinan which produces anesthetic effects in rats with a potency above dextrorphan but below dextromethorphan itself.[3]
History
Dextromethorphan was identified as one of three compounds tested as part of US Navy and CIA-funded research that sought a "nonaddictive substitute for codeine"; it is implied that the compound was first found to have clinical potential in this study.[4] It was first patented under in 1954.[5] The U.S. Food and Drug Administration (FDA) approved dextromethorphan for over-the-counter sale as a cough suppressant in 1958. This filled the need for a cough suppressant lacking the sedative side-effects, stronger potential for misuse, and physically addictive properties of codeine phosphate, the most widely used cough medication at the time.[6] In the United States, codeine phosphate syrup is still available in small quantities without a prescription in some states, but requires a signature and ID to purchase, similar to modern rules for sale of pseudoephedrine.
During the 1960s and 1970s, dextromethorphan became available in an over-the-counter tablet form by the brand name Romilar. In 1973, Romilar was taken off the shelves after a burst in sales because of frequent abuse, and was replaced by cough syrup in an attempt to cut down on abuse.[6]
Uses
The primary use of dextromethorphan is as a cough suppressant, for the temporary relief of cough caused by minor throat and bronchial irritation (such as commonly accompanies the flu and common cold), as well as those resulting from inhaled irritants.[citation needed]
A 2004 study showed that dextromethorphan was no more effective for children than a placebo.[7] Studies conducted by the American Academy of Pediatrics show that dextromethorphan is not superior to a placebo in providing nocturnal symptom relief for children with cough and sleep difficulty due to upper respiratory infections.[8]
In addition, a combination of dextromethorphan and quinidine by has been shown to alleviate symptoms of easy laughing and crying (pseudobulbar affect) in patients with amyotrophic lateral sclerosis and multiple sclerosis.[9] Dextromethorphan is also being investigated as a possible treatment for neuropathic pain and pain associated with fibromyalgia.[10] On October 29, 2010 The FDA approved the combination product (Nuedexta) manufactured by Avanir pharmaceutical corporation for the treatment of pseudobulbar affect (PBA).
Recreational use

Since their introduction, over-the-counter preparations containing dextromethorphan have been used in manners inconsistent with their labeling, often as a recreational drug.[6] At doses higher than medically recommended, dextromethorphan is classified as a dissociative psychedelic drug, with visible effects that are similar to those of ketamine and phencyclidine (PCP). It can produce distortions of the visual field, feelings of dissociation, distortions of bodily perception, excitement, as well as a loss of comprehension of time. Some users report euphoria. Dextromethorphan usually provides its recreational effects in a non-linear fashion, so that they are experienced in significantly varied stages. These stages are commonly referred to as "plateaus."[11][12][13]
Chemistry
Dextromethorphan is the dextrorotatory enantiomer of the methyl ether of levorphanol, an opioid analgesic. It is also a stereoisomer of levomethorphan, an opioid analgesic. It is named according to IUPAC rules as (+)-3-methoxy-17-methyl-9α,13α,14α-morphinan. As the pure free base, dextromethorphan occurs as an odorless, white to slightly yellow crystalline powder. It is freely soluble in chloroform and insoluble in water. Dextromethorphan is commonly available as the monohydrated hydrobromide salt, however some newer extended-release formulations contain dextromethorphan bound to an ion exchange resin based on polystyrene sulfonic acid. Dextromethorphan's specific rotation in water is +27.6° (20°C, Sodium D-line).[citation needed]
Pharmacology
Pharmacodynamics
Dextromethorphan has been shown to possess the following properties, mainly in binding assays to various receptors of animal tissues. Low Ki values mean strong binding or high affinity; high Ki values mean weak binding to the target or low affinity:
- Uncompetitive NMDA receptor (PCP site) antagonist (Ki = 7,253 nM).[14][15][16][17]
