Nitrous oxide: Difference between revisions
Updating {{chembox}} (changes to verified fields - updated 'DrugBank_Ref', 'ChEMBL_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report errors or [[user |
No edit summary Tags: Mobile edit Mobile web edit |
||
| Line 1: | Line 1: | ||
{{Short description|Colourless non-flammable gas}} |
|||
{{Redirect|N2O}} |
|||
{{cs1 config|name-list-style=vanc|display-authors=6}} |
|||
{{see also|Recreational use of nitrous oxide}} |
|||
{{Redirect|N2O|3=Nitrous oxide (disambiguation)|4=and|5=N2O (disambiguation)}} |
|||
{{Redirect|Laughing gas}} |
{{Redirect|Laughing gas}} |
||
{{ |
{{Distinguish|text=[[nitric oxide]] ({{chem|NO}}), [[nitrogen dioxide]] ({{chem|NO|2}}), or generic nitrogen oxide pollutants [[NOx]]}} |
||
{{Use British English|date=December 2018}} |
|||
{{Other uses}} |
|||
{{Use dmy dates|date=December 2018}} |
|||
For Other uses, see [[NOS]]. |
|||
{{Chembox |
|||
{{chembox |
|||
| Verifiedfields = changed |
| Verifiedfields = changed |
||
| Watchedfields = changed |
|||
| verifiedrevid = 400329520 |
|||
| verifiedrevid = 477162830 |
|||
| ImageFile = Nitrous-oxide-3D-vdW.png |
|||
| ImageFile_Ref = {{chemboximage|correct|??}} |
|||
| ImageSize = 150px |
|||
| Name = |
|||
| ImageName = Nitrous oxide – space-filling model |
|||
| |
| ImageFile = Nitrous-oxide-2D-VB.svg |
||
| ImageName = Nitrous oxide's canonical forms |
|||
| ImageSize1 = 200px |
|||
| ImageFile1 = Nitrous-oxide-dimensions-3D-balls.png |
|||
| ImageName1 = Nitrous oxide's bond lengths |
|||
| ImageName1 = Ball-and-stick model with bond lengths |
|||
| ImageFile2_Ref = {{chemboximage|correct|??}} |
|||
| ImageFile2 = Nitrous-oxide- |
| ImageFile2 = Nitrous-oxide-3D-vdW.png |
||
| ImageSize2 = |
| ImageSize2 = 150px |
||
| ImageName2 = |
| ImageName2 = Space-filling model of nitrous oxide |
||
| SystematicName = Oxodiazen-2-ium-1-ide |
|||
| IUPACName = Dinitrogen monoxide |
|||
| IUPACName = Nitrous oxide<ref>{{Cite web|url=https://www.degruyter.com/database/IUPAC/entry/iupac.compound.948/html|title=[Nitrous oxide]|website=Degruyter.com|access-date=24 July 2022}}</ref> ''(not recommended)''<br />Dinitrogen oxide<ref>[[IUPAC nomenclature of inorganic chemistry 2005]]. [http://old.iupac.org/publications/books/rbook/Red_Book_2005.pdf PDF], p. 317.</ref> ''(alternative name)'' |
|||
| OtherNames = Laughing gas, sweet air |
|||
| OtherNames = {{Unbulleted list|Laughing gas|sweet air|nitrous|nos|protoxide of nitrogen|hyponitrous oxide|dinitrogen oxide|dinitrogen monoxide}} |
|||
| Section1 = {{Chembox Identifiers |
|||
| Section1 = {{Chembox Identifiers |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
| UNII_Ref = {{fdacite|correct|FDA}} |
||
| UNII = K50XQU1029 |
| UNII = K50XQU1029 |
||
| Line 26: | Line 29: | ||
| InChIKey1 = GQPLMRYTRLFLPF-UHFFFAOYAP |
| InChIKey1 = GQPLMRYTRLFLPF-UHFFFAOYAP |
||
| SMILES = N#[N+][O-] |
| SMILES = N#[N+][O-] |
||
| SMILES1 = [N-]=[N+]=O |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChI = 1S/N2O/c1-2-3 |
| StdInChI = 1S/N2O/c1-2-3 |
||
| Line 32: | Line 36: | ||
| CASNo_Ref = {{cascite|correct|CAS}} |
| CASNo_Ref = {{cascite|correct|CAS}} |
||
| CASNo = 10024-97-2 |
| CASNo = 10024-97-2 |
||
| CAS_Ref = {{cascite|correct|??}} |
|||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
||
| ChemSpiderID = 923 |
| ChemSpiderID = 923 |
||
| ChEMBL_Ref = {{ebicite|changed|EBI}} |
|||
| ChEMBL = 1234579 |
|||
| DrugBank_Ref = {{drugbankcite|changed|drugbank}} |
|||
| DrugBank = DB06690 |
|||
| PubChem = 948 |
| PubChem = 948 |
||
| InChI =1/N2O/c1-2-3 |
| InChI = 1/N2O/c1-2-3 |
||
| UNNumber = 1070 (compressed)<br/>2201 (liquid) |
| UNNumber = 1070 (compressed)<br />2201 (liquid) |
||
| RTECS = QX1350000 |
| RTECS = QX1350000 |
||
| MeSHName = |
| MeSHName = |
||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
| ChEBI_Ref = {{ebicite|correct|EBI}} |
||
| ChEBI = 17045 |
| ChEBI = 17045 |
||
| KEGG_Ref = {{keggcite| |
| KEGG_Ref = {{keggcite|correct|kegg}} |
||
| KEGG = D00102 |
| KEGG = D00102 |
||
| Beilstein = 8137358 |
|||
| ATCCode_prefix = N01 |
|||
| Gmelin = 2153410 |
|||
| ATCCode_suffix = AX13 |
|||
}} |
}} |
||
| Section2 = {{Chembox Properties |
| Section2 = {{Chembox Properties |
||
| Formula = N |
| Formula = {{chem|N|2|O}} |
||
| MolarMass = 44.013 g/mol |
| MolarMass = 44.013 g/mol |
||
| Appearance = |
| Appearance = colourless gas |
||
| Density = 1.977 g/L (gas) |
| Density = 1.977 g/L (gas) |
||
| |
| MeltingPtC = −90.86 |
||
| MeltingPt_notes = |
|||
| BoilingPt = –88.48 °C (184.67 K) |
|||
| BoilingPtC = −88.48 |
|||
| Solubility = 0.15 g/100 ml (15 °C) |
|||
| BoilingPt_notes = |
|||
| SolubleOther = soluble in [[alcohol]], [[ether]], [[sulfuric acid]] |
|||
| Solubility = 1.5 g/L (15 °C) |
|||
| SolubleOther = soluble in [[ethanol|alcohol]], [[diethyl ether|ether]], [[sulfuric acid]] |
|||
| LogP = 0.35 |
| LogP = 0.35 |
||
| RefractIndex = 1. |
| RefractIndex = 1.000516 (0 °C, 101.325 kPa) |
||
| Viscosity = 14.90 μPa·s<ref name="TakahashiShibasaki-Kitakawa1996">{{cite journal|last1=Takahashi|first1=Mitsuo|last2=Shibasaki-Kitakawa|first2=Naomi|last3=Yokoyama|first3=Chiaki|last4=Takahashi|first4=Shinji|title=Viscosity of Gaseous Nitrous Oxide from 298.15 K to 398.15 K at Pressures up to 25 MPa|journal=Journal of Chemical & Engineering Data|volume=41|issue=6|year=1996|pages=1495–1498|issn=0021-9568|doi=10.1021/je960060d}}</ref> |
|||
| VaporPressure = 5150 kPa (20 °C) |
| VaporPressure = 5150 kPa (20 °C) |
||
| HenryConstant = |
| HenryConstant = |
||
| AtmosphericOHRateConstant = |
| AtmosphericOHRateConstant = |
||
| MagSus = −18.9·10<sup>−6</sup> cm<sup>3</sup>/mol |
|||
}} |
}} |
||
| Section3 = {{Chembox Structure |
| Section3 = {{Chembox Structure |
||
| MolShape = linear, ''C'' |
| MolShape = linear, ''C''{{ssub|∞v}} |
||
| Dipole = 0.166 [[Debye|D]] |
| Dipole = 0.166 [[Debye|D]] |
||
}} |
}} |
||
| Section4 |
| Section4 = |
||
| Section5 = {{Chembox Thermochemistry |
|||
| DeltaHf = +82.05 kJ/mol |
| DeltaHf = +82.05 kJ/mol |
||
| Entropy = 219.96 J |
| Entropy = 219.96 J/(K·mol) |
||
}} |
}} |
||
| |
| Section6 = {{Chembox Pharmacology |
||
| ATCCode_prefix = N01 |
|||
| ATCCode_suffix = AX13 |
|||
| AdminRoutes = [[Inhalation]] |
| AdminRoutes = [[Inhalation]] |
||
| Bioavail = |
| Bioavail = |
||
| Line 77: | Line 91: | ||
| ProteinBound = |
| ProteinBound = |
||
| Excretion = [[Respiratory]] |
| Excretion = [[Respiratory]] |
||
| Legal_status = <!-- All U.S. states except for [[California]] allow nitrous oxide as an [[ |
| Legal_status = <!-- All U.S. states except for [[California]] allow nitrous oxide as an [[anaesthetic]]. Also used in the [[United Kingdom]] and [[Australia]]. --> |
||
| Legal_US = |
|||
| Legal_US_comment = |
|||
| Legal_UK = |
| Legal_UK = |
||
| Legal_AU = |
| Legal_AU = |
||
| Legal_CA = |
| Legal_CA = |
||
| Pregnancy_category = |
|||
| PregCat = |
|||
| |
| Pregnancy_AU = |
||
| PregCat_US = C |
|||
}} |
}} |
||
| Section7 = {{Chembox Hazards |
| Section7 = {{Chembox Hazards |
||
| |
| ExternalSDS = [http://www.inchem.org/documents/icsc/icsc/eics0067.htm Ilo.org], ICSC 0067 |
||
| GHSPictograms = {{GHS gas cylinder}} {{GHS flame over circle}} |
|||
| EUIndex = Oxidant ['''O'''] |
|||
| GHSSignalWord = Danger |
|||
| EUClass = |
|||
| HPhrases = {{H-phrases|270|280|281}} |
|||
| RPhrases = |
|||
| PPhrases = {{P-phrases|220|244|282|336|317|370+376|403|410+403}} |
|||
| SPhrases = |
|||
| MainHazards = |
| MainHazards = |
||
| NFPA-H = 2 |
| NFPA-H = 2 |
||
| NFPA-F = 0 |
| NFPA-F = 0 |
||
| NFPA-R = 0 |
| NFPA-R = 0 |
||
| NFPA- |
| NFPA-S = OX |
||
| FlashPt = |
| FlashPt = Nonflammable |
||
| LD50 = |
| LD50 = |
||
| PEL = |
| PEL = |
||
}} |
}} |
||
| Section8 = {{Chembox Related |
| Section8 = {{Chembox Related |
||
| |
| OtherFunction = [[Nitric oxide]]<br />[[Dinitrogen trioxide]]<br />[[Nitrogen dioxide]]<br />[[Dinitrogen tetroxide]]<br />[[Dinitrogen pentoxide]] |
||
| |
| OtherFunction_label = [[nitrogen]] [[oxide]]s |
||
| |
| OtherCompounds = [[Ammonium nitrate]]<br />[[Azide]] |
||
}} |
}} |
||
}} |
}} |
||
'''Nitrous oxide''' (dinitrogen oxide or dinitrogen monoxide), commonly known as '''laughing gas''', '''nitrous''', '''nitro''', or '''nos''',<ref>{{cite book |title=Let's review: chemistry, the physical setting |edition=3rd |first1=Albert S. |last1=Tarendash |publisher=Barron's Educational Series |year=2001 |isbn=978-0-7641-1664-3 |page=[https://archive.org/details/letsreviewchemis03edtare/page/44 44] |url=https://archive.org/details/letsreviewchemis03edtare |url-access=registration }}</ref> is a [[chemical compound]], an [[Nitrogen oxide|oxide of nitrogen]] with the [[Chemical formula|formula]] {{chem|N|2|O}}. At room temperature, it is a colourless [[Flammability#Definitions|non-flammable]] [[gas]], and has a slightly sweet scent and taste.<ref>{{Cite web |last=PubChem |title=Nitrous oxide |url=https://pubchem.ncbi.nlm.nih.gov/compound/948 |access-date=2022-03-29 |website=pubchem.ncbi.nlm.nih.gov |language=en}}</ref> At elevated temperatures, nitrous oxide is a powerful [[Oxidising agent|oxidiser]] similar to molecular oxygen. |
|||
'''Nitrous oxide''', commonly known as '''laughing gas''' or '''sweet air''',<ref>{{cite book |
|||
|title=Let's review: chemistry, the physical setting |
|||
|edition=3rd |
|||
|first1=Albert S. |
|||
|last1=Tarendash |
|||
|publisher=Barron's Educational Series |
|||
|year=2001 |
|||
|isbn=0-764-11664-9 |
|||
|page=44 |
|||
|url=http://books.google.com/books?id=aOij0MVjsy0C}}, [http://books.google.com/books?id=aOij0MVjsy0C&pg=PA44 Extract of page 44] |
|||
</ref> is a [[chemical compound]] with the [[chemical formula|formula]] {{chem|N|2|O}}. It is an [[oxide]] of [[nitrogen]]. At room temperature, it is a colorless [[Flammability|non-flammable]] [[gas]], with a slightly sweet odor and taste. It is used in [[surgery]] and [[dentistry]] for its [[Anesthesia|anesthetic]] and [[analgesic]] effects. It is known as "laughing gas" due to the [[Euphoria|euphoric]] effects of inhaling it, a property that has led to its [[recreational drug use|recreational use]] as a [[dissociative]] [[anesthetic]]. It is also used as an [[Oxidizing agent|oxidizer]] in [[rocket]]ry and in [[Auto racing|motor racing]] to increase the power output of [[Piston engine|engines]]. At elevated temperatures, nitrous oxide is a powerful oxidizer similar to molecular oxygen. |
|||
Nitrous oxide has significant [[Nitrous oxide (medication)|medical uses]], especially in [[surgery]] and [[dentistry]], for its [[Anesthesia|anaesthetic]] and [[Analgesic|pain-reducing]] effects.<ref name="ACB 2020">{{cite journal |author1-last=Quax |author1-first=Marcel L. J. |author2-last=Van Der Steenhoven |author2-first=Timothy J. |author3-last=Bronkhorst |author3-first=Martinus W. G. A. |author4-last=Emmink |author4-first=Benjamin L. |date=July 2020 |title=Frostbite injury: An unknown risk when using nitrous oxide as a party drug |journal=Acta Chirurgica Belgica |publisher=[[Taylor & Francis]] on behalf of the Royal Belgian Society for Surgery |volume=120 |issue=1–4 |pages=140–143 |doi=10.1080/00015458.2020.1782160 |issn=0001-5458 |pmid=32543291 |s2cid=219702849}}</ref> Its colloquial name, "laughing gas", coined by [[Humphry Davy]], is due to the [[Euphoria|euphoric]] effects upon inhaling it, a property that has led to its [[Recreational use of nitrous oxide|recreational use]] as a [[Dissociative drug|dissociative]] anaesthetic.<ref name="ACB 2020"/> It is on the [[WHO Model List of Essential Medicines|World Health Organization's List of Essential Medicines]].<ref name="WHO21st">{{cite book | title = World Health Organization model list of essential medicines: 21st list 2019 | year = 2019 | hdl = 10665/325771 | publisher = World Health Organization | location = Geneva | hdl-access=free | last1 = Organization | first1 = World Health }}</ref> It is also used as an oxidiser in [[rocket propellant]]s, and in [[auto racing|motor racing]] to increase the [[engine power|power output]] of [[petrol engine|engines]]. |
|||
Nitrous oxide gives rise to NO (nitric oxide) on reaction with oxygen atoms, and this NO in turn reacts with [[ozone]]. As a result, it is the main naturally occurring regulator of [[stratosphere|stratospheric]] ozone. |
|||
Nitrous oxide's atmospheric concentration reached 333 [[Parts-per notation|parts per billion]] (ppb) in 2020, increasing at a rate of about 1 ppb annually.<ref name="agage" /><ref name="noaaesrl" /> It is a major scavenger of [[ozone layer|stratospheric ozone]], with an impact comparable to that of [[chlorofluorocarbon|CFCs]].<ref name="sciozo"/> About 40% of human-caused emissions are [[Greenhouse gas emissions from agriculture#Nitrous oxide emissions|from agriculture]].<ref name="HTian">{{cite journal |last1=Tian |first1=Hanqin |last2=Xu |first2=Rongting |last3=Canadell |first3=Josep G. |last4=Thompson |first4=Rona L. |last5=Winiwarter |first5=Wilfried |last6=Suntharalingam |first6=Parvadha |last7=Davidson |first7=Eric A. |last8=Ciais |first8=Philippe |last9=Jackson |first9=Robert B. |last10=Janssens-Maenhout |first10=Greet |date=October 2020 |title=A comprehensive quantification of global nitrous oxide sources and sinks |url=https://www.nature.com/articles/s41586-020-2780-0 |url-status=bot: unknown |journal=Nature |language=en |volume=586 |issue=7828 |pages=248–256 |bibcode=2020Natur.586..248T |doi=10.1038/s41586-020-2780-0 |issn=1476-4687 |pmid=33028999 |hdl=1871.1/c74d4b68-ecf4-4c6d-890d-a1d0aaef01c9 |s2cid=222217027 |archive-url=https://web.archive.org/web/20201203131716/https://www.nature.com/articles/s41586-020-2780-0 |archive-date=3 December 2020 |access-date=2020-11-09|hdl-access=free }}</ref><ref name=":0">{{cite journal |author=Thompson, R. L. |author2=Lassaletta, L. |author3=Patra, P. K. |title=Acceleration of global N<sub>2</sub>O emissions seen from two decades of atmospheric inversion |journal=Nat. Clim. Change |year=2019 |volume=9 |issue=12 |pages=993–998 |doi=10.1038/s41558-019-0613-7|bibcode=2019NatCC...9..993T |s2cid=208302708 |url=http://pure.iiasa.ac.at/id/eprint/16173/2/N2O_paper_SI_revision2_v1.docx|hdl=11250/2646484 |hdl-access=free }}</ref> Nitrogen is added to the soil via animal urine and dung, and synthetic fertilisers: micro-organisms then release it in nitrous oxide.<ref>{{Cite web |date=2021-12-13 |title=Reduce nitrous oxide emissions |url=https://www.agmatters.nz/goals/reduce-nitrous-oxide/ |access-date=2024-04-01 |website=Ag Matters |language=en}}</ref> Being the third most important [[greenhouse gas]], nitrous oxide substantially contributes to [[global warming]].<ref name="ipccar5">{{cite book |url=https://www.ipcc.ch/report/ar5/wg1/ |contribution= Chapter 8 |title=AR5 Climate Change 2013: The Physical Science Basis |pages=677–678}}</ref><ref name="physorg">{{cite news |title=Nitrous oxide emissions pose an increasing climate threat, study finds |language=en |work=phys.org |url=https://phys.org/news/2020-10-nitrous-oxide-emissions-pose-climate.html |access-date=2020-11-09}}</ref> Reduction of emissions is a popular topic in the [[politics of climate change]].<ref>{{Cite web |last=Mundschenk |first=Susanne |date=3 August 2022 |title=The Netherlands is showing how not to tackle climate change {{!}} The Spectator |url=https://www.spectator.co.uk/article/the-netherlands-is-showing-how-not-to-tackle-climate-change |access-date=2022-08-28 |website=www.spectator.co.uk |language=en}}</ref> |
|||
==History== |
|||
The gas was first synthesized by English [[natural philosopher]] and chemist [[Joseph Priestley]] in 1772, who called it ''phlogisticated nitrous air'' (see [[phlogiston]]).<ref name="Nitrous Oxide pioneers">{{cite journal |url=http://journals.lww.com/anesthesiology/citation/1941/09000/The_Development_of_Anesthesia.8.aspx |author=Keys TE|title=The_Development_of_Anesthesia|work=Anesthesiology journal |year=1941|volume=2|pages=552–574}}</ref> Priestley published his discovery in the book ''Experiments and Observations on Different Kinds of Air (1775)'', where he described how to produce the preparation of "nitrous air diminished", by heating iron filings dampened with [[nitric acid]].<ref name="Joseph Priestley">{{cite web |url=http://www.erowid.org/chemicals/nitrous/nitrous_journal1.shtml|author=Priestley J|title=Experiments and Observations on Different Kinds of Air (vol.2, sec.3)|year=1776}}</ref> |
|||
Nitrous oxide is used as a [[propellent|propellant]], and has a variety of applications from [[rocket]]ry to making whipped cream. It is used as a [[recreational drug]] for its potential to induce a brief "high". Most recreational users are unaware of its [[neurotoxic]] effects when abused. When used chronically, nitrous oxide has the potential to cause neurological damage through inactivation of [[vitamin B12]]. |
|||
===Early use (1794–1843)=== |
|||
The first important use of nitrous oxide was made possible by [[Thomas Beddoes]] and [[James Watt]], who worked together to publish the book ''Considerations on the Medical Use and on the Production of [[Factitious]] Airs (1794)''. This book was important for two reasons. First, James Watt had invented a novel machine to produce "Factitious Airs" (i.e. nitrous oxide) and a novel "breathing apparatus" to inhale the gas. Second, the book also presented the new medical theories by Thomas Beddoes, that [[tuberculosis]] and other lung diseases could be treated by inhalation of "Factitious Airs".<ref name="Drug discovery"/> |
|||
==Uses== |
|||
