Cannabidiol
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Cannabidiol |
| AHFS/Drugs.com | International Drug Names |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 13–19% (oral),[1] 11–45% (mean 31%; inhaled)[2] |
| Elimination half-life | 9 h[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.215.986 |
| Chemical and physical data | |
| Formula | C21H30O2 |
| Molar mass | 314.4636 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 66 °C (151 °F) |
| |
| |
| | |
| Part of a series on |
| Cannabis |
|---|
 |
Cannabidiol (INN:[3] CBD) is one of at least 113 active cannabinoids identified in cannabis.[4][5] It is a major phytocannabinoid, accounting for up to 40% of the plant's extract.[6]
CBD does not appear to have any intoxicating effects such as those caused by THC in marijuana, but may have effects on anxiety and an anti-psychotic effect.[7]
Anticonvulsant
Anecdotal reports and early studies suggested that CBD may be of value in treating epilepsy, but the quality of the studies was too poor to draw definitive conclusions.[8][9] A 2014 Cochrane review included four randomized controlled trials, but all were of poor quality.[10] CBD received orphan drug status in the United States for the treatment of Dravet syndrome, a rare form of intractable epilepsy.[11] Plans are underway to conduct double-blinded, randomized studies of CBD in Dravet syndrome and other treatment-resistant epilepsies.[12]
Dravet syndrome (aka myoclonic epilepsy of infancy or SMEI) is a rare form of epilepsy that begins in infancy and is difficult to treat.[13] Some cannabis/hemp extract preparations containing CBD are marketed as dietary supplements and claim efficacy against Dravet syndrome. One such preparation is marketed under the tradename Charlotte's Web Hemp Extract.[14][15] GW Pharmaceuticals is seeking FDA approval to market a liquid formulation of pure plant-derived CBD, under the trade name Epidiolex (containing 99% cannabidiol and less than 0.10% Δ9-THC) as a treatment for Dravet syndrome. Epidiolex was granted fast-track status and is in late stage trials following positive early results from the drug.[16][17][18]
Antipsychotic
There is tentative evidence that CBD has an anti-psychotic effect, but research in this area is limited.[19][20]
Safety
CBD safety in humans has been studied in multiple small studies, suggesting that it is well tolerated at doses of up to 1500 mg/day (p.o.) or 30 mg (i.v.).[21] Daily doses of CBD (700 mg) for 6 weeks did not induce any toxicity in patients being treated for Huntington's disease.[22]
Pharmacodynamics
Cannabidiol has very low affinity for the cannabinoid CB1 and CB2 receptors but acts as an indirect antagonist of these receptors.[23][24] It may potentiate THC's effects by increasing CB1 receptor density or through another CB1 receptor-related mechanism.[25] Cannabidiol may also extend the duration of the effects of THC via inhibition of the cytochrome P450 CYP3A and CYP2C enzymes.[26][unreliable source?]
Cannabidiol has been found to act as an antagonist of GPR55, a G protein-coupled receptor and putative cannabinoid receptor that is expressed in the caudate nucleus and putamen in the brain.[27] It has also been shown to act as a 5-HT1A receptor partial agonist,[28] and this action may be involved in the antidepressant,[29][30] anxiolytic,[30][31] and neuroprotective[32][33] effects of cannabidiol. It is an allosteric modulator of the μ- and δ-opioid receptors as well.[34] Cannabidiol's pharmacological effects have additionally been attributed to PPARγ agonism and intracellular calcium release.[6]
