Line 1:
Line 1:
{{Drugbox| Watchedfields = changed
{{Drugbox
| Watchedfields = changed
| verifiedrevid = 321793640
| verifiedrevid = 321793640
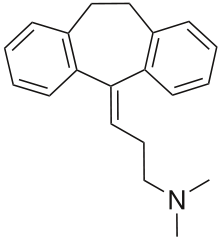
| IUPAC_name = 3-(10,11-dihydro- 5''H''-dibenzo[[''a'',''d'']]cycloheptene- 5-ylidene)- ''N'',''N''-dimethyl- 1-propanamine''
| IUPAC_name = 3-(10,11-dihydro- 5''H''-dibenzo[[''a'',''d'']]cycloheptene- 5-ylidene)- ''N'',''N''-dimethyl- 1-propanamine''
| image = Amitriptyline.svg
| image = Amitriptyline.svg
| image2 = Amitriptyline-from-picrate-xtal-3D-balls.png
| image2 = Amitriptyline-from-picrate-xtal-3D-balls.png
| CASNo_Ref = {{cascite}}
| CASNo_Ref = {{cascite}}
| InChI = 1/C20H23N/c1-21(2)15-7-12-20-18-10-5-3-8-16(18)13-14-17-9-4-6-11-19(17)20/h3-6,8-12H,7,13-15H2,1-2H3
| InChI = 1/C20H23N/c1-21(2)15-7-12-20-18-10-5-3-8-16(18)13-14-17-9-4-6-11-19(17)20/h3-6,8-12H,7,13-15H2,1-2H3
| smiles = c3cc2c(/C(c1c(cccc1)CC2)=C\CCN(C)C)cc3
| smiles = c3cc2c(/C(c1c(cccc1)CC2)=C\CCN(C)C)cc3
| InChIKey = KRMDCWKBEZIMAB-UHFFFAOYAI
| InChIKey = KRMDCWKBEZIMAB-UHFFFAOYAI
| CAS_number = 50-48-6
| CAS_number = 50-48-6
| ATC_prefix = N06
| ATC_prefix = N06
| ATC_suffix = AA09
| ATC_suffix = AA09
| PubChem = 2160
| PubChem = 2160
| ChemSpiderID = 2075
| ChemSpiderID = 2075
| DrugBank = APRD00227
| DrugBank = APRD00227
| C = 20 | H = 23 | N = 1
| C = 20 | H = 23 | N = 1
| molecular_weight = 277.403 g/mol
| molecular_weight = 277.403 g/mol
| bioavailability = 30–60% due to first pass metabolism
| bioavailability = 30–60% due to first pass metabolism
| protein_bound = > 90%
| protein_bound = > 90%
| metabolism = [[Hepatic]]<br \>[[CYP2C19]], [[CYP1A2]], [[CYP2D6]]
| metabolism = [[Hepatic]]<br \>[[CYP2C19]], [[CYP1A2]], [[CYP2D6]]
| elimination_half-life = 10–50 hours, with an average of 15 hours
| elimination_half-life = 10–50 hours, with an average of 15 hours
| excretion = [[Renal]]
| excretion = [[Renal]]
| pregnancy_US = D
| pregnancy_US = D
| pregnancy_category =
| pregnancy_category =
⚫
| legal_AU = Unscheduled
⚫ | routes_of_administration
= Oral
| legal_UK = POM
| legal_US = Unscheduled
⚫ | legal_status =
Unscheduled
⚫ | routes_of_administration= Oral
}}
}}
Line 46:
Line 44:
Amitriptyline is used in [[ankylosing spondylitis]] for [[pain]] relief and in some European countries it is officially approved as a preventive for patients with frequent/[[Chronic (medicine)|chronic]] [[migraine]]s, usually 25 to 75 mg. It is also used as a preventive for patients with recurring [[biliary dyskinesia]] ([[sphincter of Oddi]] dysfunction), usually 10 mg daily<ref name="Hubscher">S. G. Hubscher et al. (2006). Functional biliary type pain syndrome. In P. J. Pasricha, W. D. Willis & G. F. Gebhart (Eds.), ' ' italics' ' Chronic Abdominal and Visceral Pain' 'italics' '. London: Informa Healthcare, pp. 459-461.</ref>.
Amitriptyline is used in [[ankylosing spondylitis]] for [[pain]] relief and in some European countries it is officially approved as a preventive for patients with frequent/[[Chronic (medicine)|chronic]] [[migraine]]s, usually 25 to 75 mg. It is also used as a preventive for patients with recurring [[biliary dyskinesia]] ([[sphincter of Oddi]] dysfunction), usually 10 mg daily<ref name="Hubscher">S. G. Hubscher et al. (2006). Functional biliary type pain syndrome. In P. J. Pasricha, W. D. Willis & G. F. Gebhart (Eds.), ' ' italics' ' Chronic Abdominal and Visceral Pain' 'italics' '. London: Informa Healthcare, pp. 459-461.</ref>.