The machine to produce "Factitious Airs" had three parts: A furnace to burn the needed material, a vessel with water where the produced gas passed through in a spiral pipe (for impurities to be "washed off"), and finally the gas cylinder with a gasometer where the gas produced, 'air,' could be tapped into portable air bags (made of airtight oily silk). The breathing apparatus consisted of one of the portable air bags connected with a tube to a mouthpiece. With this new equipment being engineered and produced by 1794, the way was paved for [[clinical trial]]s,{{Clarify|date=April 2011}} which began when [[Thomas Beddoes]] in 1798 established the ''"[[Pneumatic Institution]] for Relieving Diseases by Medical Airs"'' in [[Clifton, Bristol|Clifton (Bristol)]]. In the basement of the building, a large scale machine was producing the gases under the supervision of a young [[Humphry Davy]], who was encouraged to experiment with new gases for patients to inhale.<ref name="Drug discovery"/> The first important work of Davy was examination of the nitrous oxide, and the publication of his results in the book: ''Researches, Chemical and Philosophical (1800)''. In that publication, Davy notes the analgesic effect of nitrous oxide at page 465 and its potential to be used for surgical operations at page 556.<ref name="Humphry Davy">{{cite book |url=http://books.google.com/?id=jhUAAAAAQAAJ&printsec=frontcover&dq=Researches,+chemical+and+philosophical&cd=1#v=onepage&q |author=Davy H|title=Researches, chemical and philosophical –chiefly concerning nitrous oxide or dephlogisticated nitrous air, and its respiration|year=1800 |publisher=Printed for J. Johnson}}</ref> |
|||
===Rocket motors=== |
|||
Despite Davy's discovery that inhalation of nitrous oxide could relieve a conscious person from pain, another 44 years elapsed before doctors attempted to use it for [[anaesthesia]]. The use of nitrous oxide as a [[recreational drug]] at "laughing gas parties", primarily arranged for the [[Social structure of Britain#Upper class|British upper class]], became an immediate success beginning in 1799. While the effects of the gas generally make the user feel stuporous, dreamy and sedated, some people also "get the giggles" in a state of euphoria, and frequently, erupt in laughter.<ref name="Illicit drugs">{{cite web |url=http://www.druglibrary.org/schaffer/Library/studies/cu/CU43.html|author=Brecher EM|title=Consumers Union Report on Licit and Illicit Drugs, Part VI – Inhalants and Solvents and Glue-Sniffing|work=Consumer Reports Magazine|year=1972}}</ref> |
|||
Nitrous oxide may be used as an [[oxidizing agent|oxidiser]] in a [[rocket]] motor. It has advantages over other oxidisers in that it is much less toxic, and because of its stability at room temperature, it is also easier to store and relatively safe to carry on a flight. As a secondary benefit, it may be decomposed readily to form breathing air. Its high density and low storage pressure (when maintained at low temperatures) enable it to be highly competitive with stored high-pressure gas systems.<ref>{{cite web|author=Berger, Bruno |date=5 October 2007 |url=http://www.spl.ch/publication/SPL_Papers/N2O_safety_e.pdf |archive-url=https://ghostarchive.org/archive/20221009/http://www.spl.ch/publication/SPL_Papers/N2O_safety_e.pdf |archive-date=2022-10-09 |url-status=live |title=Is nitrous oxide safe? |publisher=Swiss Propulsion Laboratory |pages=1–2 |quote=...Self pressurizing (Vapor pressure at 20°C is ~50.1 bar...Nontoxic, low reactivity -> rel. safe handling (General safe ???)...Additional energy from decomposition (as a monopropellant: ISP of 170 s)...Specific impulse doesn't change much with O/F...[page 2] N{{ssub|2}}O is a monopropellant (as H{{ssub|2}}O{{ssub|2}} or Hydrazine...)}}</ref> |
|||
In a 1914 patent, American rocket pioneer [[Robert Goddard]] suggested nitrous oxide and gasoline as possible propellants for a liquid-fuelled rocket.<ref>Goddard, R. H. (1914) "Rocket apparatus" {{US patent|1103503}}</ref> Nitrous oxide has been the oxidiser of choice in several [[hybrid rocket]] designs (using solid fuel with a liquid or gaseous oxidiser). The combination of nitrous oxide with [[hydroxyl-terminated polybutadiene]] fuel has been used by [[SpaceShipOne]] and others. It also is notably used in [[amateur rocketry|amateur]] and [[high power rocket]]ry with various plastics as the fuel. |
|||
===Anesthetic use=== |
|||
{{see|Nitrous oxide and oxygen}} |
|||
The first time nitrous oxide was used as an [[anesthetic]] drug in the treatment of a patient was when dentist [[Horace Wells]], with assistance by [[Gardner Quincy Colton]] and [[John Mankey Riggs]], demonstrated insensitivity to pain from a [[dental extraction]] on 11 December 1844.<ref name="Discovery of Wells">{{cite web |url=http://ukpmc.ac.uk/picrender.cgi?artid=1703729&blobtype=pdf|author=Erving HW|title=The Discoverer of Anæsthesia: Dr. Horace Wells of Hartford|work=Yale Journal of Biology and Medicine, May 1933; v.5, n.5, p.421–430|year=1933}}</ref> In the following weeks, Wells treated the first 12–15 patients with nitrous oxide in [[Hartford]], and according to his own record only failed in two cases.<ref name="Horace Wells">{{cite book |url=http://books.google.com/?id=exNtlBi8T4EC&printsec=frontcover&dq=Horace+Wells#v=onepage&q|author=Wells H|title=A history of the discovery, of the application of nitrous oxide gas, ether, and other vapors, to surgical operations|year=1847 |publisher=J. Gaylord Wells}}</ref> In spite of these convincing results being reported by Wells to the medical society in [[Boston]] already in December 1844, this new method was not immediately adopted by other dentists. The reason for this was most likely that Wells, in January 1845 at his first public demonstration towards the medical faculty in Boston, had been partly unsuccessful, leaving his colleagues doubtful regarding its efficacy and safety.<ref name="Discovery of anaesthesia">{{cite web |url=http://www.ijaweb.org/text.asp?2007/51/6/472/61183|author=Desai SP, Desai MS, Pandav CS|title=The discovery of modern anaesthesia-contributions of Davy, Clarke, Long, Wells and Morton|work=Indian J Anaesth 2007;51:472-8|year=2007}}</ref> The method did not come into general use until 1863, when [[Gardner Quincy Colton]] successfully started to use it in all his "Colton Dental Association" clinics, that he had just established in [[New Haven]] and [[New York City]].<ref name="Drug discovery"/> Over the following three years, Colton and his associates successfully administered nitrous oxide to more than 25,000 patients.<ref name="use in dentistry"/> Today, nitrous oxide is used in dentistry as an [[anxiolytic]], as an adjunct to [[local anesthetic]]. While nitrous oxide does have some anesthetic properties, especially with regards to soft tissues like the gums, it is not suitable for suppressing the pain caused by dental work. |
|||
Nitrous oxide also may be used in a [[monopropellant rocket]]. In the presence of a heated [[catalyst]], {{chem|N|2|O}} will decompose exothermically into nitrogen and oxygen, at a temperature of approximately {{convert|1070|F}}.<ref>[http://spg-corp.com/nitrous-oxide-safety.html Nitrous Oxide Safety]. Space Propulsion Group (2012)</ref> Because of the large heat release, the catalytic action rapidly becomes secondary, as thermal autodecomposition becomes dominant. In a vacuum thruster, this may provide a monopropellant [[specific impulse]] (''I''{{ssub|sp}}) of as much as 180 s. While noticeably less than the ''I''{{ssub|sp}} available from [[hydrazine]] thrusters (monopropellant or [[Bipropellant rocket|bipropellant]] with [[dinitrogen tetroxide]]), the decreased toxicity makes nitrous oxide an option worth investigating. |
|||
In hospitals, nitrous oxide was however found not to be a strong enough [[anesthetic]] for the use in large operations. Being a stronger and more potent anesthetic, [[sulfuric ether]] was instead demonstrated and accepted for use in October 1846, along with [[chloroform]] in 1847.<ref name="Drug discovery"/> When [[Joseph Thomas Clover]] invented the "gas-ether inhaler" in 1876, it however became a common practice at hospitals to initiate all anesthetic treatments with a mild flow of nitrous oxide, and then gradually increase the [[anaesthesia]] with the stronger ether/chloroform. Clover's gas-ether inhaler was designed to supply the patient with nitrous oxide and ether at the same time, with the exact mixture being controlled by the operator of the device. It remained in use by many hospitals until the 1930s.<ref name="use in dentistry"/> Although hospitals today are using a more advanced [[anaesthetic machine]], these machines still use the same principle launched with Clover's gas-ether inhaler: To initiate the anesthesia with nitrous oxide, before the administration of a more powerful anesthetic. |
|||
Nitrous oxide is said to [[deflagration|deflagrate]] at approximately {{convert|600|C}} at a pressure of 309 psi (21 atmospheres).<ref name="Munke">Munke, Konrad (2 July 2001) [http://hobbyspace.com/AAdmin/archive/SpecialTopics/Misc/eindhoven.pdf Nitrous Oxide Trailer Rupture], Report at CGA Seminar "Safety and Reliability of Industrial Gases, Equipment and Facilities", 15–17 October 2001, St. Louis, Missouri</ref> At 600 {{abbr|psi|pounds per square inch}}, for example, the required ignition energy is only 6 joules, whereas {{chem|N|2|O}} at 130 psi a 2,500-joule ignition energy input is insufficient.<ref>{{cite web|url=http://www.scaled.com/images/uploads/news/N2OSafetyGuidelines.pdf |title=Scaled Composites Safety Guidelines for {{chem|N|2|O}} |publisher=Scaled Composites |date=17 June 2009 |archive-url=https://web.archive.org/web/20110712044612/http://www.scaled.com/images/uploads/news/N2OSafetyGuidelines.pdf |access-date=29 December 2013 |archive-date=12 July 2011 |quote=For example, N2O flowing at 130 psi in an epoxy composite pipe would not react even with a 2500 J ignition energy input. At 600 psi, however, the required ignition energy was only 6 J.}}</ref><ref>[http://hobbyspace.com/AAdmin/archive/SpecialTopics/Misc/pratt-explosion.pdf FR-5904]. Pratt & Whitney Aircraft.</ref> |
|||
==Production== |
|||
[[File:Nitrous oxide production.png|thumb|right|200px|Nitrous oxide production]] |
|||
Nitrous oxide is most commonly prepared by careful heating of [[ammonium nitrate]], which decomposes into nitrous oxide and water vapor.<ref>{{cite book|last = Holleman|first = A. F.|coauthors = Wiberg, E.|title = Inorganic Chemistry|publisher = Academic Press|location = San Diego|year = 2001|isbn = 0-12-352651-5}}</ref> The addition of various [[phosphate]]s favors formation of a purer gas at slightly lower temperatures. One of the earliest commercial producers was [[George Poe]] in [[Trenton, New Jersey]].<ref name=wp>{{cite news|title=George Poe is Dead |url=http://pqasb.pqarchiver.com/washingtonpost_historical/access/243050292.html?dids=243050292:243050292&FMT=ABS&FMTS=ABS:FT&date=FEB+03%2C+1914&author=&pub=The+Washington+Post&desc=GEORGE+POE+IS+DEAD&pqatl=google |quote=Cousin of Famous Poet and Noted as a Scientist. Inventor of the Respirator. Also First to Liquefy Nitrous Oxide. Cadet at [[Virginia Military Institute]] at Time of [[Battle of Newmarket]]. Mentioned for the Nobel Prize for Scientific Attainment in Chemistry. Prof. George Poe, a cousin of the poet Edgar Allan Poe, a noted scientist and inventor, who had been mentioned for the Nobel prize for scientific attainment, a former resident of Washington, died in Norfolk, Virginia, yesterday of general paralysis. Prof. Poe was in his sixty-eighth year. |publisher=Washington Post |date=February 3, 1914 |accessdate=2007-12-29}}</ref> |
|||
===Internal combustion engine=== |
|||
:NH<sub>4</sub>NO<sub>3</sub> (s) → 2 H<sub>2</sub>O (g) + N<sub>2</sub>O (g) |
|||
{{Main|Nitrous oxide engine}} |
|||
In vehicle [[racing]], nitrous oxide (often called "[[Nitrous oxide engine|nitrous]]") allows the engine to burn more fuel by providing more oxygen during combustion. The increase in oxygen allows an increase in the injection of fuel, allowing the engine to produce more [[engine power]]. The gas is not flammable at a low pressure/temperature, but it delivers more oxygen than atmospheric air by breaking down at elevated temperatures, about 570 degrees F (~300C). Therefore, it often is mixed with another fuel that is easier to deflagrate. Nitrous oxide is a strong oxidising agent, roughly equivalent to hydrogen peroxide, and much stronger than oxygen gas. |
|||
This reaction occurs between 170 and 240 °C, temperatures where ammonium nitrate is a moderately sensitive [[explosive]] and a very powerful [[oxidizer]]. Above 240 °C the [[exothermic reaction]] may accelerate to the point of [[detonation]], so the mixture must be cooled to avoid such a disaster. Superheated steam is used to reach reaction temperature in some [[turnkey]] production plants.<ref>{{cite web |
|||
|url = http://www.sanghioverseas.com/nitrous_oxide_gas_plants/nitrous_oxide_gas_plants.htm |
|||
|publisher = Sanghi Organization |
|||
|title = Nitrous oxide plant}}</ref> |
|||
Nitrous oxide is stored as a compressed liquid; the [[heat of vaporization|evaporation]] and expansion of liquid nitrous oxide in the [[intake manifold]] causes a large drop in intake charge temperature, resulting in a denser charge, further allowing more air/fuel mixture to enter the cylinder. Sometimes nitrous oxide is injected into (or prior to) the intake manifold, whereas other systems directly inject, right before the cylinder (direct port injection) to increase power. |
|||
Downstream, the hot, corrosive mixture of gases must be cooled to condense the steam, and filtered to remove higher oxides of nitrogen. Ammonium nitrate smoke, as an extremely persistent [[colloid]], will also have to be removed. The cleanup is often done in a train of 3 gas washes; namely base, acid and base again. Any significant amounts of nitric oxide (NO) may not necessarily be absorbed directly by the base (sodium hydroxide) washes. |
|||
The technique was used during [[World War II]] by [[Luftwaffe]] aircraft with the [[GM-1]] system to boost the power output of [[aircraft engine]]s. Originally meant to provide the Luftwaffe standard aircraft with superior high-altitude performance, technological considerations limited its use to extremely high altitudes. Accordingly, it was only used by specialised planes such as high-altitude [[reconnaissance aircraft]], [[schnellbomber|high-speed bombers]] and high-altitude [[interceptor aircraft]]. It sometimes could be found on Luftwaffe aircraft also fitted with another engine-boost system, [[MW 50]], a form of [[Water injection (engine)|water injection]] for aviation engines that used [[methanol]] for its boost capabilities. |
|||
The nitric oxide impurity is sometimes [[chelate]]d out with [[ferrous sulfate]], reduced with iron metal, or oxidised and absorbed in base as a higher oxide. The first base wash may (or may not) react out much of the ammonium nitrate smoke. However, this reaction generates ammonia gas, which may have to be absorbed in the acid wash. |
|||
One of the major problems of using nitrous oxide in a reciprocating engine is that it can produce enough power to damage or destroy the engine. Very large power increases are possible, and if the mechanical structure of the engine is not properly reinforced, the engine may be severely damaged or destroyed during this type of operation. It is important with nitrous oxide augmentation of [[petrol engine]]s to maintain proper [[operating temperature]]s and fuel levels to prevent "[[pre-ignition]]",<ref>Cline, Allen W. (January 2000) [http://www.contactmagazine.com/Issue54/EngineBasics.html "Engine Basics: Detonation and Pre-Ignition"]. ''CONTACT!'' Magazine</ref> or "detonation" (sometimes referred to as "knock"). Most problems that are associated with nitrous oxide do not come from mechanical failure due to the power increases. Since nitrous oxide allows a much denser charge into the cylinder, it dramatically increases cylinder pressures. The increased pressure and temperature can cause problems such as melting the pistons or valves. It also may crack or warp the piston or cylinder head and cause pre-ignition due to uneven heating. |
|||
===Other routes=== |
|||
The direct oxidation of [[ammonia]] may someday rival the ammonium nitrate [[pyrolysis]] synthesis of nitrous oxide mentioned above. This capital-intensive process, which originates in Japan, uses a [[manganese dioxide]]-[[bismuth oxide]] catalyst:<ref>Synthesis of Nitrous Oxide by Oxidation of Ammonia T Suwa, A Matsushima, Y Suziki, Y Namina, Kohyo Kagaku Zasshi, 1961; Showa Denka Ltd.</ref> |
|||
:2 NH<sub>3</sub> + 2 O<sub>2</sub> → N<sub>2</sub>O + 3 H<sub>2</sub>O |
|||
Automotive-grade liquid nitrous oxide differs slightly from medical-grade nitrous oxide. A small amount of [[sulfur dioxide]] ({{chem|SO|2}}) is added to prevent substance abuse.<ref name="Automotive gas">{{cite web|url=https://www.holley.com/support/faq/?category=NOS |work=Holley |title=Holley performance products, FAQ for Nitrous Oxide Systems |access-date=18 December 2013}}</ref> |
|||
Higher oxides of nitrogen are formed as impurities. In comparison, [[catalysis|uncatalyzed]] ammonia oxidation (i.e. combustion or explosion) goes primarily to N<sub>2</sub> and H<sub>2</sub>O. |
|||
===Aerosol propellant=== |
|||
Nitrous oxide can be made by heating a solution of [[sulfamic acid]] and [[nitric acid]]. Many gases are made this way in Bulgaria.{{Citation needed|date=February 2008}}<ref>Brozadzhiew & Rettos, 1975.</ref> |
|||
[[File:N2O whippets.jpg|thumb|right|Food-grade {{chem|N|2|O}} [[whipped-cream charger]]s]] |
|||
The gas is approved for use as a [[food additive]] ([[E number|E number]]: E942), specifically as an [[Aerosol spray#Aerosol propellants|aerosol spray propellant]]. Its most common uses in this context are in aerosol [[whipped cream]] canisters and [[cooking spray]]s. |
|||
The gas is extremely soluble in fatty compounds. In aerosol whipped cream, it is dissolved in the fatty cream until it leaves the can, when it becomes gaseous and thus creates foam. Used in this way, it produces whipped cream which is four times the volume of the liquid, whereas whipping air into cream only produces twice the volume. If air were used as a propellant, oxygen would accelerate [[rancidification]] of the butterfat, but nitrous oxide inhibits such degradation. Carbon dioxide cannot be used for whipped cream because it is acidic in water, which would curdle the cream and give it a seltzer-like "sparkling" sensation. |