Research suggests that CBD may exert some of its pharmacological action through its inhibition of fatty acid amide hydrolase (FAAH), which may in turn increase the levels of endocannabinoids, such as anandamide, produced by the body.[6] It has also been speculated that some of the metabolites of CBD have pharmacological effects that contribute to the biological activity of CBD.[35]
Pharmacokinetic interactions
There is preclinical evidence to suggest that cannabidiol may reduce THC clearance, modestly increasing THC's plasma concentrations resulting in a greater amount of THC available to receptors, increasing the effect of THC in a dose-dependent manner.[36][37] Despite this, the available evidence in humans suggests no significant effect of CBD on THC plasma levels.[38]
Pharmaceutical preparations
Nabiximols (USAN, trade name Sativex) is an aerosolized mist for oral administration containing a near 1:1 ratio of CBD and THC. The drug was approved by Canadian authorities in 2005 to alleviate pain associated with multiple sclerosis.[39][40] Epidiolex, a drug with cannabidiol as its active pharmaceutical ingredient, received orphan drug status in the United States for treatment of Dravet syndrome in July 2015.[41]
Epidiolex is an oil formulation of CBD extracted from the cannabis plant undergoing clinical trials for refractory epilepsy syndromes.[42]
Chemistry
Cannabidiol is insoluble in water but soluble in organic solvents such as pentane. At room temperature, it is a colorless crystalline solid.[43] In strongly basic media and the presence of air, it is oxidized to a quinone.[44] Under acidic conditions it cyclizes to THC.[45] The synthesis of cannabidiol has been accomplished by several research groups.[46][47][48]
Biosynthesis
Cannabis produces CBD-carboxylic acid through the same metabolic pathway as THC, until the last step, where CBDA synthase performs catalysis instead of THCA synthase.[49]

Isomerism
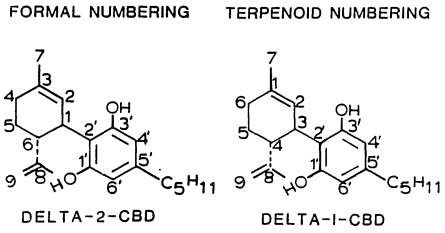
| 7 double bond isomers and their 30 stereoisomers | ||||||||
|---|---|---|---|---|---|---|---|---|
| Formal numbering | Terpenoid numbering | Number of stereoisomers | Natural occurrence | Convention on Psychotropic Substances Schedule | Structure | |||
| Short name | Chiral centers | Full name | Short name | Chiral centers | ||||
| Δ5-cannabidiol | 1 and 3 | 2-(6-isopropenyl-3-methyl-5-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ4-cannabidiol | 1 and 3 | 4 | No | unscheduled | 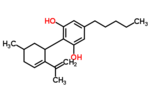
|
| Δ4-cannabidiol | 1, 3 and 6 | 2-(6-isopropenyl-3-methyl-4-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ5-cannabidiol | 1, 3 and 4 | 8 | No | unscheduled | 
|
| Δ3-cannabidiol | 1 and 6 | 2-(6-isopropenyl-3-methyl-3-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ6-cannabidiol | 3 and 4 | 4 | ? | unscheduled | 
|
| Δ3,7-cannabidiol | 1 and 6 | 2-(6-isopropenyl-3-methylenecyclohex-1-yl)-5-pentyl-1,3-benzenediol | Δ1,7-cannabidiol | 3 and 4 | 4 | No | unscheduled | 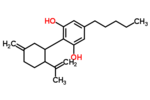
|
| Δ2-cannabidiol | 1 and 6 | 2-(6-isopropenyl-3-methyl-2-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ1-cannabidiol | 3 and 4 | 4 | Yes | unscheduled | 
|
| Δ1-cannabidiol | 3 and 6 | 2-(6-isopropenyl-3-methyl-1-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ2-cannabidiol | 1 and 4 | 4 | No | unscheduled | 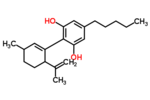
|
| Δ6-cannabidiol | 3 | 2-(6-isopropenyl-3-methyl-6-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ3-cannabidiol | 1 | 2 | No | unscheduled | 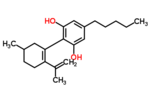
|
See also: Tetrahydrocannabinol#Isomerism, Abnormal cannabidiol.