=== Unapproved / Off-label ===
=== Unapproved/Off-label ===
Amitriptyline may be prescribed for other conditions such as [[insomnia]], [[post-traumatic stress disorder]] (PTSD),<ref>National Institute for Clinical Excellence: [http://www.nice.org.uk/nicemedia/pdf/CG026publicinfo.pdf The Treatment of PTSD in Adults and Children]</ref> [[migraine]], [[rebound headache]], [[chronic pain]], [[tinnitus]], [[chronic cough]], [[postherpetic neuralgia]] (persistent pain following a [[shingles]] attack), [[carpal tunnel syndrome]] (CTS), [[fibromyalgia]], [[vulvodynia]], [[interstitial cystitis]], [[UCPPS|male chronic pelvic pain syndrome]], [[irritable bowel syndrome]] (IBS), [[diabetic neuropathy|diabetic peripheral neuropathy]], [[neurological]] pain, and painful [[paresthesia]]s related to [[multiple sclerosis]] and at low doses as a [[prophylaxis]] (preventive) for patients with [[Chronic (medicine)|chronic]] [[migraine]]s.<ref name="pmid3579659">{{cite journal | author = Ziegler D, Hurwitz A, Hassanein R, Kodanaz H, Preskorn S, Mason J | title = Migraine prophylaxis. A comparison of propranolol and amitriptyline | journal = Arch Neurol | volume = 44 | issue = 5 | pages = 486–9 | year = 1987 | pmid = 3579659}}</ref>
Amitriptyline may be prescribed for other conditions such as [[insomnia]], [[post-traumatic stress disorder]] (PTSD),<ref>National Institute for Clinical Excellence: [http://www.nice.org.uk/nicemedia/pdf/CG026publicinfo.pdf The Treatment of PTSD in Adults and Children]</ref> [[migraine]], [[rebound headache]], [[chronic pain]], [[tinnitus]], [[chronic cough]], [[postherpetic neuralgia]] (persistent pain following a [[shingles]] attack), [[carpal tunnel syndrome]] (CTS), [[fibromyalgia]], [[vulvodynia]], [[interstitial cystitis]], [[UCPPS|male chronic pelvic pain syndrome]], [[irritable bowel syndrome]] (IBS), [[diabetic neuropathy|diabetic peripheral neuropathy]], [[neurological]] pain, and painful [[paresthesia]]s related to [[multiple sclerosis]] and at low doses as a [[prophylaxis]] (preventive) for patients with [[Chronic (medicine)|chronic]] [[migraine]]s.<ref name="pmid3579659">{{cite journal | author = Ziegler D, Hurwitz A, Hassanein R, Kodanaz H, Preskorn S, Mason J | title = Migraine prophylaxis. A comparison of propranolol and amitriptyline | journal = Arch Neurol | volume = 44 | issue = 5 | pages = 486–9 | year = 1987 | pmid = 3579659}}</ref>
Amitriptyline (Elavil , Tryptizol , Laroxyl ) is a tricyclic antidepressant (TCA).[ 2]
History
Amitriptyline, under the brand name Elavil , was approved on April 7, 1961 for the treatment of major depression in the United States .[ 3]
Indications
Approved
Amitriptyline is approved for the treatment of major depression, as well as clinical/endogenous depression and also involutional melancholia or "depression of late life", which is no longer seen as a disease in its own right. Adult typical dosages are 25 to 150 mg daily, with half this dose initially for elderly or adolescent patients.
Children between the ages of 7 to 10 years typically have a dose of 10 to 20 mg; older children 25 to 50 mg at night. It should be gradually withdrawn at the end of the course, which overall should be of no more than three months.[ 4]
Amitriptyline is used in ankylosing spondylitis for pain relief and in some European countries it is officially approved as a preventive for patients with frequent/chronic migraines , usually 25 to 75 mg. It is also used as a preventive for patients with recurring biliary dyskinesia (sphincter of Oddi dysfunction), usually 10 mg daily[ 5]
Amitriptyline may be prescribed for other conditions such as insomnia , post-traumatic stress disorder (PTSD),[ 6] migraine , rebound headache , chronic pain , tinnitus , chronic cough , postherpetic neuralgia (persistent pain following a shingles attack), carpal tunnel syndrome (CTS), fibromyalgia , vulvodynia , interstitial cystitis , male chronic pelvic pain syndrome , irritable bowel syndrome (IBS), diabetic peripheral neuropathy , neurological pain, and painful paresthesias related to multiple sclerosis and at low doses as a prophylaxis (preventive) for patients with chronic migraines .[ 7] [ 4]
Amitriptyline in low doses is also sometimes prescribed to help ease the symptoms of chronic fatigue syndrome . It is thought to help combat symptoms of insomnia primarily, in addition to other selected symptoms of the affliction.[citation needed
A randomized controlled trial published in June 2005 found that amitriptyline was effective in functional dyspepsia that did not respond to a first-line treatment (famotidine or mosapride ).[ 8]
Pharmacology
Amitriptyline acts primarily as a serotonin-norepinephrine reuptake inhibitor , with strong actions on the norepinephrine transporter , and moderate effects on the serotonin transporter .[ 9] [ 10] dopamine transporter and therefore does not affect dopamine reuptake , being nearly 1,000 times weaker on it than on serotonin .[ 10]
Amitriptyline additionally functions as a 5-HT2A , 5-HT2C , 5-HT6 , 5-HT7 , α1 -adrenergic , H1 , and mACh receptor antagonist , and σ1 receptor agonist .[ 11] [ 12] [ 13] [ 14] NMDA receptor negative allosteric modulator at the same binding site as phencyclidine .[ 15] sodium channels , L -type calcium channelsKv 1.1 , Kv 7.2 , and Kv 7.3 voltage-gated potassium channels , and therefore acts as a sodium , calcium , and potassium channel blocker as well.[ 16] [ 17] [ 18]
Recently, amitriptyline has been demonstrated to act as an agonist of the TrkA and TrkB receptors .[ 19] heterodimerization of these proteins in the absence of NGF and has potent neurotrophic activity both in-vivo and in-vitro in mouse models.[ 19]
Side effects
Common side effects of using amitriptyline are mostly due to its anticholinergic activity, including: weight gain, dry mouth, changes in appetite, drowsiness, muscle stiffness, nausea, constipation, nervousness, dizziness, blurred vision, urinary retention, insomnia and changes in sexual function. Some rare side effects include tinnitus , hypotension , mania , psychosis , sleep paralysis , hypnagogia , hypnopompia , heart block , arrhythmias , lip and mouth ulcers, extrapyramidal symptoms , depression , and hepatic toxicity .