- σ1 and σ2 sigma receptor agonist (Ki = 205 nM and 11,060 nM, respectively). In a comparative investigation of dimemorfan, dextromethorphan and dextrorphan in mouse cells, dextromethorpan binds with relatively high affinity to Sigma-1 receptors and with very low affinity to Sigma-2 receptors.[14]
- α3β4-, α4β2-, and α7-nACh receptor (Ki = in the μM range) antagonist. Dextromethorphan binds to nicotinic receptors in frog eggs (Xenopus oocytes), human embryonic kidney cells and mouse tissue. It inhibits the antinociceptive (pain killing) action of nicotine in the tail-flick test in mice, where mouse tails are exposed to heat, which makes the mouse flick its tail if it feels pain.[18][19][20]
- μ-, δ-, and κ-opioid receptor agonist (Ki = 1,280 nM, 11,500 nM, and 7,000 nM, respectively).[21]
- SERT and NET blocker (Ki = 23 nM and 240 nM, respectively).[21][22][23][24]
- NADPH oxidase inhibitor.[25]
Its affinities for some of the sites listed are relatively very low and are probably insignificant, such as binding to NMDA receptors and opioid receptors, even at high recreational doses.[citation needed] Instead of acting as a direct antagonist of the NMDA receptor itself, it is likely that dextromethorphan functions as a prodrug to its nearly 10-fold more potent metabolite dextrorphan, and this is the true mediator of its dissociative effects.[14] It is not entirely clear what role, if any, (+)-3-methoxymorphinan, dextromethorphan's other major metabolite, plays in its effects.[26]
Pharmacokinetics
Following oral administration, dextromethorphan is rapidly absorbed from the gastrointestinal tract, where it enters the bloodstream and crosses the blood-brain barrier.[citation needed]
At therapeutic doses, dextromethorphan acts centrally (meaning that it acts on the brain) as opposed to locally (on the respiratory tract). It elevates the threshold for coughing, without inhibiting ciliary activity. Dextromethorphan is rapidly absorbed from the gastrointestinal tract and converted into the active metabolite dextrorphan in the liver by the cytochrome P450 enzyme CYP2D6. The average dosage necessary for effective antitussive therapy is between 10 mg and 45 mg, depending on the individual. The International Society for the Study of Cough recommend "an adequate first dose of medication ie 60 mg in the adult and repeat dosing should be infrequent rather than the qds recommended."[27]
The duration of action after oral administration is approximately three to eight hours for dextromethorphan-hydrobromide, and ten to twelve hours for dextromethorphan-polistirex. Approximately 1 in 10 of the caucasian population has little or no CYP2D6 enzyme activity leading to long lived high drug levels.[28]
Because administration of dextromethorphan can trigger a histamine release (an allergic reaction), its use in atopic children is very limited.[citation needed]
Metabolism
The first-pass through the hepatic portal vein results in some of the drug's being metabolized by O-demethylation into an active metabolite of dextromethorphan called dextrorphan (DXO). DXO is the 3-hydroxy derivative of dextromethorphan. The therapeutic activity of dextromethorphan is believed to be caused by both the drug and this metabolite. Dextromethorphan also undergoes N-demethylation (to 3-methoxymorphinan or MEM),[29] and partial conjugation with glucuronic acid and sulfate ions. Hours after dextromethorphan therapy, (in humans) the metabolites (+)-3-hydroxy-N-methylmorphinan, (+)-3-morphinan, and traces of the unchanged drug are detectable in the urine.[30]
A major metabolic catalyst involved is the cytochrome P450 enzyme known as 2D6, or CYP2D6. A significant portion of the population has a functional deficiency in this enzyme and are known as poor CYP2D6 metabolizers. O-demethylation of DXM to DXO contributes to 100% of the DXO formed during DXM metabolism.[29] As CYP2D6 is a major metabolic pathway in the inactivation of dextromethorphan, the duration of action and effects of dextromethorphan can be increased by as much as three times in such poor metabolizers.[31] In one study on 252 Americans, 84.3% were found to be "fast" (extensive) metabolizers, 6.8% to be "intermediate" metabolizers, and 8.8% were "slow" metabolizers of DXM.[32] There are a number of known alleles for CYP2D6, including several completely inactive variants. The distribution of alleles is uneven amongst ethnic groups; see also CYP2D6 - Ethnic factors in variability.