Society and culture
Natural sources
Selective breeding by growers in the USA dramatically lowered the CBD content of cannabis; their customers preferred varietals that were more mind-altering due to a higher THC, lower CBD content.[51] To meet the demands of medical cannabis patients, growers are currently developing more CBD-dominant strains.[52]
Counterfeits
Hemp Industries Association said in 2014 that hemp seed oil is less than 25 parts per million CBD, thus hemp oil is not a source of CBD which could be used in epilepsy treatments.[53]
Legal status
CBD does not appear to be have any psychoactive ("high") effects such as those caused by ∆9-THC in marijuana, but may have effects on anxiety and anti-psychotic effect.[7] As the legal landscape and understanding about the differences in medical cannabinoids unfolds, it will be increasingly important to distinguish “medical marijuana” (with noted varying degrees of psychotropic effects and deficits in executive function) – from “medical CBD”.[7][54]
Various breeds/strains of "medical marijuana" are found to have a significant variety in the ratios of CBD-to-THC and are known to contain other non-psychotropic cannabinoids.[55] However it is only the amount of ∆9-THC that chemically gives a legal determination as to whether the plant material(s) used for the purposes of extracting CBD are considered hemp, or considered marijuana.[citation needed]
Any psychoactive marijuana, regardless of its CBD content, is derived from the flower (or bud) of the genus cannabis. Non-psychoactive hemp (also commonly-termed industrial hemp), regardless of its CBD content, is any part of the genus cannabis plant, whether growing or not, containing a ∆-9 tetrahydrocannabinol concentration of no more than three-tenths of one percent (0.3%) on a dry weight basis. Certain standards are required for the legal growth and production of hemp. The Colorado Industrial Hemp Program registers growers of industrial hemp and samples crops to verify that the THC concentration does not exceed 0.3% on dry weight basis.[56]
United Nations
Cannabidiol is not scheduled by the Convention on Psychotropic Substances.
U.S.
A CNN program that featured Charlotte's Web cannabis in 2013 brought increased demand for CBD-dominant cannabis across the US. Legislation was passed in Kentucky in 2013 to promote Hemp farming in that state as an economic development program to help tobacco farmers who were losing markets for their crops; the state lobbied for and was able to win a provision in the Agricultural Act of 2014 that legalized hemp production in states like Kentucky.[57][58][59]
As of 2017, at least 31 states had legalized industrial hemp production, including for example: California, Colorado, Indiana, Maine, Montana, North Carolina, North Dakota, Oregon, South Carolina, Tennessee, Vermont, and West Virginia. Many other states have passed legislation authorizing the cultivation of industrial hemp for pilot projects or studies, including: Connecticut, Delaware, Hawaii, Illinois, Kentucky, Nebraska, and Utah. Additionally, the National Association of State Departments of Agriculture and the National Conference of State Legislatures have both adopted resolutions supporting revisions to the federal rules and regulations authorizing commercial production of industrial hemp.[60][61][62]
In the state of Utah, people possessing CBD-dominant hemp extract are exempt from penalties for possession or use of hemp extract under the Controlled Substances Act if they have a neurologist sign an application for a Hemp Extract Registration Card.[63] The neurologist indicates if the patient exhibits signs of intractable epilepsy or if the patient may receive benefit from the use of hemp extract. The law requires the neurologist to keep and transmit data for the Utah Department of Health database of neurologist evaluations.[64] The individual possessing the hemp extract must also obtain the hemp extract's certificate of analysis from the seller.[65]
Australia
Prescription Medicine (Schedule 4) for therapeutic use containing 2 per cent (2.0%) or less of other cannabinoids commonly found in cannabis (such as ∆9-THC).[66]
New Zealand
Cannabidiol is currently a class B1 controlled drug in New Zealand under the Misuse of Drugs Act. It is also a prescription medicine under the Medicines Act. In 2017 the rules were changed so that anyone wanting to use it could go to the Health Ministry for approval. Prior to this, the only way to obtain a prescription was to seek the personal approval of the Minister of Health.
Associate Health Minister Peter Dunne said restrictions would be removed, which means a doctor will now be able to prescribe cannabidiol to patients.[67]
Canada
Cannabidiol is a Schedule II drug in Canada. As such, it is only available with a prescription.[68]
Europe
Cannabidiol is listed in the EU Cosmetics Ingredient Database.[69]
Cannabidiol is listed in the EU Novel Food Catalogue.[70] This listing only applies to isolated or synthetic CBD, not to crude hemp extracts or tinctures naturally containing CBD.
The European Industrial Hemp Association has issued a position paper suggesting regulatory framework in EU.[71]
Several industrial hemp varieties can be legally cultivated in western Europe. A variety such as "Fedora 17" has a cannabinoid profile consistently around 1% cannabidiol (CBD) with THC less than 0.1%.[72]
Although the World Health Organization listed Cannabidiolum in a list of International Nonproprietary Names for Pharmaceutical Substances (INN) on 30 June 2016. French and Spanish versions wrongly mention agonist action of CBD on cannabinoid receptors while the English version says CBD is a cannabinoid receptor antagonist.[citation needed]
Sweden
CBD is not classified in Sweden.