Overdose
The symptoms and the treatment of an overdose are largely the same as for the other TCAs.
See also
References
^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)" . nctr-crs.fda.gov . FDA . Retrieved 22 Oct 2023 .^ Barbui C, Hotopf M (2001). "Amitriptyline v. the rest: still the leading antidepressant after 40 years of randomised controlled trials" . The British Journal of Psychiatry : the Journal of Mental Science . 178 : 129–44. PMID 11157426 . ^ Fangmann P, Assion HJ, Juckel G, González CA, López-Muñoz F (2008). "Half a century of antidepressant drugs: on the clinical introduction of monoamine oxidase inhibitors, tricyclics, and tetracyclics. Part II: tricyclics and tetracyclics" . Journal of Clinical Psychopharmacology . 28 (1): 1–4. doi :10.1097/jcp.0b013e3181627b60 . PMID 18204333 . CS1 maint: multiple names: authors list (link ) ^ a b British National Formulary 45 (March 2003).^ S. G. Hubscher et al. (2006). Functional biliary type pain syndrome. In P. J. Pasricha, W. D. Willis & G. F. Gebhart (Eds.), ' ' italics' ' Chronic Abdominal and Visceral Pain' 'italics' '. London: Informa Healthcare, pp. 459-461.
^ National Institute for Clinical Excellence: The Treatment of PTSD in Adults and Children
^ Ziegler D, Hurwitz A, Hassanein R, Kodanaz H, Preskorn S, Mason J (1987). "Migraine prophylaxis. A comparison of propranolol and amitriptyline". Arch Neurol . 44 (5): 486–9. PMID 3579659 . {{cite journal }}: CS1 maint: multiple names: authors list (link )^ Otaka M, Jin M, Odashima M; et al. (2005). "New strategy of therapy for functional dyspepsia using famotidine, mosapride and amitriptyline" . Aliment. Pharmacol. Ther . 21 (Suppl 2): 42–6. doi :10.1111/j.1365-2036.2005.02473.x . PMID 15943846 . CS1 maint: multiple names: authors list (link ) ^ http://www.cnsforum.com/content/pictures/imagebank/hirespng/antidep_uptake_specific.png ^ a b Attention: This template ({{ cite pmid }} ) is deprecated. To cite the publication identified by PMID 9537821, please use {{ cite journal }} with |pmid=9537821 instead. ^ Attention: This template ({{ cite pmid }} ) is deprecated. To cite the publication identified by PMID 9400006, please use {{ cite journal }} with |pmid=9400006 instead. ^ Alan F. Schatzberg, Charles B. (2006). Essentials of clinical psychopharmacology . American Psychiatric Pub. p. 7. ISBN 1585622435, 9781585622436 ^ Attention: This template ({{ cite pmid }} ) is deprecated. To cite the publication identified by PMID 11561066, please use {{ cite journal }} with |pmid=11561066 instead. ^ Attention: This template ({{ cite pmid }} ) is deprecated. To cite the publication identified by PMID 17689532, please use {{ cite journal }} with |pmid=17689532 instead. ^ Attention: This template ({{ cite pmid }} ) is deprecated. To cite the publication identified by PMID 2568580, please use {{ cite journal }} with |pmid=2568580 instead. ^ Attention: This template ({{ cite pmid }} ) is deprecated. To cite the publication identified by PMID 9435180, please use {{ cite journal }} with |pmid=9435180 instead. ^ Attention: This template ({{ cite pmid }} ) is deprecated. To cite the publication identified by PMID 18048694, please use {{ cite journal }} with |pmid=18048694 instead. ^ Punke MA, Friederich P (2007). "Amitriptyline is a potent blocker of human Kv1.1 and Kv7.2/7.3 channels" . Anesthesia and Analgesia . 104 (5): 1256–64, tables of contents. doi :10.1213/01.ane.0000260310.63117.a2 . PMID 17456683 . ^ a b Attention: This template ({{ cite pmid }} ) is deprecated. To cite the publication identified by PMID 19549602, please use {{ cite journal }} with |pmid=19549602 instead.