|||
:HNO<sub>3</sub> + NH<sub>2</sub>SO<sub>3</sub>H → N<sub>2</sub>O + H<sub>2</sub>SO<sub>4</sub> + H<sub>2</sub>O |
|||
The whipped cream produced with nitrous oxide is unstable, and will return to a more liquid state within half an hour to one hour.<ref>{{Cite web|url=http://www.explora-science.net/nitrousoxide-use-as-a-propellant-and-in-cooking/|title=Explora Science {{!}} Nitrous use as a propellant and in cooking|language=en-US|access-date=2019-02-19|archive-date=27 February 2019|archive-url=https://web.archive.org/web/20190227015310/http://www.explora-science.net/nitrousoxide-use-as-a-propellant-and-in-cooking/}}</ref> Thus, the method is not suitable for decorating food that will not be served immediately. |
|||
There is no explosive hazard in this reaction if the mixing rate is controlled. However, as usual, toxic higher oxides of nitrogen are formed. |
|||
In December 2016, some manufacturers reported a shortage of aerosol whipped creams in the United States due to an explosion at the [[Air Liquide]] nitrous oxide facility in [[Florida]] in late August. With a major facility offline, the disruption caused a shortage resulting in the company diverting the supply of nitrous oxide to medical customers rather than to food manufacturing. The shortage came during the [[Christmas and holiday season]] when canned whipped cream use is normally at its highest.<ref>{{cite news |last=Dewey |first=Caitlin |url=https://www.washingtonpost.com/news/wonk/wp/2016/12/21/the-real-reason-you-cant-buy-whipped-cream-this-christmas/ |title=The real reason grocery stores are running out of whipped cream this Christmas |newspaper=[[The Washington Post]] |date=2016-12-21 |access-date=2016-12-22 }}</ref> |
|||
Nitrous oxide is produced in large volumes as a by-product in the synthesis of [[adipic acid]]; one of the two reactants used in nylon manufacture.<ref>{{cite journal |
|||
|title = Abatement of N<sub>2</sub>O emissions produced in the adipic acid industry |
|||
|author = Reimer R. A.; Slaten C. S.; Seapan M.; Lower M. W.; Tomlinson P. E.; |
|||
|journal = Environmental progress |
|||
|year = 1994 |
|||
|volume = 13 |
|||
|issue = 2 |
|||
|pages = 134–137 |
|||
|doi = 10.1002/ep.670130217}}</ref><ref>{{cite journal |
|||
|title = Abatement of N<sub>2</sub>O emissions produced in the adipic acid industry |
|||
|author = A. Shimizu, , K. Tanaka and M. Fujimori |
|||
|journal = Chemosphere – Global Change Science |
|||
|year = 2000 |
|||
|volume = 2 |
|||
|issue = 3–4 |
|||
|pages = 425–434 |
|||
|doi = 10.1016/S1465-9972(00)00024-6}}</ref> This might become a major commercial source, but will require the removal of higher oxides of nitrogen and organic impurities. Currently much of the gas is decomposed before release for environmental protection. Greener processes may prevail that substitute [[hydrogen peroxide]] for nitric acid oxidation; hence no generation of oxide of nitrogen by-products. |
|||
Similarly, cooking spray, which is made from various types of oils combined with [[lecithin]] (an [[emulsifier]]), may use nitrous oxide as a [[propellant]]. Other propellants used in cooking spray include food-grade [[ethanol|alcohol]] and [[propane]]. |
|||
[[Hydroxylammonium chloride]] can react with [[sodium nitrite]] to produce N<sub>2</sub>O as well: |
|||
: NH<sub>3</sub>OH<sup>+</sup>Cl<sup>−</sup> + NaNO<sub>2</sub> → N<sub>2</sub>O + NaCl + 2 H<sub>2</sub>O |
|||
If the nitrite is added to the hydroxylamine solution, the only remaining byproduct is salt water. However, if the hydroxylamine solution is added to the nitrite solution (nitrite is in excess), then toxic higher oxides of nitrogen are also formed. |
|||
Also, HNO<sub>3</sub> can be reduced to N<sub>2</sub>O by SnCl<sub>2</sub> and HCl mixture: |
|||
:2 HNO<sub>3</sub> + 8 HCl + 4 SnCl<sub>2</sub> → 5 H<sub>2</sub>O + 4 SnCl<sub>4</sub> + N<sub>2</sub>O |
|||
===Medicine=== |
|||
Natural production of N<sub>2</sub>O occurs through the process of [[denitrification]] in oxygen-poor soils and marine environments, in which denitrifying bacteria respire NO<sub>3</sub><sup>-</sup>. |
|||
{{Main|Nitrous oxide (medication)}} |
|||
[[File:N2O Medical Tanks.jpg|thumb|right|upright|Medical-grade {{chem|N|2|O}} tanks used in [[dentistry]]]] |
|||
Nitrous oxide has been used in dentistry and surgery, as an anaesthetic and analgesic, since 1844.<ref name="Drug discovery">{{cite book|url=https://books.google.com/books?id=mYQxRY9umjcC |author=Sneader W |title=Drug Discovery –A History |chapter=Systematic Medicine |pages=74–87 |date=2005 |publisher=John Wiley and Sons |isbn=978-0-471-89980-8}}</ref> In the early days, the gas was administered through simple inhalers consisting of a breathing bag made of rubber cloth.<ref name="use in dentistry">{{cite journal|author=Miller AH |title=Technical Development of Gas Anesthesia |journal=Anesthesiology |volume=2 |issue=4 |pages=398–409 |year=1941 |doi=10.1097/00000542-194107000-00004|s2cid=71117361 |doi-access=free }}</ref> Today, the gas is administered in hospitals by means of an automated [[relative analgesia machine]], with an [[anaesthetic vaporiser]] and a [[medical ventilator]], that delivers a precisely dosed and breath-actuated flow of [[nitrous oxide and oxygen|nitrous oxide mixed with oxygen]] in a 2:1 ratio. |
|||
==Applications== |
|||
===Rocket motors=== |
|||
Nitrous oxide can be used as an [[oxidizing agent|oxidizer]] in a [[rocket]] motor. This has the advantages over other oxidizers in that it is non-toxic and, due to its stability at room temperature, easy to store and relatively safe to carry on a flight. As a secondary benefit it can be readily decomposed to form breathing air. Its high density and low storage pressure enable it to be highly competitive with stored high-pressure gas systems. |
|||
Nitrous oxide is a weak [[general anaesthetic]], and so is generally not used alone in general anaesthesia, but used as a carrier gas (mixed with oxygen) for more powerful general anaesthetic drugs such as [[sevoflurane]] or [[desflurane]]. It has a [[minimum alveolar concentration]] of 105% and a [[blood/gas partition coefficient]] of 0.46. The use of nitrous oxide in anaesthesia can increase the risk of postoperative nausea and vomiting.<ref>{{Cite journal|last1=Divatia|first1=Jigeeshu V.|last2=Vaidya|first2=Jayant S.|last3=Badwe|first3=Rajendra A.|last4=Hawaldar|first4=Rohini W.|title=Omission of Nitrous Oxide during Anesthesia Reduces the Incidence of Postoperative Nausea and Vomiting|journal=Anesthesiology|volume=85|issue=5|pages=1055–1062|doi=10.1097/00000542-199611000-00014|pmid=8916823|year=1996|s2cid=41549796|doi-access=free}}</ref><ref>{{Cite journal|last=Hartung|first=John|title=Twenty-Four of Twenty-Seven Studies Show a Greater Incidence of Emesis Associated with Nitrous Oxide than with Alternative Anesthetics|journal=Anesthesia & Analgesia|volume=83|issue=1|pages=114–116|doi=10.1213/00000539-199607000-00020|year=1996}}</ref><ref>{{Cite journal|last1=Tramèr|first1=M.|last2=Moore|first2=A.|last3=McQuay|first3=H.|date=February 1996|title=Omitting nitrous oxide in general anaesthesia: meta-analysis of intraoperative awareness and postoperative emesis in randomized controlled trials|journal=British Journal of Anaesthesia|volume=76|issue=2|pages=186–193|pmid=8777095|doi=10.1093/bja/76.2.186|doi-access=free}}</ref> |
|||
In a 1914 patent, American rocket pioneer [[Robert Goddard]] suggested nitrous oxide and gasoline as possible propellants for a liquid-fueled rocket. Nitrous oxide has been the oxidizer of choice in several [[hybrid rocket]] designs (using solid fuel with a liquid or gaseous oxidizer). The combination of nitrous oxide with [[hydroxyl-terminated polybutadiene]] fuel has been used by [[SpaceShipOne]] and others. It is also notably used in [[amateur rocketry|amateur]] and [[high power rocket]]ry with various [[plastic]]s as the fuel. |
|||
Dentists use a simpler machine which only delivers an {{chem|N|2|O}}/{{chem|O|2}} mixture for the patient to inhale while conscious but must still be a recognised purpose designed dedicated relative analgesic flowmeter with a minimum 30% of oxygen at all times and a maximum upper limit of 70% nitrous oxide. The patient is kept conscious throughout the procedure, and retains adequate mental faculties to respond to questions and instructions from the dentist.<ref>{{Cite journal|last=Council on Clinical Affairs|date=2013|title=Guideline on use of nitrous oxide for pediatric dental patients|url=http://www.aapd.org/media/policies_guidelines/g_nitrous.pdf |archive-url=https://ghostarchive.org/archive/20221009/http://www.aapd.org/media/policies_guidelines/g_nitrous.pdf |archive-date=2022-10-09 |url-status=live|journal=Reference Manual V37|volume=6|pages=206–210}}</ref> |
|||
Nitrous oxide can also be used in a [[monopropellant rocket]]. In the presence of a heated [[catalyst]], N<sub>2</sub>O will decompose exothermically into nitrogen and oxygen, at a temperature of approximately 1300 °C. Because of the large heat release, the catalytic action rapidly becomes secondary as thermal autodecomposition becomes dominant. In a vacuum thruster, this can provide a monopropellant [[specific impulse]] (''I''<sub>sp</sub>) of as much as 180 s. While noticeably less than the ''I''<sub>sp</sub> available from [[hydrazine]] thrusters (monopropellant or [[Bipropellant rocket|bipropellant]] with [[Dinitrogen tetroxide|nitrogen tetroxide]]), the decreased toxicity makes nitrous oxide an option worth investigating. |
|||
Inhalation of nitrous oxide is used frequently to relieve pain associated with [[childbirth]], [[Physical trauma|trauma]], [[dentistry|oral surgery]] and [[acute coronary syndrome]] (including heart attacks). Its use during labour has been shown to be a safe and effective aid for birthing women.<ref>{{cite web |last=Copeland |first=Claudia |url=http://www.pregnancy.org/article/nitrous-oxide-analgesia-child-birth |title=Nitrous Oxide Analgesia for Childbirth |website=Pregnancy.org |archive-url=https://web.archive.org/web/20110525080809/http://www.pregnancy.org/article/nitrous-oxide-analgesia-child-birth |archive-date=25 May 2011 }}</ref> Its use for acute coronary syndrome is of unknown benefit.<ref name="AHA10">{{cite journal|author=O'Connor RE |title=Part 10: acute coronary syndromes: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care |journal=Circulation |volume=122 |issue=18 Suppl 3 |pages=S787–817 |year=2010 |pmid=20956226 |doi=10.1161/CIRCULATIONAHA.110.971028 |author2=Brady W |author3=Brooks SC |last4=Diercks |first4=D. |last5=Egan |first5=J. |last6=Ghaemmaghami |first6=C. |last7=Menon |first7=V. |last8=O'Neil |first8=B. J. |last9=Travers |first9=A. H. |last10=Yannopoulos |doi-access=free }}</ref> |
|||
Specific impulse (''I''<sub>sp</sub>) can be improved by blending a hydrocarbon fuel with the nitrous oxide inside the same storage tank, becoming a nitrous oxide fuel blend (NOFB) monopropellant. This storage mixture does not incur the danger of spontaneous ignition, since N<sub>2</sub>O is chemically stable. When the nitrous oxide decomposes by a heated catalyst, high temperature oxygen is released and rapidly ignites the hydrocarbon fuel-blend. NOFB monopropellants are capable of I{{su|b=sp}} greater than 300 seconds, while avoiding the toxicity associated with hypergolic propulsion systems.<ref>{{cite| url=http://www.faqs.org/patents/app/20090133788|title=Nitrous Oxide Fuel Blend Monopropellants|publisher=Patentdocs| accessdate=2009-11-11}}</ref><ref>{{cite| url=http://www.firestar-engineering.com/|title=FireStar Engineering, LLC| publisher=FireStar Engineering| accessdate=2009-12-11}}</ref> The low freezing point of NOFB eases thermal management compared to hydrazine and dinitrogen tetroxide—a valuable property for space storable propellants. |
|||
In Canada and the UK, [[Nitrous oxide (medication)|Entonox]] and Nitronox are used commonly by ambulance crews (including unregistered practitioners) as rapid and highly effective analgesic gas. |
|||
Nitrous oxide can also be used as a monopropellant, for example for use in rockets. For this, the nitrous oxide needs to be brought to a high enough temperature or a high enough pressure. Nitrous oxide is said to deflagrate somewhere around 600° Fahrenheit (315°Celsius). It can also easily be ignited using a combination of the two. At 600 psi for example, the required ignition energy is only 6 J, whereas N2O at 130 psi would not react even with a 2500 J ignition energy input.<ref>[http://www.scaled.com/images/uploads/news/N2OSafetyGuidelines.pdf NOX as a monopropellant 1]</ref> |
|||
<ref>[http://www.spl.ch/publication/SPL_Papers/N2O_safety_e.pdf NOX as a monopropellant 2]</ref> |
|||
<ref>[http://hobbyspace.com/AAdmin/archive/SpecialTopics/Misc/pratt-explosion.pdf NOX as a monopropellant 3]</ref><ref>[http://hobbyspace.com/AAdmin/archive/SpecialTopics/Misc/eindhoven.pdf NOX as a monopropellant 4]</ref> |
|||
Fifty per cent nitrous oxide can be considered for use by trained non-professional first aid responders in prehospital settings, given the relative ease and safety of administering 50% nitrous oxide as an analgesic. The rapid reversibility of its effect would also prevent it from precluding diagnosis.<ref>{{Cite journal|last1=Faddy|first1=S. C.|last2=Garlick|first2=S. R.|date=2005-12-01|title=A systematic review of the safety of analgesia with 50% nitrous oxide: can lay responders use analgesic gases in the prehospital setting?|journal=Emergency Medicine Journal|volume=22|issue=12|pages=901–908|doi=10.1136/emj.2004.020891|pmc=1726638|pmid=16299211}}</ref> |
|||
===Internal combustion engine=== |
|||
{{Main|Nitrous}} |
|||
===Recreational use=== |
|||
In vehicle [[racing]], nitrous oxide (often referred to as just "[[nitrous]]") allows the engine to burn more fuel and air, resulting in a more powerful combustion. The gas itself is not flammable at a low pressure/temperature, but it delivers more [[oxygen]] than atmospheric air by breaking down at elevated temperatures. Therefore, it is often mixed with another fuel that is easier to deflagrate. |
|||
{{main|Recreational use of nitrous oxide}} |
|||
[[File:Doctor and Mrs Syntax, with a party of friends, experimentin Wellcome L0022227.jpg|thumb|[[Aquatint]] depiction of a laughing gas party in the nineteenth century, by [[Thomas Rowlandson]]]] |
|||
[[File:Ban of Nitrous oxide use.jpg|thumb|Street sign indicating ban of nitrous oxide use near the Poelestraat in [[Groningen]]]] |
|||
[[File:Nitrous oxide whippits used recreationally as a drug by Dutch youngsters near a school, Utrecht, 2017 - 1.jpg|thumb|[[Whipped-cream charger|Whippit]] remnants (the small steel canisters) of recreational drug use, the Netherlands, 2017]] |
|||
[[recreational use of nitrous oxide|Recreational inhalation of nitrous oxide]], with the purpose of causing [[euphoria (emotion)|euphoria]] and/or slight [[hallucination]]s, began as a phenomenon for the British upper class in 1799, known as "laughing gas parties".<ref>{{Cite book|last=Davy|first=Humphry|url=http://archive.org/details/researcheschemic00davy|title=Researches, chemical and philosophical: chiefly concerning nitrous oxide, or diphlogisticated nitrous air, and its respiration|date=1800|publisher=London : printed for J. Johnson, St. Paul's Church-Yard, by Biggs and Cottle, Bristol|others=Francis A. Countway Library of Medicine}}</ref> |
|||
Nitrous oxide is stored as a compressed liquid; the [[heat of vaporization|evaporation]] and expansion of liquid nitrous oxide in the [[intake manifold]] causes a large drop in intake charge temperature, resulting in a denser charge, further allowing more air/fuel mixture to enter the cylinder. Nitrous oxide is sometimes injected into (or prior to) the intake manifold, whereas other systems directly inject right before the cylinder (direct port injection) to increase power. |
|||
Starting in the 19th century, the widespread availability of the gas for medical and culinary purposes allowed for recreational use to expand greatly globally. In the UK as of 2014, nitrous oxide was estimated to be used by almost half a million young people at nightspots, festivals and parties.<ref>{{cite news | title = Warning over laughing gas misuse | url = https://www.theguardian.com/politics/2014/aug/09/warning-over-laughing-gas-misuse | date = 9 August 2014 | work = [[The Guardian]] |location=London |agency=[[Press Association]] | access-date = 9 August 2014}}</ref> |
|||
The technique was used during [[World War II]] by ''[[Luftwaffe]]'' aircraft with the [[GM-1]] system to boost the power output of [[aircraft engine]]s. Originally meant to provide the ''Luftwaffe'' standard aircraft with superior high-altitude performance, technological considerations limited its use to extremely high altitudes. Accordingly, it was only used by specialized planes like high-altitude [[reconnaissance aircraft]], [[schnellbomber|high-speed bombers]], and high-altitude [[interceptor aircraft]]. |
|||
Widespread recreational use of the drug throughout the UK was featured in the 2017 [[Vice Media|Vice]] documentary ''Inside The Laughing Gas Black Market'', in which journalist [[Matt Shea (documentary filmmaker)|Matt Shea]] met with dealers of the drug who stole it from hospitals.<ref>{{Citation|last=VICE|title=Inside The Laughing Gas Black Market|date=2017-02-07|url=https://www.youtube.com/watch?v=gdhdAktIHtg| archive-url=https://ghostarchive.org/varchive/youtube/20211029/gdhdAktIHtg| archive-date=2021-10-29|access-date=2019-03-29}}{{cbignore}}</ref> |
|||