Sativex (CBD and THC) is a prescription product available for relief of severe spasticity due to multiple sclerosis.[73][74]
UK
Cannabidiol, in an oral-mucosal spray formulation combined with delta-9-tetrahydrocannabinol, is a prescription product available for relief of severe spasticity due to multiple sclerosis (where other anti-spasmodics have not been effective).[75]
As of 31 December 2016, products containing cannabidiol that are marketed for medical purposes are classed as medicines by the UK regulatory body, the Medicines and Healthcare products Regulatory Agency (MHRA) and cannot be marketed without regulatory approval for the medical claims.[76]
Switzerland
While THC remains illegal, CBD is not subjected by the swiss Narcotic Acts because this substance does not produce a comparable psychoactive effect. [77] Cannabis products containing less than 1% THC can be sold and purchased legally.[78]
References
- ^ a b Mechoulam R, Parker LA, Gallily R (November 2002). "Cannabidiol: an overview of some pharmacological aspects". J Clin Pharmacol (Review). 42 (11 Suppl): 11S–19S. doi:10.1177/0091270002238789. PMID 12412831.
- ^ Scuderi C, Filippis DD, Iuvone T, Blasio A, Steardo A, Esposito G (May 2009). "Cannabidiol in medicine: a review of its therapeutic potential in CNS disorders". Phytother Res (Review). 23 (5): 597–602. doi:10.1002/ptr.2625. PMID 18844286.
- ^ "International Nonproprietary Names for Pharmaceutical Substances (INN)" (PDF). WHO Drug Information. 30 (2): 241. 2016.
- ^ Borgelt LM, Franson KL, Nussbaum AM, Wang GS (February 2013). "The pharmacologic and clinical effects of medical cannabis". Pharmacotherapy (Review). 33 (2): 195–209. doi:10.1002/phar.1187. PMID 23386598.
- ^ Aizpurua-Olaizola, Oier; Soydaner, Umut; Öztürk, Ekin; Schibano, Daniele; Simsir, Yilmaz; Navarro, Patricia; Etxebarria, Nestor; Usobiaga, Aresatz (February 2, 2016). "Evolution of the Cannabinoid and Terpene Content during the Growth ofCannabis sativaPlants from Different Chemotypes". Journal of Natural Products. 79 (2): 324–331. doi:10.1021/acs.jnatprod.5b00949. PMID 26836472.
- ^ a b c Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimarães FS (December 2012). "Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders". Philos. Trans. R. Soc. Lond., B, Biol. Sci. (Review). 367 (1607): 3364–78. doi:10.1098/rstb.2011.0389. PMC 3481531. PMID 23108553.
- ^ a b c Iseger TA, Bossong MG (2015). "A systematic review of the antipsychotic properties of cannabidiol in humans". Schizophr. Res. 162 (1–3): 153–61. doi:10.1016/j.schres.2015.01.033. PMID 25667194.
- ^ Longo, Dan L.; Friedman, Daniel; Devinsky, Orrin (September 10, 2015). "Cannabinoids in the Treatment of Epilepsy". New England Journal of Medicine. 373 (11): 1048–1058. doi:10.1056/NEJMra1407304.
- ^ Welty, Timothy E.; Luebke, Adrienne; Gidal, Barry E. (September 2014). "Cannabidiol: Promise and Pitfalls". Epilepsy Currents. 14 (5): 250–252. doi:10.5698/1535-7597-14.5.250.
- ^ Gloss, D; Vickrey, B (March 5, 2014). "Cannabinoids for epilepsy". The Cochrane database of systematic reviews (3): CD009270. PMID 24595491.
- ^ "Cannabis-Derived Dravet Syndrome Drug Gets US Orphan Drug Approval". November 18, 2013. Retrieved July 21, 2015.
- ^ Devinsky, Orrin; Cilio, Maria Roberta; Cross, Helen; Fernandez-Ruiz, Javier; French, Jacqueline; Hill, Charlotte; Katz, Russell; Di Marzo, Vincenzo; Jutras-Aswad, Didier; Notcutt, William George; Martinez-Orgado, Jose; Robson, Philip J.; Rohrback, Brian G.; Thiele, Elizabeth; Whalley, Benjamin; Friedman, Daniel (June 2014). "Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders". Epilepsia. 55 (6): 791–802. doi:10.1111/epi.12631.
- ^ "What is Dravet Syndrome?". Dravetfoundation.org. June 20, 2014. Retrieved December 4, 2016.