Further reading
SSRIs Tooltip Selective serotonin reuptake inhibitors SNRIs Tooltip Serotonin–norepinephrine reuptake inhibitors NRIs Tooltip Norepinephrine reuptake inhibitors NDRIs Tooltip Norepinephrine–dopamine reuptake inhibitors NaSSAs Tooltip Noradrenergic and specific serotonergic antidepressants SARIs Tooltip Serotonin antagonist and reuptake inhibitors SMS Tooltip Serotonin modulator and stimulators Others
TCAs Tooltip Tricyclic antidepressants TeCAs Tooltip Tetracyclic antidepressants Others
Non-selective MAOA Tooltip Monoamine oxidase A -selectiveMAOB Tooltip Monoamine oxidase B -selective
α1
Agonists Antagonists
Abanoquil Ajmalicine Alfuzosin Anisodamine Anisodine Atiprosin Atypical antipsychotics (e.g., brexpiprazole , clozapine , olanzapine , quetiapine , risperidone )Benoxathian Beta blockers (e.g., adimolol , amosulalol , arotinolol , carvedilol , eugenodilol , labetalol )Buflomedil Bunazosin Corynanthine Dapiprazole Domesticine Doxazosin Ergolines (e.g., acetergamine , ergotamine , dihydroergotamine , lisuride , nicergoline , terguride )Etoperidone Fenspiride Hydroxyzine Indoramin Ketanserin L-765,314 mCPP Mepiprazole Metazosin Monatepil Moxisylyte Naftopidil Nantenine Neldazosin Niaprazine Niguldipine Pardoprunox Pelanserin Perlapine Phendioxan Phenoxybenzamine Phentolamine Phenylpiperazine antidepressants (e.g., hydroxynefazodone , nefazodone , trazodone , triazoledione )Piperoxan Prazosin Quinazosin Quinidine Silodosin Spegatrine Spiperone Talipexole Tamsulosin Terazosin Tiodazosin Tolazoline Tetracyclic antidepressants (e.g., amoxapine , maprotiline , mianserin )Tricyclic antidepressants (e.g., amitriptyline , clomipramine , doxepin , imipramine , trimipramine )Trimazosin Typical antipsychotics (e.g., chlorpromazine , fluphenazine , loxapine , thioridazine )Urapidil WB-4101 Zolertine
α2
Agonists Antagonists
1-PP Adimolol Amesergide Aptazapine Atipamezole Atypical antipsychotics (e.g., asenapine , brexpiprazole , clozapine , lurasidone , olanzapine , paliperidone , quetiapine , risperidone , zotepine )Azapirones (e.g., buspirone , gepirone , ipsapirone , tandospirone )BRL-44408 Buflomedil Cirazoline Efaroxan Esmirtazapine Fenmetozole Fluparoxan Idazoxan Ketanserin Lisuride mCPP Mianserin Mirtazapine NAN-190 Pardoprunox Phentolamine Phenoxybenzamine Piperoxan Piribedil Rauwolscine Rotigotine Setiptiline Spegatrine Spiroxatrine Sunepitron Terguride Tolazoline Typical antipsychotics (e.g., chlorpromazine , fluphenazine , loxapine , thioridazine )Yohimbine
β
mAChRs Tooltip Muscarinic acetylcholine receptors
Agonists Antagonists
3-Quinuclidinyl benzilate 4-DAMP Aclidinium bromide (+formoterol )Abediterol AF-DX 250 AF-DX 384 Ambutonium bromide Anisodamine Anisodine Antihistamines (first-generation) (e.g., brompheniramine , buclizine , captodiame , chlorphenamine (chlorpheniramine) , cinnarizine , clemastine , cyproheptadine , dimenhydrinate , dimetindene , diphenhydramine , doxylamine , meclizine , mequitazine , perlapine , phenindamine , pheniramine , phenyltoloxamine , promethazine , propiomazine , triprolidine )AQ-RA 741 Atropine Atropine methonitrate Atypical antipsychotics (e.g., clozapine , fluperlapine , olanzapine (+fluoxetine ), rilapine , quetiapine , tenilapine , zotepine )Benactyzine Benzatropine (benztropine) Benzilone Benzilylcholine mustard Benzydamine Bevonium BIBN 99 Biperiden Bornaprine Camylofin CAR-226,086 CAR-301,060 CAR-302,196 CAR-302,282 CAR-302,368 CAR-302,537 CAR-302,668 Caramiphen Cimetropium bromide Clidinium bromide Cloperastine CS-27349 Cyclobenzaprine Cyclopentolate Darifenacin DAU-5884 Desfesoterodine Dexetimide DIBD Dicycloverine (dicyclomine) Dihexyverine Difemerine Diphemanil metilsulfate Ditran Drofenine EA-3167 EA-3443 EA-3580 EA-3834 Emepronium bromide Etanautine Etybenzatropine (ethybenztropine) Fenpiverinium Fentonium bromide Fesoterodine Flavoxate Glycopyrronium bromide (+beclometasone/formoterol , +indacaterol , +neostigmine )Hexahydrodifenidol Hexahydrosiladifenidol Hexbutinol Hexocyclium Himbacine HL-031,120 