One of the major problems of using nitrous oxide in a reciprocating engine is that it can produce enough power to damage or destroy the engine. Very large power increases are possible, and if the mechanical structure of the engine is not properly reinforced, the engine may be severely damaged or destroyed during this kind of operation. It is very important with nitrous oxide augmentation of [[internal combustion engine]]s to maintain proper [[operating temperature]]s and fuel levels to prevent "preignition", or "detonation" (sometimes referred to as "knocking" or "pinging"). Most problems that are associated with nitrous do not come from mechanical failure due to the power increases. Since nitrous allows a much denser charge into the cylinder it dramatically increases cylinder pressures. The increased pressure and temperature can cause problems such as melting the piston or valves. It may also crack or warp the piston or head and cause preignition due to uneven heating. |
|||
A significant issue cited in London's press is the effect of nitrous oxide canister littering, which is highly visible and causes significant complaints from communities.<ref>{{Cite web|url=https://metro.co.uk/2018/07/10/recycling-used-laughing-gas-canisters-for-cash-could-help-create-a-cleaner-britain-7694925/|title=Recycling used laughing gas canisters for cash could help create a cleaner Britain|date=2018-07-10|website=Metro|language=en-US|access-date=2019-07-15}}</ref> |
|||
Automotive-grade liquid nitrous oxide differs slightly from medical-grade nitrous oxide. A small amount of [[sulfur dioxide]] is added to prevent substance abuse.<ref name="Automotive gas">{{cite web |url=http://www.holley.com/TechService/FAQ.asp?category=NOS|author=Holley|title=Holley performance products, FAQ for Nitrous Oxide Systems}}</ref> |
|||
Prior to 8 November 2023, nitrous oxide was subject to the Psychoactive Substances Act 2016 in the UK. It was already illegal to produce, supply, import or export nitrous oxide for recreational use. However, the UK government updated the law on 8 November 2023 to include possession of nitrous oxide by classifying it as a Class C drug under the Misuse of Drugs Act 1971.<ref>{{Cite web |title=Nitrous oxide ban: guidance |url=https://www.gov.uk/government/publications/nitrous-oxide-ban/nitrous-oxide-ban-guidance |access-date=2023-12-06 |website=GOV.UK |language=en}}</ref> |
|||
===Aerosol propellant=== |
|||
[[Image:Nitrous oxide - 10 x 8g.jpg|thumb|200px|An 8 g canister of nitrous oxide intended for use as a [[whipped cream]] aerating agent]] |
|||
The gas is approved for use as a [[food additive]] (also known as E942), specifically as an [[Aerosol spray#Aerosol propellants|aerosol spray propellant]]. Its most common uses in this context are in aerosol [[whipped cream]] canisters, [[cooking spray]]s, and as an inert gas used to displace oxygen, to inhibit bacterial growth, when filling packages of [[potato chips]] and other similar snack foods. |
|||
While casual use of nitrous oxide is understood by most recreational users to be a route to a "safe high", many are unaware that excessive consumption has the potential to cause neurological harm which, if left untreated, can result in permanent neurological damage.<ref name="bbc.co.uk">{{cite news |title=Nitrous oxide: Laughing gas users risk spine damage, say doctors |url=https://www.bbc.co.uk/news/health-64718233 |access-date=26 March 2023}}</ref> In Australia, recreation use became a public health concern following a rise in reported cases of neurotoxicity and a rise in [[emergency room]] admissions, and in (the state of) South Australia legislation was passed in 2020 to restrict canister sales.<ref name=nangs/> |
|||
The gas is extremely soluble in fatty compounds. In aerosol whipped cream, it is dissolved in the fatty cream until it leaves the can, when it becomes gaseous and thus creates foam. Used in this way, it produces whipped cream four times the volume of the liquid, whereas whipping air into cream only produces twice the volume. If air were used as a propellant, oxygen would accelerate [[rancidification]] of the butterfat; nitrous oxide inhibits such degradation. Carbon dioxide cannot be used for whipped cream because it is acidic in water, which would curdle the cream and give it a seltzer-like 'sparkling' sensation. |
|||
==Safety== |
|||
However, the whipped cream produced with nitrous oxide is unstable and will return to a more or less liquid state within half an hour to one hour. Thus, the method is not suitable for decorating food that will not be immediately served. |
|||
Nitrous oxide is a significant [[occupational hazard]] for surgeons, dentists and nurses. Because nitrous oxide is minimally metabolised in humans (with a rate of 0.004%), it retains its potency when exhaled into the room by the patient, and can pose an intoxicating and prolonged exposure hazard to the clinic staff if the room is poorly ventilated. Where nitrous oxide is administered, a continuous-flow fresh-air [[ventilation (architecture)|ventilation system]] or {{chem|N|2|O}} [[scavenger system]] is used to prevent a waste-gas buildup.{{citation needed|date=August 2022}} |
|||
Similarly, [[cooking spray]], which is made from various types of oils combined with [[lecithin]] (an [[emulsifier]]), may use nitrous oxide as a [[propellant]]; other propellants used in cooking spray include food-grade [[alcohol]] and [[propane]]. |
|||
The [[National Institute for Occupational Safety and Health]] recommends that workers' exposure to nitrous oxide should be controlled during the administration of anaesthetic gas in medical, dental and veterinary operators.<ref>[https://www.cdc.gov/niosh/docs/94-100/ CDC.gov NIOSH Alert: Controlling Exposures to Nitrous Oxide During Anesthetic Administration]. Cincinnati, OH: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 94-100</ref> It set a [[recommended exposure limit]] (REL) of 25 [[Parts-per notation|ppm]] (46 mg/m<sup>3</sup>) to escaped anaesthetic.<ref>{{Cite web|title =NIOSH Pocket Guide to Chemical Hazards – Nitrous oxide|url = https://www.cdc.gov/niosh/npg/npgd0465.html|website = CDC|access-date = 2015-11-21}}</ref> |
|||
Users of nitrous oxide often obtain it from whipped cream dispensers that use nitrous oxide as a propellant (see above section), for recreational use as a euphoria-inducing [[inhalant]] drug. It is not harmful in small doses, but risks due to lack of oxygen do exist (see ''[[Nitrous oxide#Recreational use|Recreational use]] below). |
|||
=== |
===Mental and manual impairment=== |
||
Exposure to nitrous oxide causes short-term decreases in mental performance, audiovisual ability and manual dexterity.<ref>[https://www.cdc.gov/niosh/docs/1970/77-140.html Criteria for a recommended standard: occupational exposure to waste anesthetic gases and vapors]. Cincinnati, OH: U.S. Department of Health, Education, and Welfare, Public Health Service, Center for Disease Control, National Institute for Occupational Safety and Health, DHEW (NIOSH) Publication No. 77B140.</ref> These effects coupled with the induced spatial and temporal disorientation could result in physical harm to the user from environmental hazards.<ref name="Mike Jay 22–25"/> |
|||
{{see|Nitrous oxide and oxygen}} |
|||
===Neurotoxicity and neuroprotection=== |
|||
[[Image:N2O Medical Tanks.jpg|thumb|100px|right|Medical grade N<sub>2</sub>O tanks used in [[dentistry]].]] |
|||
Nitrous oxide is [[neurotoxic]] and there is evidence that medium or long-term habitual consumption of significant quantities can cause neurological harm with the potential for permanent damage if left untreated.<ref name=nangs>{{cite journal |vauthors=Evans EB, Evans MR |title=Nangs, balloons and crackers: Recreational nitrous oxide neurotoxicity |journal=Aust J Gen Pract |volume=50 |issue=11 |pages=834–838 |date=November 2021 |pmid=34713284 |doi=10.31128/AJGP-10-20-5668 |s2cid=240153502 |type=Review|doi-access=free }}</ref><ref name="bbc.co.uk"/> |
|||
Like other [[NMDA receptor antagonist]]s, it has been suggested that {{chem|N|2|O}} produces [[neurotoxicity]] in the form of [[Olney's lesions]] in rodents upon prolonged (several hour) exposure.<ref name="pmid14622904">{{cite journal |year=2003|title=Prolonged exposure to inhalational anesthetic nitrous oxide kills neurons in adult rat brain|journal=Neuroscience|volume=122|issue=3|pages=609–16|doi=10.1016/j.neuroscience.2003.07.012|pmid=14622904|vauthors=Jevtovic-Todorovic V, Beals J, Benshoff N, Olney JW|s2cid=9407096}}</ref><ref name="pmid12854473">{{cite journal|year=2003|title=NMDA receptor antagonist neurotoxicity and psychotomimetic activity|journal=Masui. The Japanese Journal of Anesthesiology|language=ja|volume=52|issue=6|pages=594–602|pmid=12854473|vauthors=Nakao S, Nagata A, Masuzawa M, Miyamoto E, Yamada M, Nishizawa N, Shingu K}}</ref><ref name="pmid10928976">{{cite journal |year=2000|title=Ketamine potentiates cerebrocortical damage induced by the common anaesthetic agent nitrous oxide in adult rats|journal=British Journal of Pharmacology|volume=130|issue=7|pages=1692–8|doi=10.1038/sj.bjp.0703479|pmc=1572233|pmid=10928976 |vauthors=Jevtovic-Todorovic V, Benshoff N, Olney JW}}</ref><ref name="pmid15718054">{{cite journal|last2=Carter|year=2005|title=The anesthetics nitrous oxide and ketamine are more neurotoxic to old than to young rat brain|journal=Neurobiology of Aging|volume=26|issue=6|pages=947–56|doi=10.1016/j.neurobiolaging.2004.07.009|pmid=15718054|author=Jevtovic-Todorovic V, Carter LB|s2cid=25095727}}</ref> |
|||
Nitrous oxide has been used for [[anesthesia]] in [[dentistry]] since December 1844, where [[Horace Wells]] made the first 12–15 dental operations with the gas in [[Hartford]]. Its debut as a generally accepted method however came in 1863, when [[Gardner Quincy Colton]] introduced it more broadly at all the Colton Dental Association clinics, that he founded in [[New Haven]] and [[New York city]].<ref name="Drug discovery">{{cite book|url=http://books.google.com/?id=mYQxRY9umjcC&printsec=frontcover&dq=Drug+Discovery+history&cd=1|author=Sneader W|title=Drug Discovery –A History|work=(Part 1: Legacy of the past, chapter 8: systematic medicine, p.74-87)|year=2005|accessdate=2010-04-21|publisher=John Wiley and Sons|isbn=9780471899808}}</ref> The first devices used in dentistry to administer the gas, known as Nitrous Oxide inhalers, were designed in a very simple way with the gas stored and breathed through a breathing bag made of rubber cloth, without a [[scavenger system]] and [[flowmeter]], and with no addition of oxygen/air.<ref name="use in dentistry">{{cite web |url=http://journals.lww.com/anesthesiology/citation/1941/07000/Technical_Development_of_Gas_Anesthesia.4.aspx |author=Miller AH|title=Technical Development of Gas Anesthesia|work=Anesthesiology journal (July 1941, vol.2, is.4, p.398-409)|year=1941}}</ref> Today these simple and somewhat unreliable inhalers, of course have been replaced by the more modern [[relative analgesia machine]], which is an automated machine designed to deliver a precisely dosed and breath-actuated flow of nitrous oxide mixed with oxygen, for the patient to inhale safely. The machine used in dentistry is designed as a more simplified version of the larger [[anaesthetic machine]] used by hospitals, as it doesn't feature the additional [[anaesthetic vaporiser]] and [[medical ventilator]]. The purpose of the machine allows for a simpler design, as it only delivers a mixture of [[nitrous oxide and oxygen]] for the patient to inhale, in order to depress the feeling of pain -while keeping the patient in a conscious state. |
|||
It has been argued that, because {{chem|N|2|O}} is rapidly expelled from the body under normal circumstances, it is less likely to be neurotoxic than other NMDAR antagonists.<ref name="pmid16179534">{{cite journal |year=2005|title=Potentially neuroprotective and therapeutic properties of nitrous oxide and xenon|journal=Annals of the New York Academy of Sciences|volume=1053|issue=1|pages=289–300|bibcode=2005NYASA1053..289A|doi=10.1111/j.1749-6632.2005.tb00036.x|pmid=16179534 |vauthors=Abraini JH, David HN, Lemaire M|s2cid=34160112}}</ref> Indeed, in rodents, short-term exposure results in only mild injury that is rapidly reversible, and neuronal death occurs only after constant and sustained exposure.<ref name="pmid14622904" /> Nitrous oxide also may cause neurotoxicity after extended exposure because of [[hypoxia (medical)|hypoxia]]. This is especially true of non-medical formulations such as [[whipped-cream charger]]s (also known as "whippets" or "nangs"),<ref>{{cite journal|pmid=23801743|doi=10.1093/bja/aet215|year=2013|last1=De Vasconcellos|first1=K.|title=Nitrous oxide: Are we still in equipoise? A qualitative review of current controversies|journal=British Journal of Anaesthesia|volume=111|issue=6|pages=877–85|last2=Sneyd|first2=J. R.|doi-access=free}}</ref> which never contain oxygen, since oxygen makes cream rancid.<ref name="Middleton 2012 p.">{{cite book|title=Physics in anaesthesia|last=Middleton|first=Ben|publisher=Scion Pub. Ltd|year=2012|isbn=978-1-904842-98-9|location=Banbury, Oxfordshire, UK}}</ref> |
|||
In heavy (≥400 g or ≥200 L of {{N2O}} gas in one session) or frequent (regular, i.e., daily or weekly) users reported to poison control centers, signs of [[peripheral neuropathy]] have been noted: the presence of [[ataxia]] (gait abnormalities) or [[paresthesia]] (perception of abnormal sensations, e.g. tingling, numbness, prickling, mostly in the extremities). These are considered an early sign of neurological damage and indicates [[chronic toxicity]].<ref>{{cite journal|doi=10.1016/j.drugpo.2021.103519|year=2022|last1=van Riel|first1=A.J.H.P.|title=Alarming increase in poisonings from recreational nitrous oxide use after a change in EU-legislation, inquiries to the Dutch Poisons Information Center|journal=International Journal of Drug Policy|volume=100|page=103519|pmid=34753046|doi-access=free}}</ref> |
|||
The relative analgesia machine typically feature a constant-supply [[flowmeter]], which allow the proportion of nitrous oxide and the combined gas flow rate to be individually adjusted. The gas is administered by dentists through a [[Demand valve|demand-valve]] inhaler over the nose, which will only release gas when the patient inhales through the nose. Because nitrous oxide is minimally metabolized in humans (with a rate of 0.004%), it retains its potency when exhaled into the room by the patient, and can pose an intoxicating and prolonged exposure hazard to the clinic staff if the room is poorly ventilated. Where nitrous oxide is administered, a continuous-flow fresh-air [[Ventilation (architecture)|ventilation system]] or nitrous [[scavenger system]] is used to prevent a waste-gas buildup. |
|||
Nitrous oxide at 75% by volume reduces ischemia-induced neuronal death induced by occlusion of the middle cerebral artery in rodents, and decreases NMDA-induced Ca<sup>2+</sup> influx in neuronal cell cultures, a critical event involved in [[excitotoxicity]].<ref name="pmid16179534" /> |
|||
Hospitals are administering nitrous oxide as one of the [[anesthetic]] drugs delivered by [[anaesthetic machine]]s. Nitrous oxide is a weak [[general anesthetic]], and so is generally not used alone in [[general anesthesia]]. In general anesthesia it is used as a carrier gas in a 2:1 ratio with oxygen for more powerful general anesthetic drugs such as [[sevoflurane]] or [[desflurane]]. It has a [[minimum alveolar concentration]] of 105% and a blood:gas partition coefficient of 0.46. |
|||
===DNA damage=== |
|||
When nitrous oxide is inhaled as the only anesthetic drug, it is normally administered as a mixture with 30% gas and 70% oxygen.<ref name=Dentalfear>{{cite web|url=http://www.dentalfearcentral.org/laughing_gas.html|author=Dental Fear Central|title=Inhalation sedation (aka Laughing Gas)|year=2004|accessdate=2010-04-18}}</ref> The medical grade gas tanks, with the tradename [[Entonox|Entonox and Nitronox]] contain a mixture with 50%, but this will normally be diluted to a lower percentage upon the operational delivery to the patient. Inhalation of nitrous oxide is frequently used to relieve pain associated with [[childbirth]], [[Physical trauma|trauma]], [[dentistry|oral surgery]], and [[acute coronary syndrome]] (includes heart attacks). Its use during labor has been shown to be a safe and effective aid for women wanting to give birth without an epidural.<ref>Nitrous Oxide Analgesia for Childbirth, by Claudia Copeland, Ph.D. <http://www.pregnancy.org/article/nitrous-oxide-analgesia-child-birth></ref> Its use for acute coronary syndrome is of unknown benefit.<ref name=AHA10>{{cite journal |author=O'Connor RE, Brady W, Brooks SC, ''et al.'' |title=Part 10: acute coronary syndromes: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care |journal=Circulation |volume=122 |issue=18 Suppl 3 |pages=S787–817 |year=2010 |pmid=20956226 |doi=10.1161/CIRCULATIONAHA.110.971028 |url=}}</ref> |
|||
Occupational exposure to ambient nitrous oxide has been associated with DNA damage, due to interruptions in DNA synthesis.<ref>{{cite web |last1=Randhawa |first1=G. |last2=Bodenham |first2=A. |title=The increasing recreational use of nitrous oxide: history revisited |url=https://academic.oup.com/bja/article/116/3/321/2566058 |journal=British Journal of Anaesthesia |volume=116 |issue=3 |pages=321–324 |language=en |doi=10.1093/bja/aev297 |pmid=26323292 |date=1 March 2016}}</ref> This correlation is dose-dependent<ref>{{cite journal |last1=Wrońska-Nofer |first1=Teresa |last2=Nofer |first2=Jerzy-Roch |last3=Jajte |first3=Jolanta |last4=Dziubałtowska |first4=Elżbieta |last5=Szymczak |first5=Wiesław |last6=Krajewski |first6=Wojciech |last7=Wąsowicz |first7=Wojciech |last8=Rydzyński |first8=Konrad |title=Oxidative DNA damage and oxidative stress in subjects occupationally exposed to nitrous oxide (N<sub>2</sub>O) |journal=Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis |date=1 March 2012 |volume=731 |issue=1 |pages=58–63 |doi=10.1016/j.mrfmmm.2011.10.010 |pmid=22085808 }}</ref><ref>{{cite journal |title=DNA damage induced by nitrous oxide: Study in medical personnel of operating rooms |journal=Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis |date=18 June 2009 |volume=666 |issue=1–2 |pages=39–43 |doi=10.1016/j.mrfmmm.2009.03.012 |pmid=19439331 |last1=Wrońska-Nofer |first1=Teresa |last2=Palus |first2=Jadwiga |last3=Krajewski |first3=Wojciech |last4=Jajte |first4=Jolanta |last5=Kucharska |first5=Małgorzata |last6=Stetkiewicz |first6=Jan |last7=Wąsowicz |first7=Wojciech |last8=Rydzyński |first8=Konrad }}</ref> and does not appear to extend to casual recreational use; however, further research is needed to confirm the duration and quantity of exposure needed to cause damage. |