- ^ Maa, Edward; Figi, Paige (2014). "The case for medical marijuana in epilepsy". Epilepsia. 55 (6): 783–786. doi:10.1111/epi.12610. ISSN 0013-9580.
- ^ Young, Saundra. "Marijuana stops child's severe seizures" (PDF). CNN. CNN. Retrieved January 7, 2016.
- ^ Melville, Nancy A. (August 14, 2013), Seizure Disorders Enter Medical Marijuana Debate, Medscape Medical News., retrieved January 14, 2014
{{citation}}: Italic or bold markup not allowed in:|publisher=(help) - ^ Throckmorton, Douglas (June 24, 2015). "Cannabidiol: Barriers to Research and Potential Medical Benefits". FDA. FDA. Retrieved December 15, 2015.
- ^ Angus, Chen (December 8, 2015). "Marijuana's Main Ingredient, Cannabidiol, May Be An Effective Way To Treat Epilepsy". Medical Daily. Retrieved December 14, 2015.
- ^ Leweke FM, Mueller JK, Lange B, Rohleder C (2016). "Therapeutic Potential of Cannabinoids in Psychosis". Biol. Psychiatry. 79 (7): 604–12. doi:10.1016/j.biopsych.2015.11.018. PMID 26852073.
- ^ Schubart CD, Sommer IE, Fusar-Poli P, de Witte L, Kahn RS, Boks MP (2013). "Cannabidiol as a potential treatment for psychosis". European Neuropsychopharmacology. 24 (1): 51–64. doi:10.1016/j.euroneuro.2013.11.002. PMID 24309088.
- ^ Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, et al. (June 2014). "Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders". Epilepsia. 55 (6): 791–802. doi:10.1111/epi.12631. PMC 4707667. PMID 24854329.
- ^ Zuardi AW, Crippa JA, Hallak JE, Moreira FA, Guimarães FS (2006). "Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug". Braz. J. Med. Biol. Res. 39 (4): 421–9. PMID 16612464.
- ^ Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO (August 2007). "Cannabidiol—recent advances". Chem. Biodivers. (Review). 4 (8): 1678–92. doi:10.1002/cbdv.200790147. PMID 17712814.
- ^ Pertwee RG (2008). "The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin". British Journal of Pharmacology. 153 (2): 199–215. doi:10.1038/sj.bjp.0707442. PMC 2219532. PMID 17828291.
- ^ Hayakawa K, Mishima K, Hazekawa M, Sano K, Irie K, Orito K, Egawa T, Kitamura Y, Uchida N, Nishimura R, Egashira N, Iwasaki K, Fujiwara M (2008). "Cannabidiol potentiates pharmacological effects of Δ9-tetrahydrocannabinol via CB1 receptor-dependent mechanism". Brain Research. 1188: 157–164. doi:10.1016/j.brainres.2007.09.090. PMID 18021759.
- ^ Alchimia Blog, Cannabinoids and their medicinal properties
- ^ Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ (2007). "The orphan receptor GPR55 is a novel cannabinoid receptor". British Journal of Pharmacology. 152 (7): 1092–101. doi:10.1038/sj.bjp.0707460. PMC 2095107. PMID 17876302.
- ^ Russo EB, Burnett A, Hall B, Parker KK (August 2005). "Agonistic properties of cannabidiol at 5-HT1a receptors". Neurochemical Research. 30 (8): 1037–43. doi:10.1007/s11064-005-6978-1. PMID 16258853.
- ^ Zanelati TV, Biojone C, Moreira FA, Guimarães FS, Joca SR (January 2010). "Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT1A receptors". British Journal of Pharmacology. 159 (1): 122–8. doi:10.1111/j.1476-5381.2009.00521.x. PMC 2823358. PMID 20002102.
- ^ a b Resstel LB, Tavares RF, Lisboa SF, Joca SR, Corrêa FM, Guimarães FS (January 2009). "5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats". British Journal of Pharmacology. 156 (1): 181–8. doi:10.1111/j.1476-5381.2008.00046.x. PMC 2697769. PMID 19133999.
- ^ Campos AC, Guimarães FS (August 2008). "Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats". Psychopharmacology. 199 (2): 223–30. doi:10.1007/s00213-008-1168-x. PMID 18446323.