Homatropine Imidafenacin Ipratropium bromide (+salbutamol )Isopropamide J-104,129 Hyoscyamine Mamba toxin 3 Mamba toxin 7 Mazaticol Mebeverine Meladrazine Mepenzolate Methantheline Methoctramine Methylatropine Methylhomatropine Methylscopolamine Metixene Muscarinic toxin 7 N-Ethyl-3-piperidyl benzilate N-Methyl-3-piperidyl benzilate Nefopam Octatropine methylbromide (anisotropine methylbromide) Orphenadrine Otenzepad (AF-DX 116) Otilonium bromide Oxapium iodide Oxitropium bromide Oxybutynin Oxyphencyclimine Oxyphenonium bromide PBID PD-102,807 PD-0298029 Penthienate Pethidine pFHHSiD Phenglutarimide Phenyltoloxamine Pipenzolate bromide Piperidolate Pirenzepine Piroheptine Pizotifen Poldine Pridinol Prifinium bromide Procyclidine Profenamine (ethopropazine) Propantheline bromide Propiverine Quinidine 3-Quinuclidinyl thiochromane-4-carboxylate Revefenacin Rociverine RU-47,213 SCH-57,790 SCH-72,788 SCH-217,443 Scopolamine (hyoscine) Scopolamine butylbromide (hyoscine butylbromide) Silahexacyclium Sofpironium bromide Solifenacin SSRIs Tooltip Selective serotonin reuptake inhibitors (e.g., femoxetine , paroxetine )Telenzepine Terodiline Tetracyclic antidepressants (e.g., amoxapine , maprotiline , mianserin , mirtazapine )Tiemonium iodide Timepidium bromide Tiotropium bromide Tiquizium bromide Tofenacin Tolterodine Tricyclic antidepressants (e.g., amitriptyline (+perphenazine ), amitriptylinoxide , butriptyline , cidoxepin , clomipramine , desipramine , desmethyldesipramine , dibenzepin , dosulepin (dothiepin) , doxepin , imipramine , lofepramine , nitroxazepine , northiaden (desmethyldosulepin) , nortriptyline , protriptyline , quinupramine , trimipramine )Tridihexethyl Trihexyphenidyl Trimebutine Tripitamine (tripitramine) Tropacine Tropatepine Tropicamide Trospium chloride Typical antipsychotics (e.g., chlorpromazine , chlorprothixene , cyamemazine (cyamepromazine) , loxapine , mesoridazine , thioridazine )Umeclidinium bromide (+vilanterol )WIN-2299 Xanomeline Zamifenacin
Precursors (and prodrugs )
nAChRs Tooltip Nicotinic acetylcholine receptors
Agonists PAMs Tooltip positive allosteric modulators )
5-HIAA 6-Chloronicotine A-84,543 A-366,833 A-582,941 A-867,744 ABT-202 ABT-418 ABT-560 ABT-894 Acetylcholine Altinicline Anabasine Anatabine Anatoxin-a AR-R17779 Bephenium hydroxynaphthoate Butinoline Butyrylcholine Carbachol Choline Cotinine Cytisine Decamethonium Desformylflustrabromine Dianicline Dimethylphenylpiperazinium Epibatidine Epiboxidine Ethanol (alcohol) Ethoxysebacylcholine EVP-4473 EVP-6124 Galantamine GTS-21 Ispronicline Ivermectin JNJ-39393406 Levamisole Lobeline MEM-63,908 (RG-3487) Morantel Nicotine (tobacco )NS-1738 PHA-543,613 PHA-709,829 PNU-120,596 PNU-282,987 Pozanicline Pyrantel Rivanicline RJR-2429 Sazetidine A SB-206553 Sebacylcholine SIB-1508Y SIB-1553A SSR-180,711 Suberyldicholine Suxamethonium (succinylcholine) Suxethonium (succinyldicholine) TC-1698 TC-1734 TC-1827 TC-2216 TC-5214 TC-5619 TC-6683 Tebanicline Tribendimidine Tropisetron UB-165 Varenicline WAY-317,538 XY-4083 Antagonists NAMs Tooltip negative allosteric modulators )
Precursors (and prodrugs )
AMPAR Tooltip α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor KAR Tooltip Kainate receptor NMDAR Tooltip N-Methyl-D-aspartate receptor
Group I
mGluR1 Tooltip Metabotropic glutamate receptor 1 mGluR5 Tooltip Metabotropic glutamate receptor 5
Group II
mGluR2 Tooltip Metabotropic glutamate receptor 2 mGluR3 Tooltip Metabotropic glutamate receptor 3
Group III
mGluR4 Tooltip Metabotropic glutamate receptor 4 mGluR6 Tooltip Metabotropic glutamate receptor 6 mGluR7 Tooltip Metabotropic glutamate receptor 7 mGluR8 Tooltip Metabotropic glutamate receptor 8
H1
Agonists Antagonists