|||
===Oxygen deprivation=== |
|||
In Britain and British Columbia, Canada, Entonox and Nitronox are commonly used by ambulance crews (including unregistered practitioners) as a rapid and highly effective analgesic gas. |
|||
If pure nitrous oxide is inhaled without oxygen, oxygen deprivation can occur, resulting in low blood pressure, fainting, and even heart attacks. This can occur if the user inhales large quantities continuously, as with a strap-on mask connected to a gas canister. It can also happen if the user engages in excessive breath-holding or uses any other inhalation system that cuts off a supply of fresh air.<ref>[http://justsayn2o.com/nitrous.dangers.html#oxygendep Dangers of Nitrous Oxide]. Just Say N2O</ref> |
|||
===Vitamin B{{ssub|12}} deficiency=== |
|||
Nitrous oxide has been shown to be effective in treating a number of addictions including alcohol withdrawal.<ref>Gillman M.A, Lichtigfeld, F.J. Enlarged double-blind randomised trial of benzodiazepines against psychotropic analgesic nitrous oxide for alcohol withdrawal, Addictive Behaviors, Volume 29, Issue 6, August 2004, Pages 1183–1187</ref><ref>[http://www.sabri.org.za www.sabri.org.za]</ref> |
|||
Long-term exposure to nitrous oxide may cause [[Vitamin B12 deficiency|vitamin B{{ssub|12}} deficiency]]. This can cause serious neurotoxicity if the user has preexisting vitamin B{{ssub|12}} deficiency.<ref>{{Cite journal|last2=Holder|first2=W. D. Jr.|year=1993|title=Neurologic Degeneration Associated with Nitrous Oxide Anesthesia in Patients with Vitamin B12 Deficiency|journal=Archives of Surgery|volume=128|issue=12|pages=1391–5|doi=10.1001/archsurg.1993.01420240099018|pmid=8250714|last1=Flippo|first1=T. S.}}</ref> It inactivates the cobalamin form of vitamin B{{ssub|12}} by oxidation. Symptoms of vitamin B{{ssub|12}} deficiency, including [[Peripheral neuropathy|sensory neuropathy]], [[myelopathy]] and [[encephalopathy]], may occur within days or weeks of exposure to nitrous oxide anaesthesia in people with subclinical vitamin B{{ssub|12}} deficiency. |
|||
===Recreational use=== |
|||
Nitrous oxide can cause [[Analgesic|analgesia]], [[depersonalization]], [[derealization]], [[dizziness]], [[Euphoria (emotion)|euphoria]], and some sound distortion.<ref>AJ Giannini. Volatiles. In NS Miller (Ed.). [http://books.google.com/books?doi=VgetLfJBQv0C&pg=PA396 Comprehensive Handbook of Drug and Alcohol Addiction]. NY, Marcel Dekker, 1991 ISBN 0-8247-8474-X</ref> Research has also found that it increases [[suggestibility]] and [[imagination]].<ref>Whalley MG, Brooks GB. (2009). Enhancement of suggestibility and imaginative ability with nitrous oxide. Psychopharmacology (Berl). 203(4):745-52. 10.1007/s00213-008-1424-0 PMID 19057896</ref> Inhalation of nitrous oxide for recreational use, with the purpose to cause euphoria and slight hallucinations, began as a phenomenon for the British upper class in 1799, known as "laughing gas parties". Until at least 1863, a low availability of equipment to produce the gas, combined with a low usage of the gas for medical purposes, meant it was a relatively rare phenomenon that mainly happened among students at medical universities. When equipment became more widely available for dentistry and hospitals, most countries also restricted the legal access to buy pure nitrous oxide [[gas cylinder]]s to those sectors. As only medical staff and dentists today are legally allowed to buy the pure gas, the recreational use is also believed to be somewhat limited. The consumers union report from 1972, however found that the use of the gas for recreational purpose still take place in present time, based upon reports of its use in [[Maryland]] 1971, [[Vancouver]] 1972, and a survey made by Dr.Edward J.Lynn of its nonmedical use in [[Michigan]] 1970.<ref name="Illicit drugs"/> |
|||
Symptoms are treated with high doses of vitamin B{{ssub|12}}, but recovery can be slow and incomplete.<ref>{{cite book |last=Giannini |first=A.J. |year=1999 |title=Drug Abuse |place=Los Angeles |publisher=Health Information Press |isbn=978-1-885987-11-2 |url-access=registration |url=https://archive.org/details/drugabuse00ajam }}</ref> |
|||
*'''Citation of the results from the Michigan survey in 1970:''' ''"It was not uncommon [in the interviews] to hear from individuals who had been to parties where a professional (doctor, nurse, scientist, inhalation therapist, researcher) had provided nitrous oxide. There also were those who work in restaurants who used the N<sub>2</sub>O stored in tanks for the preparation of whip cream. Reports were received from people who used the gas contained in aerosol cans both of food and non-food products. At a recent rock festival nitrous oxide was widely sold for 25 cents a balloon. Contact was made with a "mystical-religious" group that used the gas to accelerate arriving at their [[transcendental meditation|transcendental-meditative]] state of choice. Although a few, more sophisticated users employed nitrous oxide-oxygen mixes with elaborate equipment, most users used balloons or plastic bags. They either held a breath of N<sub>2</sub>O or rebreathed the gas. There were no adverse effects reported in the more than one hundred individuals surveyed."''<ref name="Illicit drugs"/> |
|||
People with normal vitamin B{{ssub|12}} levels have stores to make the effects of nitrous oxide insignificant, unless exposure is repeated and prolonged (nitrous oxide abuse). Vitamin B{{ssub|12}} levels should be checked in people with risk factors for vitamin B{{ssub|12}} deficiency prior to using nitrous oxide anaesthesia.<ref>{{Cite web |last=Conrad |first=Marcel |title=Pernicious Anemia |website=Medscape |date=4 October 2006 |url=http://www.emedicine.com/med/topic1799.htm |access-date=2 June 2008}}</ref> |
|||
Inhaling nitrous oxide from tanks used in automotive systems is unsafe, because the toxic gas [[sulfur dioxide]] is mixed in around 100 ppm, specifically to discourage recreational use.<ref name="Automotive gas"/> |
|||
===Prenatal development=== |
|||
==Neuropharmacology== |
|||
Several experimental studies in rats indicate that chronic exposure of pregnant females to nitrous oxide may have adverse effects on the developing fetus.<ref name="Vieira1980">{{cite journal|pmid=7189346 |year=1980 |last1=Vieira |first1=E. |last2=Cleaton-Jones |first2=P. |last3=Austin |first3=J.C. |last4=Moyes |first4=D.G. |last5=Shaw |first5=R. |title=Effects of low concentrations of nitrous oxide on rat fetuses |volume=59 |issue=3 |pages=175–7 |journal=Anesthesia and Analgesia |doi=10.1213/00000539-198003000-00002|s2cid=41966990 |doi-access=free }}</ref><ref>{{cite journal|pmid=465253 |year=1979 |last1=Vieira |first1=E. |title=Effect of the chronic administration of nitrous oxide 0.5% to gravid rats |volume=51 |issue=4 |pages=283–7 |journal=British Journal of Anaesthesia |doi=10.1093/bja/51.4.283|doi-access=free }}</ref><ref>{{cite journal|pmid=6821624 |year=1983 |last1=Vieira |first1=E |last2=Cleaton-Jones |first2=P |last3=Moyes |first3=D. |title=Effects of low intermittent concentrations of nitrous oxide on the developing rat fetus |volume=55 |issue=1 |pages=67–9 |journal=British Journal of Anaesthesia |doi=10.1093/bja/55.1.67|doi-access=free }}</ref> |
|||
===Chemical/physical risks=== |
|||
The pharmacological [[mechanism of action]] of N<sub>2</sub>O in medicine is not fully known. However, it has been shown to directly modulate a broad range of [[ligand-gated ion channel]]s, and this likely plays a major role in many of its effects. It moderately blocks [[NMDA receptor|NMDA]] and [[CHRNB2|β<sub>2</sub>-subunit]]-containing [[nicotinic acetylcholine receptor|nACh channel]]s, weakly inhibits [[AMPA receptor|AMPA]], [[kainate receptor|kainate]], [[GABAC receptor|GABA<sub>C</sub>]], and [[5-HT3 receptor|5-HT<sub>3</sub> receptor]]s, and slightly potentiates [[GABAA receptor|GABA<sub>A</sub>]] and [[glycine receptor]]s.<ref name="pmid11020766">{{cite journal|author = Yamakura T, Harris RA|title = Effects of gaseous anesthetics nitrous oxide and xenon on ligand-gated ion channels. Comparison with isoflurane and ethanol|journal = Anesthesiology|volume = 93|issue = 4|pages = 1095–101|year = 2000|pmid = 11020766|url = http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0003-3022&volume=93&issue=4&spage=1095|doi = 10.1097/00000542-200010000-00034}}</ref><ref name="pmid9822732">{{cite journal|author = Mennerick S, Jevtovic-Todorovic V, Todorovic SM, Shen W, Olney JW, Zorumski CF|title = Effect of nitrous oxide on excitatory and inhibitory synaptic transmission in hippocampal cultures|journal = Journal of Neuroscience|volume = 18|issue = 23|pages = 9716–26|year = 1998|pmid = 9822732}}</ref> It has also been shown to activate [[tandem pore domain potassium channel|two-pore-domain K<sup>+</sup> channel]]s.<ref name="pmid14742687">{{cite journal|author = Gruss M, Bushell TJ, Bright DP, Lieb WR, Mathie A, Franks NP|title = Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane|journal = Molecular Pharmacology|volume = 65|issue = 2|pages = 443–52|year = 2004|pmid = 14742687|doi = 10.1124/mol.65.2.443}}</ref> While N<sub>2</sub>O affects quite a few ion channels, its [[anesthetic]], [[hallucinogenic]], and [[euphoriant]] effects are likely caused predominantly or fully via inhibition of NMDAR-mediated currents.<ref name="pmid11020766"/><ref name="pmid17352529">{{cite journal|author = Emmanouil DE, Quock RM|title = Advances in understanding the actions of nitrous oxide|journal = Anesthesia Progress|volume = 54|issue = 1|pages = 9–18|year = 2007|pmid = 17352529|pmc = 1821130|doi = 10.2344/0003-3006(2007)54[9:AIUTAO]2.0.CO;2|url = http://www.anesthesiaprogress.org/doi/abs/10.2344/0003-3006(2007)54[9:AIUTAO]2.0.CO;2}}</ref> In addition to its effects on ion channels, N<sub>2</sub>O may act to imitate [[nitric oxide]] (NO) in the central nervous system as well, and this may relate to its [[analgesic]] and [[anxiolytic]] properties.<ref name="pmid17352529"/> |
|||
At room temperature ({{Convert|20|C|disp=sqbr}}) the saturated vapour pressure is 50.525 bar, rising up to 72.45 bar at {{convert|36.4|C}}—the [[Critical point (thermodynamics)|critical temperature]]. The pressure curve is thus unusually sensitive to temperature.<ref>[http://encyclopedia.airliquide.com/encyclopedia.asp?LanguageID=11&CountryID=19&Formula=&GasID=55&UNNumber= Nitrous oxide] {{Webarchive|url=https://web.archive.org/web/20160330004038/http://encyclopedia.airliquide.com/encyclopedia.asp?LanguageID=11&CountryID=19&Formula=&GasID=55&UNNumber= |date=30 March 2016 }}. Air Liquide Gas Encyclopedia.</ref> |
|||
As with many strong oxidisers, contamination of parts with fuels have been implicated in rocketry accidents, where small quantities of nitrous/fuel mixtures explode due to "[[water hammer]]"-like effects (sometimes called "dieseling"—heating due to [[adiabatic]] compression of gases can reach decomposition temperatures).<ref>{{cite web|url=http://www.ukrocketman.com/rocketry/hybridukhistory.shtml |title=Vaseline triggered explosion of hybrid rocket |publisher=Ukrocketman.com}}</ref> Some common building materials such as stainless steel and aluminium can act as fuels with strong oxidisers such as nitrous oxide, as can contaminants that may ignite due to adiabatic compression.<ref>{{cite web|url=http://www.airproducts.com/nr/rdonlyres/8c46596e-2f7d-4895-b12a-e54cd63e1996/0/safetygram20.pdf |archive-url=https://web.archive.org/web/20060901093045/http://www.airproducts.com/nr/rdonlyres/8c46596e-2f7d-4895-b12a-e54cd63e1996/0/safetygram20.pdf |archive-date=1 September 2006 |title=Safetygram 20: Nitrous Oxide |publisher=Airproducts.com}}</ref> |
|||
===Anxiolytic effect=== |
|||
There also have been incidents where nitrous oxide decomposition in plumbing has led to the explosion of large tanks.<ref name="Munke" /> |
|||
In behavioral tests of [[anxiety]], a low dose of N<sub>2</sub>O is an effective [[anxiolytic]], and this anti-anxiety effect is associated with enhanced activity of GABA<sub>A</sub> receptors as it is partially reversed by [[GABAA receptor|benzodiazepine receptor]] [[receptor antagonist|antagonist]]s. Mirroring this, animals which have developed tolerance to the anxiolytic effects of [[benzodiazepine]]s are partially tolerant to N<sub>2</sub>O.<ref name="emmanouil">{{cite journal|title = Nitrous oxide anxiolytic effect in mice in the elevated plus maze: mediation by benzodiazepine receptors|author = Emmanouil, D.E., Johnson, C.H. & Quock, R.M.|journal = Psychopharmacology|volume = 115|issue = 1–2|pages = 167–72|year = 1994|doi = 10.1007/BF02244768|pmid = 7862891}}</ref> Indeed, in humans given 30% N<sub>2</sub>O, benzodiazepine receptor antagonists reduced the subjective reports of feeling "high", but did not alter [[psycho-motor]] performance, in human clinical studies.<ref name="zacny">{{cite journal|title = Flumazenil may attenuate some subjective effects of nitrous oxide in humans: a preliminary report|author = Zacny, J.P., Yajnik, S., Coalson, D., Lichtor, J.L., Apfelbaum, J.L., Rupani, G., Young, C., Thapar, P. & Klafta, J.|journal = Pharmacology Biochemistry and Behavior|volume = 51|issue = 4|pages = 815–9|year = 1995|doi = 10.1016/0091-3057(95)00039-Y|pmid = 7675863}}</ref> |
|||
==Mechanism of action== |
|||
The pharmacological [[mechanism of action]] of {{chem|N|2|O}} in medicine is not fully known. However, it has been shown to directly modulate a broad range of [[ligand-gated ion channel]]s, and this likely plays a major role in many of its effects. It moderately blocks [[NMDA receptor|NMDAR]] and [[CHRNB2|β{{ssub|2}}-subunit]]-containing [[nicotinic acetylcholine receptor|nACh channels]], weakly inhibits [[AMPA receptor|AMPA]], [[kainate receptor|kainate]], [[GABAA-rho receptor|GABA{{ssub|C}}]] and [[5-HT3 receptor|5-HT{{ssub|3}} receptors]], and slightly potentiates [[GABAA receptor|GABA{{ssub|A}}]] and [[glycine receptor]]s.<ref name="pmid11020766">{{cite journal|vauthors=Yamakura T, Harris RA |title=Effects of gaseous anaesthetics nitrous oxide and xenon on ligand-gated ion channels. Comparison with isoflurane and ethanol |journal=Anesthesiology |volume=93 |issue=4 |pages=1095–101 |year=2000 |pmid=11020766 |doi=10.1097/00000542-200010000-00034|s2cid=4684919 |doi-access=free }}</ref><ref name="pmid9822732">{{cite journal |vauthors=Mennerick S, Jevtovic-Todorovic V, Todorovic SM, Shen W, Olney JW, Zorumski CF |title=Effect of nitrous oxide on excitatory and inhibitory synaptic transmission in hippocampal cultures |journal=Journal of Neuroscience |volume=18 |issue=23 |pages=9716–26 |year=1998 |pmid=9822732 |pmc=6793274 |doi=10.1523/JNEUROSCI.18-23-09716.1998 }}</ref> It also has been shown to activate [[Two-pore-domain potassium channel|two-pore-domain {{chem|K|+}} channels]].<ref name="pmid14742687">{{cite journal |vauthors=Gruss M, Bushell TJ, Bright DP, Lieb WR, Mathie A, Franks NP |title=Two-pore-domain K<sup>+</sup> channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane |journal=Molecular Pharmacology |volume=65 |issue=2 |pages=443–52 |year=2004 |pmid=14742687 |doi=10.1124/mol.65.2.443|s2cid=7762447 }}</ref> While {{chem|N|2|O}} affects quite a few ion channels, its anaesthetic, [[hallucinogenic]] and [[euphoriant]] effects are likely caused predominantly, or fully, via inhibition of NMDA receptor-mediated currents.<ref name="pmid11020766" /><ref name="pmid17352529">{{cite journal|vauthors=Emmanouil DE, Quock RM |title=Advances in Understanding the Actions of Nitrous Oxide |journal=Anesthesia Progress |volume=54 |issue=1 |pages=9–18 |year=2007 |pmid=17352529 |pmc=1821130 |doi=10.2344/0003-3006(2007)54[9:AIUTAO]2.0.CO;2}}</ref> In addition to its effects on ion channels, {{chem|N|2|O}} may act to imitate [[nitric oxide]] (NO) in the central nervous system, and this may be related to its [[analgesic]] and [[anxiolytic]] properties.<ref name="pmid17352529" /> Nitrous oxide is 30 to 40 times more soluble than nitrogen. |
|||
The effects of inhaling sub-anaesthetic doses of nitrous oxide have been known to vary, based on several factors, including settings and individual differences;<ref>{{Cite journal|last1=Atkinson|first1=Roland M.|last2=Green|first2=J. DeWayne|last3=Chenoweth|first3=Dennis E.|last4=Atkinson|first4=Judith Holmes|date=1979-10-01|title=Subjective Effects of Nitrous Oxide: Cognitive, Emotional, Perceptual and Transcendental Experiences|journal=Journal of Psychedelic Drugs|volume=11|issue=4|pages=317–330|doi=10.1080/02791072.1979.10471415|pmid=522172}}</ref><ref>{{Cite journal|last1=Walker|first1=Diana J.|last2=Zacny|first2=James P.|date=2001-09-01|title=Within- and between-subject variability in the reinforcing and subjective effects of nitrous oxide in healthy volunteers|journal=Drug and Alcohol Dependence|volume=64|issue=1|pages=85–96|doi=10.1016/s0376-8716(00)00234-9|pmid=11470344}}</ref> however, from his discussion, Jay (2008)<ref name="Mike Jay 22–25">{{Cite journal|vauthors=Jay M |date=2008-09-01|title=Nitrous oxide: recreational use, regulation and harm reduction|journal=Drugs and Alcohol Today|volume=8|issue=3|pages=22–25|doi=10.1108/17459265200800022}}</ref> suggests that it has been reliably known to induce the following states and sensations: |
|||
* Intoxication |
|||
* Euphoria/dysphoria |
|||
* Spatial disorientation |
|||