A large number of medications are potent inhibitors of CYP2D6. Some types of medications known to inhibit CYP2D6 include certain SSRI and tricyclic antidepressants, some antipsychotics, and the commonly-available antihistamine diphenhydramine – also known as Benadryl. There exists, therefore, the potential of interactions between dextromethorphan and medications that inhibit this enzyme, particularly in slow metabolizers.[citation needed] See also CYP2D6 - Ligands.
DXM is also metabolized by CYP3A4. N-demethylation is primarily accomplished by CYP3A4, contributing to at least 90% of the MEM formed as a primary metabolite of DXM.[29]
A number of other CYP enzymes are implicated as minor pathways of DXM metabolism. CYP2B6 is actually more effective than CYP3A4 at N-demethylation of DXM, but, since the average individual has a much lower CYP2B6 content in his/her liver relative to CYP3A4, most N-demethylation of DXM is catalyzed by CYP3A4.[29]
Side effects
Side-effects of dextromethorphan use can include:[30]
At normal doses:
|
|
At dosages 12.5 to 75 times the recommended therapeutic dose:
|
|
Dextromethorphan can also cause other gastrointestinal disturbances. Dextromethorphan had been thought to cause Olney's Lesions when administered intravenously; however, this was later proven inconclusive, due to lack of research on humans. Tests were performed on rats, giving them 50 mg and up every day up to a month. Neurotoxic changes, including vacuolation, have been observed in posterior cingulate and retrosplenial cortices of rats administered other NMDA antagonists such as PCP, but not with dextromethorphan.[34][35] In some rare documented cases, dextromethorphan has produced psychological dependence in some people who used it recreationally. However, it does not produce physical addiction, according to the WHO Committee on Drug Dependence.[36]
Contraindications
Because dextromethorphan can trigger a histamine release (allergic reaction), atopic children, who are especially susceptible to allergic reactions, should be administered dextromethorphan only if absolutely necessary, and only under the strict supervision of a health care professional.[30]
Drug interactions
Dextromethorphan should not be taken with Monoamine oxidase inhibitors (MAOIs)[30] or Serotonin reuptake inhibitors (like SSRIs, SNRis, TCAs, etc.)[30] due to the potential for serotonin syndrome, which is a potentially life-threatening condition that can occur rapidly, due to a buildup of an excessive amount of serotonin in the body.[citation needed]
Food interactions
Caution should be exercised when taking Dextromethorphan when drinking grapefruit juice or eating grapefruits, as compounds in grapefruit affect a number of drugs.
See also
- Antitussive
- Cough syrup
- Hallucinogen
- Dissociatives
- Morphinans
- (+)-Naloxone - another dextrorotatory enantiomer of an opioid drug with useful non-opioid effects
References
- ^ Kukanich, B.; Papich, M. G. (2004). "Plasma profile and pharmacokinetics of dextromethorphan after intravenous and oral administration in healthy dogs". Journal of Veterinary Pharmacology and Therapeutics. 27 (5): 337–41. doi:10.1111/j.1365-2885.2004.00608.x. PMID 15500572.
- ^ "Dextromethorphan". Drugs and Chemicals of Concern. Drug Enforcement Administration. 2010.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ Hou, C; Tzeng, J; Chen, Y; Lin, C; Lin, M; Tu, C; Wang, J (2006). "Dextromethorphan, 3-methoxymorphinan, and dextrorphan have local anaesthetic effect on sciatic nerve blockade in rats". European Journal of Pharmacology. 544 (1–3): 10–6. doi:10.1016/j.ejphar.2006.06.013. PMID 16844109.