Others: Atypical antipsychotics (e.g., aripiprazole , asenapine , brexpiprazole , brilaroxazine , clozapine , iloperidone , olanzapine , paliperidone , quetiapine , risperidone , ziprasidone , zotepine )Phenylpiperazine antidepressants (e.g., hydroxynefazodone , nefazodone , trazodone , triazoledione )Tetracyclic antidepressants (e.g., amoxapine , loxapine , maprotiline , mianserin , mirtazapine , oxaprotiline )Tricyclic antidepressants (e.g., amitriptyline , butriptyline , clomipramine , desipramine , dosulepin (dothiepin) , doxepin , imipramine , iprindole , lofepramine , nortriptyline , protriptyline , trimipramine )Typical antipsychotics (e.g., chlorpromazine , flupenthixol , fluphenazine , loxapine , perphenazine , prochlorperazine , thioridazine , thiothixene )
H2
H3
H4
5-HT1
5-HT1A
Agonists: 8-OH-DPAT Adatanserin Amphetamine Antidepressants (e.g., etoperidone , hydroxynefazodone , nefazodone , trazodone , triazoledione , vilazodone , vortioxetine )Atypical antipsychotics (e.g., aripiprazole , asenapine , brexpiprazole , cariprazine , clozapine , lurasidone , quetiapine , ziprasidone )Azapirones (e.g., buspirone , eptapirone , gepirone , perospirone , tandospirone )Bay R 1531 Befiradol BMY-14802 Cannabidiol Dimemebfe Dopamine Ebalzotan Eltoprazine Enciprazine Ergolines (e.g., bromocriptine , cabergoline , dihydroergotamine , ergotamine , lisuride , LSD , methylergometrine (methylergonovine) , methysergide , pergolide )F-11,461 F-12826 F-13714 F-14679 F-15063 F-15,599 Flesinoxan Flibanserin Flumexadol Hypidone Lesopitron LY-293284 LY-301317 mCPP MKC-242 Naluzotan NBUMP Osemozotan Oxaflozane Pardoprunox Piclozotan Rauwolscine Repinotan Roxindole RU-24,969 S-14,506 S-14671 S-15535 Sarizotan Serotonin (5-HT) SSR-181507 Sunepitron Tryptamines (e.g., 5-CT , 5-MeO-DMT , 5-MT , bufotenin , DMT , indorenate , N-Me-5-HT , psilocin , psilocybin )TGBA01AD U-92,016-A Urapidil Vilazodone Xaliproden Yohimbine
Antagonists: Atypical antipsychotics (e.g., iloperidone , risperidone , sertindole )AV965 Beta blockers (e.g., alprenolol , carteolol , cyanopindolol , iodocyanopindolol , isamoltane , oxprenolol , penbutolol , pindobind , pindolol , propranolol , tertatolol )BMY-7,378 CSP-2503 Dotarizine Ergolines (e.g., metergoline )FCE-24379 Flopropione GR-46611 Isamoltane Lecozotan Mefway Metitepine (methiothepin) MIN-117 (WF-516) MPPF NAN-190 Robalzotan S-15535 SB-649,915 SDZ 216-525 Spiperone Spiramide Spiroxatrine UH-301 WAY-100135 WAY-100635 Xylamidine
5-HT1B
Agonists: Anpirtoline CGS-12066A CP-93129 CP-94253 CP-122,288 CP-135807 Eltoprazine Ergolines (e.g., bromocriptine , dihydroergotamine , ergotamine , methylergometrine (methylergonovine) , methysergide , pergolide )mCPP RU-24,969 Serotonin (5-HT) Triptans (e.g., avitriptan , donitriptan , eletriptan , sumatriptan , zolmitriptan )TFMPP Tryptamines (e.g., 5-BT , 5-CT , 5-MT , DMT )Vortioxetine
5-HT1D
Agonists: CP-122,288 CP-135807 CP-286601 Ergolines (e.g., bromocriptine , cabergoline , dihydroergotamine , ergotamine , LSD , methysergide )GR-46611 L-694247 L-772405 mCPP PNU-109291 PNU-142633 Serotonin (5-HT) TGBA01AD Triptans (e.g., almotriptan , avitriptan , donitriptan , eletriptan , frovatriptan , naratriptan , rizatriptan , sumatriptan , zolmitriptan )Tryptamines (e.g., 5-BT , 5-CT , 5-Et-DMT , 5-MT , 5-(nonyloxy)tryptamine , DMT )
5-HT1E
5-HT1F
5-HT2
5-HT2A
Agonists: 25H/NB series (e.g., 25I-NBF , 25I-NBMD , 25I-NBOH , 25I-NBOMe , 25B-NBOMe , 25C-NBOMe , 25TFM-NBOMe , 2CBCB-NBOMe , 25CN-NBOH , 2CBFly-NBOMe )2Cs (e.g., 2C-B , 2C-E , 2C-I , 2C-T-2 , 2C-T-7 , 2C-T-21 )2C-B-FLY 2CB-Ind 5-Methoxytryptamines (5-MeO-DET , 5-MeO-DiPT , 5-MeO-DMT , 5-MeO-DPT , 5-MT )α-Alkyltryptamines (e.g., 5-Cl-αMT , 5-Fl-αMT , 5-MeO-αET , 5-MeO-αMT , α-Me-5-HT , αET , αMT )AL-34662 AL-37350A Bromo-DragonFLY Dimemebfe DMBMPP DOx (e.g., DOB , DOC , DOI , DOM )Efavirenz Ergolines (e.g., 1P-LSD , ALD-52 , bromocriptine , cabergoline , ergine (LSA) , ergometrine (ergonovine) , ergotamine , lisuride , LA-SS-Az , LSB , LSD , LSD-Pip , LSH , LSP , methylergometrine (methylergonovine) , pergolide )Flumexadol IHCH-7113 Jimscaline Lorcaserin MDxx (e.g., MDA (tenamfetamine) , MDMA (midomafetamine) , MDOH , MMDA )O-4310 Oxaflozane PHA-57378 PNU-22394 PNU-181731 RH-34 SCHEMBL5334361 Phenethylamines (e.g., lophophine , mescaline )Piperazines (e.g., BZP , quipazine , TFMPP )Serotonin (5-HT) TCB-2 TFMFly Tryptamines (e.g., 5-BT , 5-CT , bufotenin , DET , DiPT , DMT , DPT , psilocin , psilocybin , tryptamine )