* Temporal disorientation |
|||
* Reduced pain sensitivity |
|||
A minority of users also will present with uncontrolled vocalisations and muscular spasms. These effects generally disappear minutes after removal of the nitrous oxide source.<ref name="Mike Jay 22–25"/> |
|||
===Anxiolytic effect=== |
|||
In behavioural tests of [[anxiety]], a low dose of {{chem|N|2|O}} is an effective anxiolytic, and this anti-anxiety effect is associated with enhanced activity of GABA{{ssub|A}} receptors, as it is partially reversed by [[GABAA receptor|benzodiazepine receptor]] [[receptor antagonist|antagonists]]. Mirroring this, animals that have developed tolerance to the anxiolytic effects of [[benzodiazepine]]s are partially tolerant to {{chem|N|2|O}}.<ref name="emmanouil">{{cite journal|title=Nitrous oxide anxiolytic effect in mice in the elevated plus maze: mediation by benzodiazepine receptors |vauthors=Emmanouil DE, Johnson CH, Quock RM |journal=Psychopharmacology |volume=115 |issue=1–2 |pages=167–72 |year=1994 |doi=10.1007/BF02244768 |pmid=7862891|s2cid=21652496 }}</ref> Indeed, in humans given 30% {{chem|N|2|O}}, benzodiazepine receptor antagonists reduced the subjective reports of feeling "high", but did not alter [[psychomotor learning|psychomotor]] performance, in human clinical studies.<ref name="zacny">{{cite journal|title=Flumazenil may attenuate some subjective effects of nitrous oxide in humans: a preliminary report |vauthors=Zacny JP, Yajnik S, Coalson D, Lichtor JL, Apfelbaum JL, Rupani G, Young C, Thapar P, Klafta J |journal=Pharmacology Biochemistry and Behavior |volume=51 |issue=4 |pages=815–9 |year=1995 |doi=10.1016/0091-3057(95)00039-Y |pmid=7675863|s2cid=39068081 }}</ref><ref>{{Cite journal |last=Gillman |first=Mark Akfred |date=2022 |title=What is better for psychiatry: Titrated or fixed concentrations of nitrous oxide? |journal=Front. Psychiatry |volume=13 |issue=773190 |pages=460–3|doi=10.3389/fpsyt.2022.773190 |pmid=36072452 |pmc=9441863 |doi-access=free }}</ref> |
|||
===Analgesic effect=== |
===Analgesic effect=== |
||
The analgesic effects of N |
The analgesic effects of {{chem|N|2|O}} are linked to the interaction between the [[Opioid#Endogenous opioids|endogenous opioid]] system and the descending [[Norepinephrine|noradrenergic]] system. When animals are given morphine chronically, they develop tolerance to its pain-killing effects, and this also renders the animals tolerant to the analgesic effects of {{chem|N|2|O}}.<ref>{{cite journal|title=Tolerance to nitrous oxide analgesia in rats and mice |vauthors=Berkowitz BA, Finck AD, Hynes MD, Ngai SH |journal=Anesthesiology |volume=51 |issue=4 |pages=309–12 |year=1979 |doi=10.1097/00000542-197910000-00006 |pmid=484891|s2cid=26281498 |doi-access=free }}</ref> Administration of [[antibodies]] that bind and block the activity of some endogenous opioids (not [[Beta-Endorphin|β-endorphin]]) also block the antinociceptive effects of {{chem|N|2|O}}.<ref name="branda">{{cite journal|title=Role of brain dynorphin in nitrous oxide antinociception in mice |vauthors=Branda EM, Ramza JT, Cahill FJ, Tseng LF, Quock RM |journal=Pharmacology Biochemistry and Behavior |volume=65 |pages=217–21 |year=2000 |doi=10.1016/S0091-3057(99)00202-6 |pmid=10672972 |issue=2|s2cid=1978597 }}</ref> Drugs that inhibit the breakdown of endogenous opioids also potentiate the antinociceptive effects of {{chem|N|2|O}}.<ref name="branda" /> Several experiments have shown that opioid receptor antagonists applied directly to the brain block the antinociceptive effects of {{chem|N|2|O}}, but these drugs have no effect when injected into the [[spinal cord]]. |
||
Apart from an indirect action, nitrous oxide, like morphine <ref>Gillman M.A. [1986a]. Minireview: Analgesic [sub anaesthetic] nitrous oxide interacts with the endogenous opioid system : A review of the evidence. Life Sciences 39: l209-l22l</ref> also interacts directly with the endogenous opioid system by binding at opioid receptor binding sites.<ref>(Daras, C., Cantrill, R. C., Gillman, M. A. [1983]. 3[H]-Naloxone displacement: evidence for nitrous oxide as an opioid agonist. European Journal of Pharmacology 89: 177-8.</ref><ref>Ori, C., Ford-Rice, F., London, E. D. [1989]. Effects of nitrous oxide and halothane on mu and kappa opioid receptors in guinea-pig brain. Anesthesiology 70: 541-544.)</ref> |
|||
Conversely, [[alpha-2 adrenergic receptor|α<sub>2</sub>-adrenoceptor]] antagonists block the antinociceptive effects of N<sub>2</sub>O when given directly to the spinal cord, but not when applied directly to the brain.<ref name="guo">{{cite journal|title = Nitrous oxide produces antinociceptive response via alpha2B and/or alpha2C adrenoceptor subtypes in mice|author = Guo, T.Z., Davies, M.F., Kingery, W.S., Patterson, A.J., Limbird, L.E. & Maze, M.|journal = Anesthesiology|volume = 90|issue =2|pages = 470–6|year = 1999|pmid = 9952154|doi = 10.1097/00000542-199902000-00022}}</ref> Indeed, [[alpha-2B adrenergic receptor|α<sub>2B</sub>-adrenoceptor]] knockout mice or animals depleted in [[norepinephrine]] are nearly completely resistant to the antinociceptive effects of N<sub>2</sub>O.<ref>{{cite journal|title = Antinociceptive action of nitrous oxide is mediated by stimulation of noradrenergic neurons in the brainstem and activation of [alpha]<sub>2B</sub> adrenoceptors|author = Sawamura, S., Kingery, W.S., Davies, M.F., Agashe, G.S., Clark, J.D., Koblika, B.K., Hashimoto, T. & Maze, M.|journal = J. Neurosci.|volume = 20|issue = 24|pages = 9242–51|year = 2000|pmid = 11125002}}</ref> It seems N<sub>2</sub>O-induced release of endogenous opioids causes disinhibition of [[brain stem]] noradrenergic neurons, which release [[norepinephrine]] into the spinal cord and inhibit pain signaling.<ref name="pmid10781114">{{cite journal|author = Maze M, Fujinaga M|title = Recent advances in understanding the actions and toxicity of nitrous oxide|journal = Anaesthesia|volume = 55|issue = 4|pages = 311–4|year = 2000|pmid = 10781114|doi = 10.1046/j.1365-2044.2000.01463.x}}</ref> Exactly how N<sub>2</sub>O causes the release of endogenous opioid peptides is still uncertain. |
|||
Conversely, [[alpha-2 adrenergic receptor|α{{ssub|2}}-adrenoceptor]] antagonists block the pain-reducing effects of {{chem|N|2|O}} when given directly to the spinal cord, but not when applied directly to the brain.<ref name="guo">{{cite journal|title=Nitrous oxide produces antinociceptive response via alpha2B and/or alpha2C adrenoceptor subtypes in mice |vauthors=Guo TZ, Davies MF, Kingery WS, Patterson AJ, Limbird LE, Maze M |journal=Anesthesiology |volume=90 |issue=2 |pages=470–6 |year=1999 |pmid=9952154 |doi=10.1097/00000542-199902000-00022|doi-access=free }}</ref> Indeed, [[alpha-2B adrenergic receptor|α{{ssub|2B}}-adrenoceptor]] knockout mice or animals depleted in [[norepinephrine]] are nearly completely resistant to the antinociceptive effects of {{chem|N|2|O}}.<ref>{{cite journal|title=Antinociceptive action of nitrous oxide is mediated by stimulation of noradrenergic neurons in the brainstem and activation of [alpha]{{ssub|2B}} adrenoceptors |vauthors=Sawamura S, Kingery WS, Davies MF, Agashe GS, Clark JD, Koblika BK, Hashimoto T, Maze M |journal=J. Neurosci. |volume=20 |issue=24 |pages=9242–51 |year=2000 |pmid=11125002 |pmc=6773006 |doi=10.1523/JNEUROSCI.20-24-09242.2000 }}</ref> Apparently {{chem|N|2|O}}-induced release of endogenous opioids causes disinhibition of [[brainstem]] noradrenergic neurons, which release norepinephrine into the spinal cord and inhibit pain signalling.<ref name="pmid10781114">{{cite journal|vauthors=Maze M, Fujinaga M |title=Recent advances in understanding the actions and toxicity of nitrous oxide |journal=Anaesthesia |volume=55 |issue=4 |pages=311–4 |year=2000 |pmid=10781114 |doi=10.1046/j.1365-2044.2000.01463.x|s2cid=39823627 |doi-access=free }}</ref> Exactly how {{chem|N|2|O}} causes the release of endogenous opioid peptides remains uncertain. |
|||
===Euphoric effect=== |
|||
In rats, N<sub>2</sub>O stimulates the [[mesolimbic pathway|mesolimbic reward pathway]] via inducing [[dopamine]] release and activating [[dopaminergic]] [[neuron]]s in the [[ventral tegmental area]] and [[nucleus accumbens]], presumably through [[NMDA receptor antagonist|antagonization of NMDA receptors]] localized in the system.<ref name="pmid17122223">{{cite journal|author = Sakamoto S, Nakao S, Masuzawa M, ''et al.''|title = The differential effects of nitrous oxide and xenon on extracellular dopamine levels in the rat nucleus accumbens: a microdialysis study|journal = Anesthesia and Analgesia|volume = 103|issue = 6|pages = 1459–63|year = 2006|pmid = 17122223|doi = 10.1213/01.ane.0000247792.03959.f1}}</ref><ref name="pmid18571333">{{cite journal|author = Benturquia N, Le Marec T, Scherrmann JM, Noble F|title = Effects of nitrous oxide on dopamine release in the rat nucleus accumbens and expectation of reward|journal = Neuroscience|volume = 155|issue = 2|pages = 341–4|year = 2008|pmid = 18571333|doi = 10.1016/j.neuroscience.2008.05.015}}</ref><ref name="pmid8726543">{{cite journal|author = Lichtigfeld FJ, Gillman MA|title = Role of dopamine mesolimbic system in opioid action of psychotropic analgesic nitrous oxide in alcohol and drug withdrawal|journal = Clinical Neuropharmacology|volume = 19|issue = 3|pages = 246–51|year = 1996|pmid = 8726543|doi = 10.1097/00002826-199619030-00006}}</ref><ref name="pmid18499630">{{cite journal|author = Koyanagi S, Himukashi S, Mukaida K, Shichino T, Fukuda K|title = Dopamine D2-like receptor in the nucleus accumbens is involved in the antinociceptive effect of nitrous oxide|journal = Anesthesia and Analgesia|volume = 106|issue = 6|pages = 1904–9|year = 2008|pmid = 18499630|doi = 10.1213/ane.0b013e318172b15b}}</ref> This action has been implicated in its euphoric effects, and notably, appears to augment its analgesic properties as well.<ref name="pmid17122223"/><ref name="pmid18571333"/><ref name="pmid8726543"/><ref name="pmid18499630"/> |
|||
==Properties and reactions== |
|||
However, it is remarkable that in mice, N<sub>2</sub>O blocks [[amphetamine]]-induced carrier-mediated dopamine release in the nucleus accumbens and [[behavioral sensitization]], abolishes the [[conditioned place preference]] (CPP) of [[cocaine]] and [[morphine]], and does not produce reinforcing (or aversive) effects of its own.<ref name="pmid16427030">{{cite journal|author = David HN, Ansseau M, Lemaire M, Abraini JH|title = Nitrous oxide and xenon prevent amphetamine-induced carrier-mediated dopamine release in a memantine-like fashion and protect against behavioral sensitization|journal = Biological Psychiatry|volume = 60|issue = 1|pages = 49–57|year = 2006|pmid = 16427030|doi = 10.1016/j.biopsych.2005.10.007}}</ref><ref name="pmid17905521">{{cite journal|author = Benturquia N, Le Guen S, Canestrelli C, ''et al.''|title = Specific blockade of morphine- and cocaine-induced reinforcing effects in conditioned place preference by nitrous oxide in mice|journal = Neuroscience|volume = 149|issue = 3|pages = 477–86|year = 2007|pmid = 17905521|doi = 10.1016/j.neuroscience.2007.08.003}}</ref> Studies on CPP of N<sub>2</sub>O in rats is mixed, consisting of reinforcement, aversion, and no change.<ref name="pmid12543228">{{cite journal|author = Ramsay DS, Watson CH, Leroux BG, Prall CW, Kaiyala KJ|title = Conditioned place aversion and self-administration of nitrous oxide in rats|journal = Pharmacology, Biochemistry, and Behavior|volume = 74|issue = 3|pages = 623–33|year = 2003|pmid = 12543228|doi = 10.1016/S0091-3057(02)01048-1}}</ref> In contrast, it is a positive reinforcer in squirrel monkeys,<ref name="pmid408480">{{cite journal|author = Wood RW, Grubman J, Weiss B|title = Nitrous oxide self-administration by the squirrel monkey|journal = The Journal of Pharmacology and Experimental Therapeutics|volume = 202|issue = 3|pages = 491–9|year = 1977|pmid = 408480}}</ref> and is well known as a [[drug abuse|drug of abuse]] in humans.<ref name="pmid9915336">{{cite journal|author = Zacny JP, Galinkin JL|title = Psychotropic drugs used in anesthesia practice: abuse liability and epidemiology of abuse|journal = Anesthesiology|volume = 90|issue = 1|pages = 269–88|year = 1999|pmid = 9915336|url = http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0003-3022&volume=90&issue=1&spage=269}}</ref> These discrepancies in response to N<sub>2</sub>O may reflect species variation or methodological differences.<ref name="pmid17905521"/> Though, it is noteworthy that in human clinical studies, N<sub>2</sub>O was found to produce mixed responses similarly to rats, reflecting high subjective individual variability.<ref name="pmid8462415">{{cite journal|author = Dohrn CS, Lichtor JL, Coalson DW, Uitvlugt A, de Wit H, Zacny JP|title = Reinforcing effects of extended inhalation of nitrous oxide in humans|journal = Drug and Alcohol Dependence|volume = 31|issue = 3|pages = 265–80|year = 1993|pmid = 8462415|doi = 10.1016/0376-8716(93)90009-F}}</ref><ref name="pmid11470344">{{cite journal|author = Walker DJ, Zacny JP|title = Within- and between-subject variability in the reinforcing and subjective effects of nitrous oxide in healthy volunteers|journal = Drug and Alcohol Dependence|volume = 64|issue = 1|pages = 85–96|year = 2001|pmid = 11470344|doi = 10.1016/S0376-8716(00)00234-9}}</ref> |
|||
Nitrous oxide is a colourless gas with a faint, sweet odour. |
|||
Nitrous oxide supports combustion by releasing the [[Coordinate covalent bond|dipolar bonded]] oxygen radical, and can thus relight a glowing [[Splint (laboratory equipment)|splint]]. |
|||
===Neurotoxicity=== |
|||
Similarly to other [[NMDA antagonist]]s like [[ketamine]], N<sub>2</sub>O has been demonstrated to produce [[neurotoxicity]] in the form of [[Olney's lesions]] (damage to the [[posterior cingulate]] and [[retrosplenial region|retrosplenial cortices]] of the [[brain]]) in rodents upon prolonged (e.g., several hour) exposure.<ref name="pmid14622904">{{cite journal|author = Jevtovic-Todorovic V, Beals J, Benshoff N, Olney JW|title = Prolonged exposure to inhalational anesthetic nitrous oxide kills neurons in adult rat brain|journal = Neuroscience|volume = 122|issue = 3|pages = 609–16|year = 2003|pmid = 14622904|doi = 10.1016/j.neuroscience.2003.07.012}}</ref><ref name="pmid12854473">{{cite journal|author = Nakao S, Nagata A, Masuzawa M, ''et al.''|url = NMDA|title = receptor antagonist neurotoxicity and psychotomimetic activity|language = Japanese|journal = Masui. the Japanese Journal of Anesthesiology|volume = 52|issue = 6|pages = 594–602|year = 2003|pmid = 12854473}}</ref><ref name="pmid10928976">{{cite journal|author = Jevtovic-Todorovic V, Benshoff N, Olney JW|title = Ketamine potentiates cerebrocortical damage induced by the common anaesthetic agent nitrous oxide in adult rats|journal = British Journal of Pharmacology|volume = 130|issue = 7|pages = 1692–8|year = 2000|pmid = 10928976|pmc = 1572233|doi = 10.1038/sj.bjp.0703479}}</ref><ref name="pmid15718054">{{cite journal|author = Jevtovic-Todorovic V, Carter LB|title = The anesthetics nitrous oxide and ketamine are more neurotoxic to old than to young rat brain|journal = Neurobiology of Aging|volume = 26|issue = 6|pages = 947–56|year = 2005|pmid = 15718054|doi = 10.1016/j.neurobiolaging.2004.07.009}}</ref> However, it also simultaneously exerts widespread [[neuroprotective]] effects via inhibiting [[glutamate]]-induced [[excitotoxicity]], and it has been argued that on account of its very short duration under normal circumstances, N<sub>2</sub>O may not share the neurotoxicity of other NMDA antagonists.<ref name="pmid16179534">{{cite journal|author = Abraini JH, David HN, Lemaire M|title = Potentially neuroprotective and therapeutic properties of nitrous oxide and xenon|journal = Annals of the New York Academy of Sciences|volume = 1053|pages = 289–300|year = 2005|pmid = 16179534|doi = 10.1196/annals.1344.025}}</ref> Indeed, in rodents, short-term exposure results in only mild injury that is rapidly reversible, and permanent neuronal death only occurs after constant and sustained exposure.<ref name="pmid14622904"/> Moreover, Olney's lesions have never been observed in primates (including humans). However, Olney's lesions must be observed within a few hours of death, which may explain why they have not been observed in primates. After a few hours, depending on dose, the vacuoles that have appeared in the neurons resolve. If the dose is large enough to kill neurons, glial cells fill in any spaces left by the dead neurons within a short time, making it impossible to tell that neurons were even there.<ref>{{cite journal|last=Brosnan-Watters|first=Gayle|coauthors=Wozniak, Nardi, Olney|title=Parallel recovery of MK-801-induced spatial learning impairment and neuronal injury in male mice|journal=Pharmacology, Biochemistry, and Behavior|year=1999|year=1997|volume=66|issue=1|pages=111–122}}</ref><ref>{{cite journal|last=Wozniak|first=David|coauthors=Brosnan-Watters, Nardi, McEwen, Corso, Olney, Fix|title=MK-801 Neurotoxicity in male mice: histological effects and chronic impairment in spatial learning|journal=Brain Research|year=1996|volume=707|pages=165–179}}</ref> Humans cannot be exposed to nitrous oxide and killed in order to investigate whether brain injury has occurred, and in most cases, primates are not killed either. While it is then impossible to determine if brain injury does result from the use of nitrous oxide, it is most likely that it does not cause cell death because exposure is typically not long enough to do so. |
|||
{{chem|N|2|O}} is inert at room temperature and has few reactions. At elevated temperatures, its reactivity increases. For example, nitrous oxide reacts with {{chem|link=Sodium amide|NaNH|2}} at {{convert|187|C}} to give {{chem|link=Sodium azide|NaN|3}}: |
|||
==Safety== |
|||
:{{chem2|2 NaNH2 + N2O -> NaN3 + NaOH + NH3 }} |
|||