- ^ Memorandum for the Secretary of Defense"
- ^ "Process for the preparation of optically active 3-methoxy-n-methylmorphinans and salts thereof" Schnider O and Grüssner A (inventors). US Patent 2 676 177. 20 April 1954. Retrieved 08 February 2011
- ^ a b c Dextromethorphan (DXM) | CESAR
- ^ "Kids' cough medicine no better than placebo" San Francisco Chronicle, July 8, 2004
- ^ Paul, I. M.; Yoder, KE; Crowell, KR; Shaffer, ML; McMillan, HS; Carlson, LC; Dilworth, DA; Berlin Jr, CM (2004). "Effect of Dextromethorphan, Diphenhydramine, and Placebo on Nocturnal Cough and Sleep Quality for Coughing Children and Their Parents". Pediatrics. 114 (1): e85–90. doi:10.1542/peds.114.1.e85. PMID 15231978.
- ^ Brooks, BR; Thisted, RA; Appel, SH; Bradley, WG; Olney, RK; Berg, JE; Pope, LE; Smith, RA; Avp-923 Als Study, Group (2004). "Treatment of pseudobulbar affect in ALS with dextromethorphan/quinidine: a randomized trial". Neurology. 63 (8): 1364–70. PMID 15505150.
{{cite journal}}:|first9=has generic name (help)CS1 maint: numeric names: authors list (link) - ^ "Cough Drug May Help Fibromyalgia Pain". WebMD.
- ^ White, William. "The DXM Experience". Retrieved December 21, 2010.
- ^ AJ Giannini. Drugs of Abuse--Second Edition. Los Angeles, Practice Management Information Corp, 1997.[page needed]
- ^ http://www.erowid.org/chemicals/dxm/dxm.shtml
- ^ a b c Chou, Y; Liao, JF; Chang, WY; Lin, MF; Chen, CF (1999). "Binding of dimemorfan to sigma-1 receptor and its anticonvulsant and locomotor effects in mice, compared with dextromethorphan and dextrorphan". Brain Research. 821 (2): 516–9. doi:10.1016/S0006-8993(99)01125-7. PMID 10064839.
- ^ Wong, B; Coulter, D; Choi, D; Prince, D (1988). "Dextrorphan and dextromethorphan, common antitussives, are antiepileptic and antagonize N-methyl-d-aspartate in brain slices". Neuroscience Letters. 85 (2): 261–6. doi:10.1016/0304-3940(88)90362-X. PMID 2897648.
- ^ Church, J; Jones, MG; Davies, SN; Lodge, D (1989). "Antitussive agents as N-methylaspartate antagonists: further studies". Canadian journal of physiology and pharmacology. 67 (6): 561–7. PMID 2673498.
- ^ Kamel, Ihab R.; Wendling, Woodrow W.; Chen, Dong; Wendling, Karen S.; Harakal, Concetta; Carlsson, Christer (2008). "N-Methyl-D-Aspartate (NMDA) Antagonists—S(+)-ketamine, Dextrorphan, and Dextromethorphan—Act as Calcium Antagonists on Bovine Cerebral Arteries". Journal of Neurosurgical Anesthesiology. 20 (4): 241–8. doi:10.1097/ANA.0b013e31817f523f. PMID 18812887.
- ^ Damaj, M. I.; Flood, P; Ho, KK; May, EL; Martin, BR (2004). "Effect of Dextrometorphan and Dextrorphan on Nicotine and Neuronal Nicotinic Receptors: In Vitro and in Vivo Selectivity". Journal of Pharmacology and Experimental Therapeutics. 312 (2): 780–5. doi:10.1124/jpet.104.075093. PMID 15356218.
- ^ Lee, J; Shin, E; Jeong, S; Kim, J; Lee, B; Yoon, I; Lee, J; Choi, S; Lee, S (2006). "Effects of dextrorotatory morphinans on α3β4 nicotinic acetylcholine receptors expressed in Xenopus oocytes". European Journal of Pharmacology. 536 (1–2): 85–92. doi:10.1016/j.ejphar.2006.02.034. PMID 16563374.