Antagonists: 5-I-R91150 5-MeO-NBpBrT AC-90179 Adatanserin Altanserin Antihistamines (e.g., cyproheptadine , hydroxyzine , ketotifen , perlapine )AMDA Atypical antipsychotics (e.g., amperozide , aripiprazole , asenapine , blonanserin , brexpiprazole , carpipramine , clocapramine , clorotepine , clozapine , fluperlapine , gevotroline , iloperidone , lurasidone , melperone , mosapramine , ocaperidone , olanzapine , paliperidone , quetiapine , risperidone , sertindole , zicronapine , ziprasidone , zotepine )Chlorprothixene Cinanserin CSP-2503 Deramciclane Dotarizine Eplivanserin Ergolines (e.g., amesergide , LY-53857 , LY-215,840 , mesulergine , metergoline , methysergide , sergolexole )Fananserin Flibanserin Glemanserin Irindalone Ketanserin KML-010 Landipirdine LY-393558 mCPP Medifoxamine Metitepine (methiothepin) MIN-117 (WF-516) Naftidrofuryl Nantenine Nelotanserin Opiranserin (VVZ-149) Pelanserin Phenoxybenzamine Pimavanserin Pirenperone Pizotifen Pruvanserin Rauwolscine Ritanserin Roluperidone S-14671 Sarpogrelate Serotonin antagonists and reuptake inhibitors (e.g., etoperidone , hydroxynefazodone , lubazodone , mepiprazole , nefazodone , triazoledione , trazodone )SR-46349B TGBA01AD Teniloxazine Temanogrel Tetracyclic antidepressants (e.g., amoxapine , aptazapine , esmirtazapine , maprotiline , mianserin , mirtazapine )Tricyclic antidepressants (e.g., amitriptyline )Typical antipsychotics (e.g., chlorpromazine , fluphenazine , haloperidol , loxapine , perphenazine , pimozide , pipamperone , prochlorperazine , setoperone , spiperone , spiramide , thioridazine , thiothixene , trifluoperazine )Volinanserin Xylamidine Yohimbine
5-HT2B
Agonists: 4-Methylaminorex Aminorex Amphetamines (e.g., chlorphentermine , cloforex , dexfenfluramine , fenfluramine , levofenfluramine , norfenfluramine )BW-723C86 DOx (e.g., DOB , DOC , DOI , DOM )Ergolines (e.g., cabergoline , dihydroergocryptine , dihydroergotamine , ergotamine , methylergometrine (methylergonovine) , methysergide , pergolide )Lorcaserin MDxx (e.g., MDA (tenamfetamine) , MDMA (midomafetamine) , MDOH , MMDA )Piperazines (e.g., TFMPP )PNU-22394 Ro60-0175 Serotonin (5-HT) Tryptamines (e.g., 5-BT , 5-CT , 5-MT , α-Me-5-HT , bufotenin , DET , DiPT , DMT , DPT , psilocin , psilocybin , tryptamine )
Antagonists: Agomelatine Atypical antipsychotics (e.g., amisulpride , aripiprazole , asenapine , brexpiprazole , cariprazine , clozapine , N-desalkylquetiapine (norquetiapine) , N-desmethylclozapine (norclozapine) , olanzapine , pipamperone , quetiapine , risperidone , ziprasidone )Cyproheptadine EGIS-7625 Ergolines (e.g., amesergide , bromocriptine , lisuride , LY-53857 , LY-272015 , mesulergine )Ketanserin LY-393558 mCPP Metadoxine Metitepine (methiothepin) Pirenperone Pizotifen Propranolol PRX-08066 Rauwolscine Ritanserin RS-127445 Sarpogrelate SB-200646 SB-204741 SB-206553 SB-215505 SB-221284 SB-228357 SDZ SER-082 Tegaserod Tetracyclic antidepressants (e.g., amoxapine , mianserin , mirtazapine )Trazodone Typical antipsychotics (e.g., chlorpromazine )TIK-301 Yohimbine
5-HT2C
Agonists: 2Cs (e.g., 2C-B , 2C-E , 2C-I , 2C-T-2 , 2C-T-7 , 2C-T-21 )5-Methoxytryptamines (5-MeO-DET , 5-MeO-DiPT , 5-MeO-DMT , 5-MeO-DPT , 5-MT )α-Alkyltryptamines (e.g., 5-Cl-αMT , 5-Fl-αMT , 5-MeO-αET , 5-MeO-αMT , α-Me-5-HT , αET , αMT )A-372159 AL-38022A Alstonine CP-809101 Dimemebfe DOx (e.g., DOB , DOC , DOI , DOM )Ergolines (e.g., ALD-52 , cabergoline , dihydroergotamine , ergine (LSA) , ergotamine , lisuride , LA-SS-Az , LSB , LSD , LSD-Pip , LSH , LSP , pergolide )Flumexadol Lorcaserin MDxx (e.g., MDA (tenamfetamine) , MDMA (midomafetamine) , MDOH , MMDA )MK-212 ORG-12962 ORG-37684 Oxaflozane PHA-57378 Phenethylamines (e.g., lophophine , mescaline )Piperazines (e.g., aripiprazole , BZP , mCPP , quipazine , TFMPP )PNU-22394 PNU-181731 Ro60-0175 Ro60-0213 Serotonin (5-HT) Tryptamines (e.g., 5-BT , 5-CT , bufotenin , DET , DiPT , DMT , DPT , psilocin , psilocybin , tryptamine )Vabicaserin WAY-629 WAY-161503 YM-348