The major safety hazards of nitrous oxide come from the fact that it is a compressed liquefied gas, an asphyxiation risk, and a [[dissociative]] [[anaesthetic]]. Exposure to nitrous oxide causes short-term decreases in mental performance, audiovisual ability, and manual dexterity.<ref>Criteria for a recommended standard: occupational exposure to waste anesthetic gases and vapors. Cincinnati, OH: U.S. Department of Health, Education, and Welfare, Public Health Service, Center for Disease Control, National Institute for Occupational Safety and Health, DHEW (NIOSH) Publication No. 77B140.</ref> Long term exposure can cause vitamin B{{ssub|12}} deficiency, numbness, reproductive side effects (in pregnant females), and other problems (see ''[[Nitrous oxide#Recreational use|Recreational use]]'' and ''[[Nitrous oxide#Biological|Biological]]'' factors in this article). |
|||
The above reaction is the route adopted by the commercial chemical industry to produce [[azide]] salts, which are used as detonators.<ref name="InorgChem">{{cite book|title=Inorganic Chemistry|url=https://archive.org/details/inorganicchemist00hous_159|url-access=limited|publisher=Pearson|year=2008|isbn=978-0-13-175553-6|edition=3rd|page=[https://archive.org/details/inorganicchemist00hous_159/page/n502 464]|chapter=Chapter 15: The group 15 elements|author1=Housecroft, Catherine E.|author2=Sharpe, Alan G.}}</ref> |
|||
The [[National Institute for Occupational Safety and Health]] recommends that workers' exposure to nitrous oxide should be controlled during the administration of anesthetic gas in medical, dental, and veterinary operators.<ref>[http://www.cdc.gov/niosh/noxidalr.html CDC.gov NIOSH Alert: Controlling Exposures to Nitrous Oxide During Anesthetic Administration]. Cincinnati, OH: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 94-100</ref> |
|||
==History== |
|||
===Chemical/physical=== |
|||
The gas was first synthesised in 1772 by English [[Natural philosophy|natural philosopher]] and chemist [[Joseph Priestley]] who called it ''dephlogisticated nitrous air'' (see [[phlogiston theory]])<ref name="Nitrous Oxide pioneers">{{cite journal|last=Keys|first=T.E.|year=1941|title=The Development of Anesthesia|journal=Anesthesiology|volume=2|issue=5|pages=552–574|bibcode=1982AmSci..70..522D|doi=10.1097/00000542-194109000-00008|s2cid=73062366|doi-access=free}}</ref> or ''inflammable nitrous air''.<ref>{{cite journal|last1=McEvoy|first1=J. G.|title=Gases, God and the balance of nature: a commentary on Priestley (1772) 'Observations on different kinds of air'|journal=Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences|date=6 March 2015|volume=373|issue=2039|page=20140229|doi=10.1098/rsta.2014.0229|pmc=4360083|pmid=25750146|bibcode=2015RSPTA.37340229M}}</ref> Priestley published his discovery in the book [[Experiments and Observations on Different Kinds of Air|''Experiments and Observations on Different Kinds of Air (1775)'']], where he described how to produce the preparation of "nitrous air diminished", by heating iron filings dampened with [[nitric acid]].<ref name="Joseph Priestley">{{cite web |year=1776|title=Experiments and Observations on Different Kinds of Air |website=Erowid |url=http://www.erowid.org/chemicals/nitrous/nitrous_journal1.shtml |author=Priestley J}}</ref> |
|||
At room temperature (20°C) the saturated vapor pressure is 58.5 bar, rising up to 72.45 bar at 36.4°C — the [[critical temperature]]. The pressure curve is thus unusually sensitive to temperature.<ref>[http://encyclopedia.airliquide.com/encyclopedia.asp?LanguageID=11&CountryID=19&Formula=&GasID=55&UNNumber= Air Liquid data on Nitrous oxide]</ref> Liquid nitrous oxide acts as a good solvent for many [[organic compounds]]; liquid mixtures may form shock sensitive explosives.{{Citation needed|date=August 2007}} |
|||
===Early use=== |
|||
As with many strong oxidizers, contamination of parts with fuels have been implicated in rocketry accidents, where small quantities of nitrous/fuel mixtures explode due to 'water hammer' like effects (sometimes called 'dieseling' — heating due to [[adiabatic]] compression of gases can reach decomposition temperatures).<ref>[http://www.ukrocketman.com/rocketry/hybridukhistory.shtml Vaseline triggered explosion of hybrid rocket]</ref> Some common building materials such as stainless steel and aluminium can act as fuels with strong oxidisers such as nitrous oxide, as can contaminants, which can ignite due to adiabatic compression.<ref>[http://www.airproducts.com/nr/rdonlyres/8c46596e-2f7d-4895-b12a-e54cd63e1996/0/safetygram20.pdf Safetygram 20: Nitrous Oxide]</ref> |
|||
[[File:Laughing_gas_Rumford_Davy.jpg|upright=1.4|thumb|"Living Made Easy": A satirical print from 1830 depicting [[Humphry Davy]] administering a dose of laughing gas to a woman|left]] |
|||
The first important use of nitrous oxide was made possible by [[Thomas Beddoes]] and [[James Watt]], who worked together to publish the book ''Considerations on the Medical Use and on the Production of Factitious Airs (1794)''. This book was important for two reasons. First, James Watt had invented a novel machine to produce "[[factitious airs]]" (including nitrous oxide) and a novel "breathing apparatus" to inhale the gas. Second, the book also presented the new medical theories by Thomas Beddoes, that [[tuberculosis]] and other lung diseases could be treated by inhalation of "Factitious Airs".<ref name="Drug discovery" /> |
|||
[[File:Anaesthesia exhibition, 1946 Wellcome M0009908.jpg|thumb|right|Sir [[Humphry Davy]]'s ''Researches chemical and philosophical: chiefly concerning nitrous oxide'' (1800), pages 556 and 557 (right), outlining potential anaesthetic properties of nitrous oxide in relieving pain during surgery]] |
|||
There have also been accidents where nitrous oxide decomposition in plumbing has led to the explosion of large tanks.<ref>[http://www.hobbyspace.com/AAdmin/archive/SpecialTopics/Misc/eindhoven.pdf Nitrous Oxide Trailer Rupture July 2, 2001] Report at CGA Seminar “Safety and Reliability of Industrial Gases, Equipment and Facilities”, October 15–17, 2001, St. Louis, Missouri by Konrad Munke, LindeGas AG</ref> |
|||
The machine to produce "Factitious Airs" had three parts: a furnace to burn the needed material, a vessel with water where the produced gas passed through in a spiral pipe (for impurities to be "washed off"), and finally the gas cylinder with a gasometer where the gas produced, "air", could be tapped into portable air bags (made of airtight oily silk). The breathing apparatus consisted of one of the portable air bags connected with a tube to a mouthpiece. With this new equipment being engineered and produced by 1794, the way was paved for [[clinical trial]]s,{{Clarify|date=April 2011}} which began in 1798 when Thomas Beddoes established the ''"[[Pneumatic Institution]] for Relieving Diseases by Medical Airs"'' in [[Hotwells]] ([[Bristol]]). In the basement of the building, a large-scale machine was producing the gases under the supervision of a young Humphry Davy, who was encouraged to experiment with new gases for patients to inhale.<ref name="Drug discovery" /> The first important work of Davy was examination of the nitrous oxide, and the publication of his results in the book: ''Researches, Chemical and Philosophical (1800)''. In that publication, Davy notes the analgesic effect of nitrous oxide at page 465 and its potential to be used for surgical operations at page 556.<ref name="Humphry Davy">{{cite book|url=https://books.google.com/books?id=jhUAAAAAQAAJ|title=Researches, chemical and philosophical –chiefly concerning nitrous oxide or dephlogisticated nitrous air, and its respiration|publisher=Printed for J. Johnson|year=1800|author=Davy H}}</ref> Davy coined the name "laughing gas" for nitrous oxide.<ref>{{cite book|last1=Hardman|first1=Jonathan G.|title=Oxford Textbook of Anaesthesia|date=2017|publisher=Oxford University Press|page=529|isbn=978-0-19-964204-5}}</ref> |
|||
Despite Davy's discovery that inhalation of nitrous oxide could relieve a conscious person from pain, another 44 years elapsed before doctors attempted to use it for [[Anesthesia|anaesthesia]]. The use of nitrous oxide as a [[Recreational drug use|recreational drug]] at "laughing gas parties", primarily arranged for the [[Social class in the United Kingdom#Upper class|British upper class]], became an immediate success beginning in 1799. While the effects of the gas generally make the user appear stuporous, dreamy and sedated, some people also "get the giggles" in a state of euphoria, and frequently erupt in laughter.<ref name="Illicit drugs">{{cite web|url=http://www.druglibrary.org/schaffer/Library/studies/cu/CU43.html|title=Consumers Union Report on Licit and Illicit Drugs, Part VI – Inhalants and Solvents and Glue-Sniffing|year=1972|author=Brecher EM|work=Consumer Reports Magazine|access-date=18 December 2013}}</ref> |
|||
===Biological=== |
|||
Nitrous oxide inactivates the cobalamin form of vitamin B{{ssub|12}} by oxidation. Symptoms of vitamin B{{ssub|12}} deficiency, including [[sensory neuropathy]], [[myelopathy]], and [[encephalopathy]], can occur within days or weeks of exposure to nitrous oxide [[anesthesia]] in people with subclinical vitamin B{{ssub|12}} deficiency.{{Citation needed|date=April 2008}} Symptoms are treated with high doses of vitamin B{{ssub|12}}, but recovery can be slow and incomplete.<ref>AJ Giannini. Drug Abuse. Los Angeles, Health Information Press, 1999 ISBN 1-885987-11-0</ref> People with normal vitamin B{{ssub|12}} levels have stores to make the effects of nitrous oxide insignificant, unless exposure is repeated and prolonged (nitrous oxide abuse).{{Citation needed|date=April 2008}} Vitamin B{{sub|12}} levels should be checked in people with risk factors for vitamin B{{ssub|12}} deficiency prior to using nitrous oxide anesthesia. |
|||
One of the earliest commercial producers in the U.S. was [[George Poe]], cousin of the poet [[Edgar Allan Poe]], who also was the first to liquefy the gas.<ref name="wp">{{cite news|url=https://pqasb.pqarchiver.com/washingtonpost_historical/access/243050292.html?dids=243050292:243050292&FMT=ABS&FMTS=ABS:FT&date=FEB+03%2C+1914&author=&pub=The+Washington+Post&desc=GEORGE+POE+IS+DEAD&pqatl=google|title=George Poe is Dead|date=3 February 1914|newspaper=Washington Post|access-date=29 December 2007|archive-date=1 March 2013|archive-url=https://web.archive.org/web/20130301050848/http://pqasb.pqarchiver.com/washingtonpost_historical/access/243050292.html?dids=243050292:243050292&FMT=ABS&FMTS=ABS:FT&date=FEB+03%2C+1914&author=&pub=The+Washington+Post&desc=GEORGE+POE+IS+DEAD&pqatl=google}}</ref> |
|||
A study of workers<ref>{{cite journal|doi=10.1056/NEJM199210013271405|pmid=1298226|year=1992|last1=Rowland|first1=AS|last2=Baird|first2=DD|last3=Weinberg|first3=CR|last4=Shore|first4=DL|last5=Shy|first5=CM|last6=Wilcox|first6=AJ|title=Reduced fertility among women employed as dental assistants exposed to high levels of nitrous oxide.|volume=327|issue=14|pages=993–7|journal=The New England journal of medicine}}</ref> |
|||
and several experimental animal studies<ref name="ReferenceA">{{cite journal|pmid=7189346|year=1980|last1=Vieira|first1=E|last2=Cleaton-Jones|first2=P|last3=Austin|first3=JC|last4=Moyes|first4=DG|last5=Shaw|first5=R|title=Effects of low concentrations of nitrous oxide on rat fetuses.|volume=59|issue=3|pages=175–7|journal=Anesthesia and analgesia}}</ref><ref name="ReferenceA"/><ref>{{cite journal|pmid=465253|year=1979|last1=Vieira|first1=E|title=Effect of the chronic administration of nitrous oxide 0.5% to gravid rats.|volume=51|issue=4|pages=283–7|journal=British journal of anaesthesia|doi=10.1093/bja/51.4.283}}</ref><ref>{{cite journal|pmid=6821624|year=1983|last1=Vieira|first1=E|last2=Cleaton-Jones|first2=P|last3=Moyes|first3=D|title=Effects of low intermittent concentrations of nitrous oxide on the developing rat fetus.|volume=55|issue=1|pages=67–9|journal=British journal of anaesthesia|doi=10.1093/bja/55.1.67}}</ref> indicate that adverse reproductive effects for pregnant females may also result from chronic exposure to nitrous oxide. |
|||
=== |
=== Anaesthetic use === |
||
N<sub>2</sub>O is a greenhouse gas with tremendous global warming potential (GWP). When compared to carbon dioxide (CO<sub>2</sub>), N<sub>2</sub>O has 310 times the ability to trap heat in the atmosphere.<ref>[http://www.epa.gov/nitrousoxide/scientific.html Science | Nitrous Oxide | Climate Change | U.S. EPA]. Epa.gov (2006-06-28). Retrieved on 2011-04-11.</ref> N<sub>2</sub>O is produced naturally in the soil during the microbial processes of [[nitrification]] and [[denitrification]]. |
|||
{{Further|Nitrous oxide (medication)}} |
|||
The United States of America signed and ratified the United Nations Framework Convention on Climate Change ([http://unfccc.int/2860.php UNFCC]) in 1992, agreeing to inventory and assess the various sources of greenhouse gases that contribute to climate change.<ref name="epa.gov">[http://www.epa.gov/climatechange/emissions/usinventoryreport.html 2011 U.S. Greenhouse Gas Inventory Report | Climate Change – Greenhouse Gas Emissions | U.S. EPA]. Epa.gov. Retrieved on 2011-04-11.</ref> The agreement requires parties to “develop, periodically update, publish and make available…national inventories of anthropogenic emissions by sources and removals by sinks of all greenhouse gases not controlled by the Montreal Protocol, using comparable methodologies…”.<ref>[http://unfccc.int/essential_background/convention/background/items/1362.php FULL TEXT OF THE CONVENTION, ARTICLE 4(1) (a)]. Unfccc.int (1998-12-31). Retrieved on 2011-04-11.</ref> In response to this agreement, the U.S. is obligated to inventory anthropogenic emissions by sources and sinks, of which agriculture is a key contributor. In 2008, agriculture contributed 6.1% of the total U.S. greenhouse gas emissions and cropland contributed nearly 69% of total direct nitrous oxide (N<sub>2</sub>O) emissions. Additionally, estimated emissions from agricultural soils were 6% higher in 2008 than 1990.<ref name="epa.gov"/> |
|||
The first time nitrous oxide was used as an [[anaesthetic]] drug in the treatment of a patient was when dentist [[Horace Wells]], with assistance by [[Gardner Quincy Colton]] and [[John Mankey Riggs]], demonstrated insensitivity to pain from a [[dental extraction]] on 11 December 1844.<ref name="Discovery of Wells">{{Cite journal|year=1933|title=The Discoverer of Anæsthesia: Dr. Horace Wells of Hartford.|journal=The Yale Journal of Biology and Medicine|volume=5|issue=5|pages=421–430|pmc=2606479|pmid=21433572|last1=Erving|first1=H. W.}}</ref> In the following weeks, Wells treated the first 12 to 15 patients with nitrous oxide in [[Hartford, Connecticut]], and, according to his own record, only failed in two cases.<ref name="Horace Wells">{{cite book|url=https://books.google.com/books?id=exNtlBi8T4EC|title=A history of the discovery, of the application of nitrous oxide gas, ether, and other vapours, to surgical operations|publisher=J. Gaylord Wells|year=1847|author=Wells H}}</ref> In spite of these convincing results having been reported by Wells to the medical society in [[Boston]] in December 1844, this new method was not immediately adopted by other dentists. The reason for this was most likely that Wells, in January 1845 at his first public demonstration to the medical faculty in Boston, had been partly unsuccessful, leaving his colleagues doubtful regarding its efficacy and safety.<ref name="Discovery of anaesthesia">{{cite journal|year=2007|title=The discovery of modern anaesthesia-contributions of Davy, Clarke, Long, Wells and Morton|url=http://www.ijaweb.org/text.asp?2007/51/6/472/61183|journal=Indian J Anaesth|volume=51|issue=6|pages=472–8|vauthors=Desai SP, Desai MS, Pandav CS}}</ref> The method did not come into general use until 1863, when Gardner Quincy Colton successfully started to use it in all his "Colton Dental Association" clinics, that he had just established in [[New Haven, Connecticut|New Haven]] and [[New York City]].<ref name="Drug discovery" /> Over the following three years, Colton and his associates successfully administered nitrous oxide to more than 25,000 patients.<ref name="use in dentistry" /> Today, nitrous oxide is used in dentistry as an anxiolytic, as an adjunct to [[Local anesthetic|local anaesthetic]]. |
|||
According to 2006 data from the [[United States Environmental Protection Agency]], industrial sources make up only about 20% of all [[human impact on the environment|anthropogenic]] sources, and include the production of [[nylon]], and the burning of fossil fuel in internal combustion engines. Human activity is thought to account for 30%; tropical soils and oceanic release account for 70%.<ref>{{cite web |
|||
|url = http://www.epa.gov/nitrousoxide/sources.html |
|||
Nitrous oxide was not found to be a strong enough anaesthetic for use in major surgery in hospital settings, however. Instead, [[diethyl ether]], being a stronger and more potent anaesthetic, was demonstrated and accepted for use in October 1846, along with [[chloroform]] in 1847.<ref name="Drug discovery" /> When [[Joseph Thomas Clover]] invented the "gas-ether inhaler" in 1876, however, it became a common practice at hospitals to initiate all anaesthetic treatments with a mild flow of nitrous oxide, and then gradually increase the anaesthesia with the stronger ether or chloroform. Clover's gas-ether inhaler was designed to supply the patient with nitrous oxide and ether at the same time, with the exact mixture being controlled by the operator of the device. It remained in use by many hospitals until the 1930s.<ref name="use in dentistry" /> Although hospitals today use a more advanced [[anaesthetic machine]], these machines still use the same principle launched with Clover's gas-ether inhaler, to initiate the anaesthesia with nitrous oxide, before the administration of a more powerful anaesthetic. |
|||