- ^ Hernandez, SC; Bertolino, M; Xiao, Y; Pringle, KE; Caruso, FS; Kellar, KJ (2000). "Dextromethorphan and its metabolite dextrorphan block alpha3beta4 neuronal nicotinic receptors". The Journal of pharmacology and experimental therapeutics. 293 (3): 962–7. PMID 10869398.
- ^ a b Codd, EE; Shank, RP; Schupsky, JJ; Raffa, RB (1995). "Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: structural determinants and role in antinociception". The Journal of pharmacology and experimental therapeutics. 274 (3): 1263–70. PMID 7562497.
- ^ Henderson, M; Fuller, RW (1992). "Dextromethorphan antagonizes the acute depletion of brain serotonin by p-chloroamphetamine and H75/12 in rats". Brain Research. 594 (2): 323–6. doi:10.1016/0006-8993(92)91144-4. PMID 1280529.
- ^ Gillman, P. K. (2005). "Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity". British Journal of Anaesthesia. 95 (4): 434–41. doi:10.1093/bja/aei210. PMID 16051647.
- ^ Schwartz, AR; Pizon, AF; Brooks, DE (2008). "Dextromethorphan-induced serotonin syndrome". Clinical toxicology (Philadelphia, Pa.). 46 (8): 771–3. PMID 19238739.
- ^ Zhang, W. (2004). "Neuroprotective effect of dextromethorphan in the MPTP Parkinson's disease model: role of NADPH oxidase". The FASEB Journal: 589–91. doi:10.1096/fj.03-0983fje.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Schmider, JÜRgen; Greenblatt, David J.; Fogelman, Steven M.; Von Moltke, Lisa L.; Shader, Richard I. (1997). "Metabolism of Dextromethorphanin vitro: Involvement of Cytochromes P450 2D6 AND 3A3/4, with a Possible Role of 2E1". Biopharmaceutics & Drug Disposition. 18: 227–40. doi:10.1002/(SICI)1099-081X(199704)18:3<227::AID-BDD18>3.0.CO;2-L.
- ^ Professor Alyn H Morice paper titled 'Cough' par. 'Dextromethorphan' http://www.issc.info/cough.html
- ^ Professor Alyn H Morice paper titled 'Cough' http://www.issc.info/cough.html
- ^ a b c d Yu, A; Haining, RL (2001). "Comparative contribution to dextromethorphan metabolism by cytochrome P450 isoforms in vitro: can dextromethorphan be used as a dual probe for both CTP2D6 and CYP3A activities?". Drug metabolism and disposition: the biological fate of chemicals. 29 (11): 1514–20. PMID 11602530.
- ^ a b c d e "Dextromethorphan". NHTSA.
- ^ Capon, Deborah A.; Bochner, Felix; Kerry, Nicole; Mikus, Gerd; Danz, Catherine; Somogyi, Andrew A. (1996). "The influence of CYP2D6 polymorphism and quinidine on the disposition and antitussive effect of dextromethorphan in humans*". Clinical Pharmacology & Therapeutics. 60: 295–307. doi:10.1016/S0009-9236(96)90056-9.
- ^ Woodworth, JR; Dennis, SR; Moore, L; Rotenberg, KS (1987). "The polymorphic metabolism of dextromethorphan". Journal of clinical pharmacology. 27 (2): 139–43. PMID 3680565.
- ^ "Child deaths lead to FDA hearing on cough, cold meds - CNN.com". CNN. 2007-10-17.
- ^ Olney, J.; Labruyere, J; Price, M. (1989). "Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs". Science. 244 (4910): 1360–2. doi:10.1126/science.2660263. PMID 2660263.
- ^ Carliss, R; Radovsky, A; Chengelis, C; Oneill, T; Shuey, D (2007). "Oral administration of dextromethorphan does not produce neuronal vacuolation in the rat brain". NeuroToxicology. 28 (4): 813–8. doi:10.1016/j.neuro.2007.03.009. PMID 17573115.
- ^ WHO Expert Committee on Drug Dependence (1970). "Seventeenth Report" (PDF). World Health Organization. Retrieved 2008-12-29.
{{cite journal}}: Cite journal requires|journal=(help)