- ^ Mishima K, Hayakawa K, Abe K, Ikeda T, Egashira N, Iwasaki K, Fujiwara M (May 2005). "Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine1A receptor-dependent mechanism". Stroke; a Journal of Cerebral Circulation. 36 (5): 1077–82. doi:10.1161/01.STR.0000163083.59201.34. PMID 15845890.
- ^ Hayakawa K, Mishima K, Nozako M, Ogata A, Hazekawa M, Liu AX, Fujioka M, Abe K, Hasebe N, Egashira N, Iwasaki K, Fujiwara M (March 2007). "Repeated treatment with cannabidiol but not Delta9-tetrahydrocannabinol has a neuroprotective effect without the development of tolerance". Neuropharmacology. 52 (4): 1079–87. doi:10.1016/j.neuropharm.2006.11.005. PMID 17320118.
- ^ Kathmann M, Flau K, Redmer A, Tränkle C, Schlicker E (2006). "Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors". Naunyn-Schmiedeberg's Archives of Pharmacology. 372 (5): 354–361. doi:10.1007/s00210-006-0033-x. PMID 16489449.
- ^ Ujváry I, Hanus L (2014). "Human metabolites of cannabidiol: a review on their formation, biological activity, and relevance in therapy". Cannabis and Cannabinoid Research. 1 (1): 90–101. doi:10.1089/can.2015.0012.
- ^ Bornheim LM, Kim KY, Li J, Perotti BY, Benet LZ (August 1995). "Effect of cannabidiol pretreatment on the kinetics of tetrahydrocannabinol metabolites in mouse brain". Drug Metabolism and Disposition. 23 (8): 825–831. PMID 7493549.
- ^ Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, Gunasekaran N, Karl T, Long LE, Huang XF, Liu K, Arnold JC, McGregor IS (November 2011). "Cannabidiol potentiates Δ⁹-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats". Psychopharmacology. 218 (2): 443–457. doi:10.1007/s00213-011-2342-0. PMID 21667074.
- ^ Hunt CA, Jones RT, Herning RI, Bachman J (June 1981). "Evidence that Cannabidiol Does Not Significantly Alter the Pharmacokinetics of Tetrahydrocannabinol in Man". Journal of Pharmacokinetics and Biopharmaceutics. 9 (3): 245–260. doi:10.1007/BF01059266. PMID 6270295.
- ^ United States Adopted Names Council: Statement on a nonproprietary name
- ^ "Fact Sheet — Sativex". Health Canada. Retrieved May 16, 2013.
- ^ "Cannabis-Derived Dravet Syndrome Drug Gets US Orphan Drug Approval". November 18, 2013. Retrieved July 21, 2015.
- ^ Torres, Kristina (September 30, 2015). "Georgia doctors encouraged in study of medical marijuana". The Atlanta Journal.
- ^ Jones PG, Falvello L, Kennard O, Sheldrick GM, Mechoulam R (1977). "Cannabidiol". Acta Crystallogr. B. 33 (10): 3211–3214. doi:10.1107/S0567740877010577.
- ^ Mechoulam R, Ben-Zvi Z, Gaoni Y (1968). "Hashish—XIII On the nature of the beam test". Tetrahedron. 24 (16): 5615–5624. doi:10.1016/0040-4020(68)88159-1. PMID 5732891.
- ^ Gaoni Y, Mechoulam R (1966). "Hashish—VII The isomerization of cannabidiol to tetrahydrocannabinols". Tetrahedron. 22 (4): 1481–1488. doi:10.1016/S0040-4020(01)99446-3.
- ^ Petrzilka T, Haefliger W, Sikemeier C, Ohloff G, Eschenmoser A (1967). "Synthese und Chiralität des (−)-Cannabidiols". Helv. Chim. Acta. 50 (2): 719–723. doi:10.1002/hlca.19670500235. PMID 5587099.
- ^ Gaoni Y, Mechoulam R (1985). "Boron trifluoride etherate on alumuna — a modified Lewis acid reagent. An improved synthesis of cannabidiol". Tetrahedron Letters. 26 (8): 1083–1086. doi:10.1016/S0040-4039(00)98518-6.
- ^ Kobayashi Y, Takeuchi A, Wang YG (2006). "Synthesis of cannabidiols via alkenylation of cyclohexenyl monoacetate". Org. Lett. 8 (13): 2699–2702. doi:10.1021/ol060692h. PMID 16774235.