Antagonists: Adatanserin Agomelatine Atypical antipsychotics (e.g., asenapine , clorotepine , clozapine , fluperlapine , iloperidone , melperone , olanzapine , paliperidone , quetiapine , risperidone , sertindole , ziprasidone , zotepine )Captodiame CEPC Cinanserin Cyproheptadine Deramciclane Desmetramadol Dotarizine Eltoprazine Ergolines (e.g., amesergide , bromocriptine , LY-53857 , LY-215,840 , mesulergine , metergoline , methysergide , sergolexole )Etoperidone Fluoxetine FR-260010 Irindalone Ketanserin Ketotifen Latrepirdine (dimebolin) Medifoxamine Metitepine (methiothepin) Nefazodone Pirenperone Pizotifen Propranolol Ritanserin RS-102221 S-14671 SB-200646 SB-206553 SB-221284 SB-228357 SB-242084 SB-243213 SDZ SER-082 Tedatioxetine Tetracyclic antidepressants (e.g., amoxapine , aptazapine , esmirtazapine , maprotiline , mianserin , mirtazapine )TIK-301 Tramadol Trazodone Tricyclic antidepressants (e.g., amitriptyline , nortriptyline )Typical antipsychotics (e.g., chlorpromazine , loxapine , pimozide , pipamperone , thioridazine )Xylamidine
5-HT3 –7
5-HT3
Agonists: Alcohols (e.g., butanol , ethanol (alcohol) , trichloroethanol )m-CPBG Phenylbiguanide Piperazines (e.g., BZP , mCPP , quipazine )RS-56812 Serotonin (5-HT) SR-57227 SR-57227A Tryptamines (e.g., 2-Me-5-HT , 5-CT , bufotenidine (5-HTQ) )Volatiles/gases (e.g., halothane , isoflurane , toluene , trichloroethane )YM-31636
Antagonists: Alosetron Anpirtoline Arazasetron AS-8112 Atypical antipsychotics (e.g., clozapine , olanzapine , quetiapine )Azasetron Batanopride Bemesetron (MDL-72222) Bupropion Cilansetron CSP-2503 Dazopride Dolasetron Galanolactone Granisetron Hydroxybupropion Lerisetron Memantine Ondansetron Palonosetron Ramosetron Renzapride Ricasetron Tedatioxetine Tetracyclic antidepressants (e.g., amoxapine , mianserin , mirtazapine )Thujone Tropanserin Tropisetron Typical antipsychotics (e.g., loxapine )Volatiles/gases (e.g., nitrous oxide , sevoflurane , xenon )Vortioxetine Zacopride Zatosetron
5-HT4
5-HT5A
5-HT6
Agonists: Ergolines (e.g., dihydroergocryptine , dihydroergotamine , ergotamine , lisuride , LSD , mesulergine , metergoline , methysergide )Hypidone Serotonin (5-HT) Tryptamines (e.g., 2-Me-5-HT , 5-BT , 5-CT , 5-MT , Bufotenin , E-6801 , E-6837 , EMD-386088 , EMDT , LY-586713 , N-Me-5-HT , ST-1936 , tryptamine )WAY-181187 WAY-208466
Antagonists: ABT-354 Atypical antipsychotics (e.g., aripiprazole , asenapine , clorotepine , clozapine , fluperlapine , iloperidone , olanzapine , tiospirone )AVN-101 AVN-211 AVN-322 AVN-397 BGC20-760 BVT-5182 BVT-74316 Cerlapirdine EGIS-12,233 GW-742457 Idalopirdine Ketanserin Landipirdine Latrepirdine (dimebolin) Masupirdine Metitepine (methiothepin) MS-245 PRX-07034 Ritanserin Ro 04-6790 Ro 63-0563 SB-258585 SB-271046 SB-357134 SB-399885 SB-742457 Tetracyclic antidepressants (e.g., amoxapine , mianserin )Tricyclic antidepressants (e.g., amitriptyline , clomipramine , doxepin , nortriptyline )Typical antipsychotics (e.g., chlorpromazine , loxapine )
5-HT7
Antagonists: Atypical antipsychotics (e.g., amisulpride , aripiprazole , asenapine , brexpiprazole , clorotepine , clozapine , fluperlapine , olanzapine , risperidone , sertindole , tiospirone , ziprasidone , zotepine )Butaclamol DR-4485 EGIS-12,233 Ergolines (e.g., 2-Br-LSD (BOL-148) , amesergide , bromocriptine , cabergoline , dihydroergotamine , ergotamine , LY-53857 , LY-215,840 , mesulergine , metergoline , methysergide , sergolexole )JNJ-18038683 Ketanserin LY-215,840 Metitepine (methiothepin) Ritanserin SB-258719 SB-258741 SB-269970 SB-656104 SB-656104A SB-691673 SLV-313 SLV-314 Spiperone SSR-181507 Tetracyclic antidepressants (e.g., amoxapine , maprotiline , mianserin , mirtazapine )Tricyclic antidepressants (e.g., amitriptyline , clomipramine , imipramine )Typical antipsychotics (e.g., acetophenazine , chlorpromazine , chlorprothixene , fluphenazine , loxapine , pimozide )Vortioxetine