- ^ Marks MD, Tian L, Wenger JP, Omburo SN, Soto-Fuentes W, He J, Gang DR, Weiblen GD, Dixon RA (2009). "Identification of candidate genes affecting Δ9-tetrahydrocannabinol biosynthesis in Cannabis sativa". Journal of Experimental Botany. 60 (13): 3715–3726. doi:10.1093/jxb/erp210. PMC 2736886. PMID 19581347.
- ^ Taura, F., Sirikantaramas, S., Shoyama, Y., Yoshikai, K., Shoyama, Y., Morimoto, S. (2007). "Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa". FEBS Letters. 581 (16): 2929–34. doi:10.1016/j.febslet.2007.05.043. PMID 17544411.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Romney, Lee (September 13, 2012). "On the frontier of medical pot to treat boy's epilepsy". Los Angeles Times.
- ^ Good, Alastair (October 26, 2010). "Growing marijuana that won't get you high". The Daily Telegraph. London.
- ^ Jones, Whitney. "Hemp Oil Not a Source of CBD Which Could Be Used in Epilepsy Treatments".
- ^ Sachs J, McGlade E, Yurgelun-Todd D (October 2015). "Safety and Toxicology of Cannabinoids". Neurotherapeutics. 12 (4): 735–746. doi:10.1007/s13311-015-0380-8. PMC 4604177. PMID 26269228.
- ^ Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R (2009). "Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb" (PDF). Trends Pharmacol. Sci. 30 (10): 515–27. doi:10.1016/j.tips.2009.07.006. PMID 19729208.
- ^ "Industrial Hemp | Department of Agriculture – Plants". Colorado.gov. Retrieved December 4, 2016.
- ^ Firger, Jessica (October 23, 2015). "The Great Kentucky Hemp Experiment". Newsweek.com. Retrieved December 4, 2016.
- ^ Shear, Michael D. (February 7, 2014) In Signing Farm Bill, Obama Extols Rural Growth. New York Times.
- ^ Patton, Janet (May 5, 2015). "Hemp industry is growing in Kentucky, attracting processors, investment | Lexington Herald-Leader". Kentucky.com. Retrieved December 4, 2016.
- ^ An act relating to authorizing the growing of industrial hemp (PDF) (SSB 5012). March 23, 2015. Retrieved May 1, 2017.
- ^ Engrossed Substitute Senate Bill 6206 (PDF). March 1, 2016. Retrieved May 1, 2017.
- ^ State Industrial Hemp Statutes. U.S. National Conference of State Legislatures (August 19, 2016)
- ^ https://health.utah.gov/wp-content/uploads/HempExtractNew.pdf
- ^ http://le.utah.gov/~2014/bills/static/HB0105.html
- ^ http://health.utah.gov/hempregistry/
- ^ "Poisons Standard March 2016". Legislation.gov.au. Retrieved December 4, 2016.
- ^ "Doctors now able to prescribe cannabidiol". radionz.co.nz. Retrieved June 2, 2017.
- ^ "Controlled Drugs and Substances Act – Schedule II". Laws-lois.justice.gc.ca. Retrieved December 4, 2016.
- ^ "CosIng – Cosmetics – GROWTH – European Commission". Ec.europa.eu. Retrieved December 4, 2016.
- ^ "Food - European Commission".
- ^ "Support the EIHA CBD position paper - EIHA European Industrial Hemp Association".
- ^ Fournier, G.; Beherec, O.; Bertucelli, S. (2003). "Intérêt du rapport Δ-9-THC / CBD dans le contrôle des cultures de chanvre industriel". Annales de Toxicologie Analytique. 15 (4): 250–259. doi:10.1051/ata/2003003.
- ^ https://lakemedelsverket.se/LMF/?q=Sativex
- ^ https://www.fass.se/LIF/product?userType=2&nplId=20101019000051
- ^ "Sativex Oromucosal Spray – Summary of Product Characteristics (SPC) – (eMC)". Medicines.org.uk. Retrieved December 4, 2016.
- ^ "MHRA statement on products containing Cannabidiol (CBD)". Gov.uk. December 14, 2016.
- ^ "Products containing cannabidiol (CBD) – overview". swissmedic.ch. Retrieved May 20, 2017.
- ^ "Cannabis à faible teneur en THC et CBD". bag.admin.ch. Retrieved May 20, 2017.
External links
- Project CBD Non-profit educational service dedicated to promoting and publicizing research into the medical utility of cannabidiol.