|title = Sources and Emissions – Where Does Nitrous Oxide Come From? |
|||
|publisher = U. S. Environmental Protection Agency |
|||
===As a patent medicine=== |
|||
|accessdate = 2008-02-02 |
|||
Colton's popularisation of nitrous oxide led to its adoption by a number of less than reputable [[Quackery|quacksalvers]], who touted it as a cure for [[tuberculosis|consumption]], [[Mycobacterial cervical lymphadenitis|scrofula]], [[catarrh]] and other diseases of the blood, throat and lungs. Nitrous oxide treatment was administered and licensed as a [[patent medicine]] by the likes of [[C. L. Blood]] and Jerome Harris in Boston and Charles E. Barney of Chicago.<ref name="alleged">{{cite news|url=https://www.newspapers.com/clip/3461701/alleged_forgery/|title=Alleged Forgery|date=1877-09-28|page=8|author=<!--Staff writers; no byline.-->|newspaper=[[The Inter Ocean]]|access-date=2015-10-26}}</ref><ref name="man">{{cite news|url=https://www.newspapers.com/clip/3461943/dr_blood_and_the_sawtelles/|title=A Man of Ominous Name|date=1890-02-19|author=<!--Staff writers; no byline.-->|newspaper=[[The Inter Ocean]]|access-date=2015-10-26}}</ref> |
|||
|year = 2006 |
|||
}}</ref> However, a 2008 study by Nobel Laureatte [[Paul Crutzen]] suggests that the amount of nitrous oxide release attributable to agricultural nitrate fertilizers has been seriously underestimated, most of which would presumably come under soil and oceanic release in the Environmental Protection Agency data.<ref>{{cite web|url=http://www.atmos-chem-phys.net/8/389|title=N<sub>2</sub>O release from agro-biofuel production negates global warming reduction by replacing fossil fuels}}</ref> Atmospheric levels have risen by more than 15% since 1750.<ref>{{cite web|title=Climate Change 2007: The Physical Sciences Basis| url=http://ipcc-wg1.ucar.edu/wg1/wg1-report.html|publisher=[[Intergovernmental Panel on Climate Change|IPCC]]|accessdate=2007-04-30}}</ref> Nitrous oxide also causes [[ozone depletion]]. A new study suggests that N{{ssub|2}}O emission currently is the single most important ozone-depleting substance (ODS) emission and is expected to remain the largest throughout the 21st century.<ref>{{cite journal|doi=10.1126/science.1176985 }}</ref><ref>Lisa Grossman [http://www.newscientist.com/article/dn17698-laughing-gas-is-biggest-threat-to-ozone-layer.html Laughing gas is biggest threat to ozone layer]. Newscientist, 28 August 2009</ref> |
|||
==Production== |
|||
Reviewing various methods of producing nitrous oxide is published.<ref name=CatalysisToday2005>{{cite journal |last1=Parmon |first1=V. N. |last2=Panov |first2=G. I. |last3=Uriarte |first3=A. |last4=Noskov |first4=A. S. |title=Nitrous oxide in oxidation chemistry and catalysis application and production |journal=Catalysis Today |volume=100 |issue=2005 |pages=115–131|doi=10.1016/j.cattod.2004.12.012|year=2005 }}</ref> |
|||
===Industrial methods=== |
|||
[[File:Nitrous oxide production.png|thumb|Nitrous oxide production]] |
|||
Nitrous oxide is prepared on an industrial scale by carefully heating [[ammonium nitrate]]<ref name=CatalysisToday2005 /> at about 250 °C, which decomposes into nitrous oxide and water vapour.<ref>{{cite book|last=Holleman |first=A. F. |author2=Wiberg, E. |title=Inorganic Chemistry |publisher=Academic Press |location=San Diego |year=2001 |isbn=978-0-12-352651-9}}</ref> |
|||
:{{chem2 | NH4NO3 -> 2 H2O + N2O }} |
|||
The addition of various [[phosphate]] salts favours formation of a purer gas at slightly lower temperatures. This reaction may be difficult to control, resulting in [[detonation]].<ref>{{cite web|url=http://www.sanghioverseas.com/nitrous_oxide_gas_plants/nitrous_oxide_gas_plants.htm |publisher=Sanghi Organization |title=Nitrous oxide plant |access-date=18 December 2013 |archive-url=https://web.archive.org/web/20131127131246/http://sanghioverseas.com/nitrous_oxide_gas_plants/nitrous_oxide_gas_plants.htm |archive-date=27 November 2013 }}</ref> |
|||
===Laboratory methods=== |
|||
The decomposition of ammonium nitrate is also a common laboratory method for preparing the gas. Equivalently, it can be obtained by heating a mixture of [[sodium nitrate]] and [[ammonium sulfate]]:<ref>[http://chemistry.tutorvista.com/inorganic-chemistry/nitrogen-family.html "Nitrogen Family"] {{Webarchive|url=https://web.archive.org/web/20141021035916/http://chemistry.tutorvista.com/inorganic-chemistry/nitrogen-family.html |date=21 October 2014 }}. chemistry.tutorvista.com</ref> |
|||
:{{chem2 | 2 NaNO3 + (NH4)2SO4 -> Na2SO4 + 2 N2O + 4 H2O }} |
|||
Another method involves the reaction of urea, nitric acid and sulfuric acid:<ref>[https://www.erowid.org/archive/rhodium/chemistry/nitrous.html "Preparation of Nitrous Oxide from Urea, Nitric Acid and Sulfuric Acid"].</ref> |
|||
:{{chem2 | 2 (NH2)2CO + 2 HNO3 + H2SO4 -> 2 N2O + 2 CO2 + (NH4)2SO4 + 2 H2O }} |
|||
Direct oxidation of ammonia with a [[manganese dioxide]]-[[Bismuth(III) oxide|bismuth oxide]] catalyst has been reported:<ref>{{cite journal|vauthors=Suwa T, Matsushima A, Suziki Y, Namina Y |title= Manufacture of Nitrous Oxide by the Catalytic Oxidation of Ammonia|journal= The Journal of the Society of Chemical Industry, Japan|volume=64|issue=11|pages= 1879–1888|year=1961|doi=10.1246/nikkashi1898.64.11_1879|doi-access=free}}</ref> cf. [[Ostwald process]]. |
|||
:{{chem2 | 2 NH3 + 2 O2 -> N2O + 3 H2O }} |
|||
[[Hydroxylammonium chloride]] reacts with [[sodium nitrite]] to give nitrous oxide. If the nitrite is added to the hydroxylamine solution, the only remaining by-product is salt water. If the hydroxylamine solution is added to the nitrite solution (nitrite is in excess), however, then toxic higher oxides of nitrogen also are formed: |
|||
:{{chem2 | NH3OHCl + NaNO2 -> N2O + NaCl + 2 H2O }} |
|||
Treating {{chem|HNO|3}} with {{chem|SnCl|2}} and HCl also has been demonstrated: |
|||
:{{chem2 | 2 HNO3 + 8 HCl + 4 SnCl2 -> 5 H2O + 4 SnCl4 + N2O }} |
|||
[[Hyponitrous acid]] decomposes to N{{ssub|2}}O and water with a [[half-life]] of 16 days at 25 °C at pH 1–3.<ref name="Wiberg&Holleman">Egon Wiberg, Arnold Frederick Holleman (2001) ''Inorganic Chemistry'', Elsevier {{ISBN|0-12-352651-5}}</ref> |
|||
:{{chem2 | H2N2O2 -> H2O + N2O }} |
|||
==Atmospheric occurrence== |
|||
[[File:N2O mm.png|thumb|upright=1.2|Nitrous oxide (N<sub>2</sub>O) measured by the Advanced Global Atmospheric Gases Experiment ([http://agage.mit.edu/ AGAGE]) in the lower atmosphere ([[troposphere]]) at stations around the world. Abundances are given as pollution free monthly mean mole fractions in [[Parts-per notation|parts-per-billion]].]] |
|||
[[File:HATS Nitrous Oxide concentration.png|thumb|right|Nitrous oxide atmospheric concentration since 1978]] |
|||
[[File:HATS Nitrous Oxide growth rate.png|thumb|right|Annual growth rate of atmospheric nitrous oxide since 2000]] |
|||
[[File:Global Nitrous Oxide Budget 2020.png|thumb|Earth's nitrous oxide budget from the [[Global Carbon Project]] (2020)<ref>{{cite web |url=https://www.globalcarbonproject.org/nitrousoxidebudget/index.htm |title={{chem|N|2|O}} Budget |publisher=Global Carbon Project |access-date=2020-11-09}}</ref>]] |
|||
Nitrous oxide is a [[Atmospheric chemistry#Atmospheric composition|minor component of Earth's atmosphere]] and is an active part of the planetary [[nitrogen cycle]]. Based on analysis of air samples gathered from sites around the world, its [[concentration]] surpassed 330 [[Parts-per notation|ppb]] in 2017.<ref name="agage">{{cite web |url=https://agage2.eas.gatech.edu/data_archive/data_figures/monthly/pdf/N2O_mm.pdf |archive-url=https://ghostarchive.org/archive/20221009/https://agage2.eas.gatech.edu/data_archive/data_figures/monthly/pdf/N2O_mm.pdf |archive-date=2022-10-09 |url-status=live |title=Nitrous Oxide (N2O) Mole Fraction |publisher=Massachusetts Institute of Technology |access-date=2021-02-15}}</ref> The growth rate of about 1 ppb per year has also accelerated during recent decades.<ref name="noaaesrl">{{cite web |url=https://www.esrl.noaa.gov/gmd/ccgg/trends_n2o/ |title=Trends in Atmospheric Nitrous Oxide |publisher=National Oceanic and Atmospheric Administration / Earth System Research Laboratories |access-date=2021-02-15}}</ref> Nitrous oxide's atmospheric abundance has grown more than 20% from a base level of about 270 ppb in year 1750.<ref name="tar">{{cite book |url=https://www.ipcc.ch/report/ar3/wg1/|contribution= Chapter 6 |title=TAR Climate Change 2001: The Scientific Basis |page=358}}</ref> |
|||
Important atmospheric properties of {{chem|N|2|O}} are summarized in the following table: |
|||
{| class="wikitable" |
|||
! Property |
|||
! Value |
|||
|- |
|||
| [[Ozone depletion potential]] (ODP) |
|||
| 0.017<ref name=Ravishankara>{{Citation|url=https://www.sciencemag.org/content/suppl/2009/08/27/1176985.DC1/Ravishankara.SOM.pdf |archive-url=https://ghostarchive.org/archive/20221009/https://www.sciencemag.org/content/suppl/2009/08/27/1176985.DC1/Ravishankara.SOM.pdf |archive-date=2022-10-09 |url-status=live|title=Supporting Online Material for - Nitrous Oxide (N<sub>2</sub>O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century|last1=Ravishankara|first1=A. R.|last2=Daniel|first2=John S.|last3=Portmann|first3=Robert W.|date=2009-08-27|journal= Science |volume=326|issue=5949|pages=123–125|doi=10.1126/science.1176985|pmid=19713491|bibcode=2009Sci...326..123R|s2cid=2100618}}</ref> ([[Trichlorofluoromethane|CCl<sub>3</sub>F]] = 1) |
|||
|- |
|||
| [[Global warming potential]] (GWP: 100-year) |
|||
| 265<ref name="ar5">{{cite book |url=https://www.ipcc.ch/report/ar5/wg1/ |contribution= Chapter 8 |title=AR5 Climate Change 2013: The Physical Science Basis |page=731}}</ref> ([[Carbon dioxide|CO<sub>2</sub>]] = 1) |
|||
|- |
|||
| [[Greenhouse gas#Atmospheric lifetime|Atmospheric lifetime]] |
|||
| 116 ± 9 years<ref name="ar6"/> |
|||
|- |
|||
|} |
|||
In 2022 the IPCC reported that: "The human perturbation of the natural nitrogen cycle through the use of synthetic fertilizers and manure, as well as nitrogen deposition resulting from land-based agriculture and fossil fuel burning has been the largest driver of the increase in atmospheric N2O of 31.0 ± 0.5 ppb (10%) between 1980 and 2019."<ref name="ar6">{{Cite report |title=Chapter 5: Global Carbon and other Biogeochemical Cycles and Feedbacks |url=https://www.ipcc.ch/report/ar6/wg1/chapter/chapter-5/ |access-date=2023-05-06 |website=www.ipcc.ch |language=en}}</ref> |
|||
===Emissions by source=== |
|||
17.0 (12.2 to 23.5) million tonnes total annual average nitrogen in {{chem|N|2|O}} was emitted in 2007–2016.<ref name="ar6"/> About 40% of {{chem|N|2|O}} emissions are from humans and the rest are part of the natural [[nitrogen cycle]].<ref>{{Cite web |last=US EPA |first=OAR |date=2015-12-23 |title=Overview of Greenhouse Gases |url=https://www.epa.gov/ghgemissions/overview-greenhouse-gases |access-date=2023-05-04 |website=www.epa.gov |language=en}}</ref> The {{chem|N|2|O}} emitted each year by humans has a greenhouse effect equivalent to about 3 billion tonnes of carbon dioxide: for comparison humans emitted 37 billion tonnes of actual carbon dioxide in 2019, and methane equivalent to 9 billion tonnes of carbon dioxide.<ref>{{Cite web |title={{!}} Greenhouse Gas (GHG) Emissions {{!}} Climate Watch |url=https://www.climatewatchdata.org/ghg-emissions?breakBy=gas&end_year=2019&gases=n2o&start_year=1990 |access-date=2023-05-04 |website=www.climatewatchdata.org}}</ref> |
|||
Most of the {{chem|N|2|O}} emitted into the atmosphere, from natural and anthropogenic sources, is produced by [[microorganism]]s such as [[denitrifying bacteria]] and [[fungus|fungi]] in soils and oceans.<ref name="Sloss1992">{{cite book|last=Sloss |first=Leslie L. |title=Nitrogen Oxides Control Technology Fact Book |url=https://books.google.com/books?id=--C_JAU7W8QC&pg=PA6 |year=1992 |publisher=William Andrew |isbn=978-0-8155-1294-3 |page=6}}</ref> Soils under natural vegetation are an important source of nitrous oxide, accounting for 60% of all naturally produced emissions. Other natural sources include the oceans (35%) and atmospheric chemical reactions (5%).<ref name="inputs">U.S. Environmental Protection Agency (2010), "[https://nepis.epa.gov/Exe/ZyPDF.cgi/P100717T.PDF?Dockey=P100717T.PDF Methane and Nitrous Oxide Emissions from Natural Sources]". Report EPA 430-R-10-001.</ref> [[Wetland]]s can also be [[Greenhouse gas emissions from wetlands|emitters of nitrous oxide]].<ref name=":4">{{Cite journal |last=Bange |first=Hermann W. |date=2006 |title=Nitrous oxide and methane in European coastal waters |url=https://linkinghub.elsevier.com/retrieve/pii/S0272771406002496 |journal=Estuarine, Coastal and Shelf Science |language=en |volume=70 |issue=3 |pages=361–374 |bibcode=2006ECSS...70..361B |doi=10.1016/j.ecss.2006.05.042}}</ref><ref name=":3">{{cite journal |last1=Thompson |first1=A. J. |last2=Giannopoulos |first2=G. |last3=Pretty |first3=J. |last4=Baggs |first4=E. M. |last5=Richardson |first5=D. J. |date=2012 |title=Biological sources and sinks of nitrous oxide and strategies to mitigate emissions |journal=Philosophical Transactions of the Royal Society B |volume=367 |issue=1593 |pages=1157–1168 |doi=10.1098/rstb.2011.0415 |pmc=3306631 |pmid=22451101}}</ref> Emissions from thawing [[permafrost]] may be significant, but as of 2022 this is not certain.<ref name="ar6" /> |
|||
The main components of anthropogenic emissions are fertilised agricultural soils and livestock manure (42%), runoff and leaching of fertilisers (25%), biomass burning (10%), fossil fuel combustion and industrial processes (10%), biological degradation of other nitrogen-containing atmospheric emissions (9%) and human [[sewage]] (5%).<ref name="denman">K. L. Denman, G. Brasseur, et al. (2007), "Couplings Between Changes in the Climate System and Biogeochemistry". In ''Fourth Assessment Report of the Intergovernmental Panel on Climate Change'', Cambridge University Press.</ref><ref>{{Cite book |url=http://www.fao.org/docrep/010/a0701e/a0701e00.HTM |title=Livestock's long shadow: Environmental issues and options |publisher=Fao.org |author1=Steinfeld, H. |author2=Gerber, P. |author3=Wassenaar, T. |author4=Castel, V. |author5=Rosales, M. |author6=de Haan, C. |access-date=2 February 2008 |year=2006}}</ref><ref name="epaUpdated">{{cite web|url=https://www3.epa.gov/climatechange/ghgemissions/gases/n2o.html |title=Overview of Greenhouse Gases: Nitrous Oxide |publisher=U.S. Environmental Protection Agency |access-date=31 March 2016|date=23 December 2015 |url-status=live |archive-url= https://web.archive.org/web/20160812082641/https://www.epa.gov/ghgemissions/overview-greenhouse-gases |archive-date=12 August 2016 }}</ref><ref name="epa">{{cite web |url= http://www.epa.gov/nitrousoxide/sources.html |title=Nitrous Oxide: Sources and Emissions |publisher=U.S. Environmental Protection Agency |access-date=2 February 2008 |year=2006 |archive-url= https://web.archive.org/web/20080116204312/http://www.epa.gov/nitrousoxide/sources.html |archive-date=16 January 2008}}</ref><ref>IPCC. 2013. Climate change: the physical basis (WG I, full report). p. 512.</ref> Agriculture enhances nitrous oxide production through soil cultivation, the use of nitrogen [[Fertilizer|fertilisers]] and animal waste handling.<ref>{{Cite journal|last1=Thompson|first1=R. L.|last2=Lassaletta|first2=L.|last3=Patra|first3=P. K.|last4=Wilson|first4=C. |last5=Wells|first5=K. C.|last6=Gressent|first6=A.|last7=Koffi|first7=E. N.|last8=Chipperfield|first8=M. P.|last9=Winiwarter|first9=W. |last10=Davidson|first10=E. A.|last11=Tian|first11=H.|date=2019-11-18|title=Acceleration of global N 2 O emissions seen from two decades of atmospheric inversion|journal=Nature Climate Change|language=en|volume=9|issue=12 |pages=993–998|doi=10.1038/s41558-019-0613-7|issn=1758-6798|bibcode=2019NatCC...9..993T|s2cid=208302708|url=http://pure.iiasa.ac.at/id/eprint/16173/2/N2O_paper_SI_revision2_v1.docx|hdl=11250/2646484|hdl-access=free}}</ref> These activities stimulate naturally occurring bacteria to produce more nitrous oxide. Nitrous oxide emissions from soil can be challenging to measure as they vary markedly over time and space,<ref>{{cite journal |last1=Molodovskaya |first1=Marina |last2=Warland |first2=Jon |last3=Richards |first3=Brian K. |last4=Öberg |first4=Gunilla |last5=Steenhuis |first5=Tammo S. |title=Nitrous Oxide from Heterogeneous Agricultural Landscapes: Source Contribution Analysis by Eddy Covariance and Chambers |journal=Soil Science Society of America Journal |date=2011 |volume=75 |issue=5 |page=1829 |doi=10.2136/SSSAJ2010.0415|bibcode=2011SSASJ..75.1829M }}</ref> and the majority of a year's emissions may occur when conditions are favorable during "hot moments"<ref>{{cite journal | last1 = Molodovskaya | first1 = M. | last2 = Singurindy | first2 = O. | last3 = Richards | first3 = B. K. | last4 = Warland | first4 = J. S. | last5 = Johnson | first5 = M. | last6 = Öberg | first6 = G. | last7 = Steenhuis | first7 = T. S. | year = 2012 | title = Temporal variability of nitrous oxide from fertilized croplands: hot moment analysis | journal = Soil Science Society of America Journal | volume = 76 | issue = 5| pages = 1728–1740 | doi = 10.2136/sssaj2012.0039 | bibcode = 2012SSASJ..76.1728M | s2cid = 54795634 }}</ref><ref>{{cite journal |last1=Singurindy |first1=Olga |last2=Molodovskaya |first2=Marina |last3=Richards |first3=Brian K. |last4=Steenhuis |first4=Tammo S. |title=Nitrous oxide emission at low temperatures from manure-amended soils under corn (Zea mays L.) |journal=Agriculture, Ecosystems & Environment |date=July 2009 |volume=132 |issue=1–2 |pages=74–81 |doi=10.1016/j.agee.2009.03.001|bibcode=2009AgEE..132...74S }}</ref> and/or at favorable locations known as "hotspots".<ref>{{cite journal | last1 = Mason | first1 = C.W. | last2 = Stoof | first2 = C.R. | last3 = Richards | first3 = B.K. | last4 = Das | first4 = S. | last5 = Goodale | first5 = C.L. | last6 = Steenhuis | first6 = T.S. | year = 2017 | title = Hotspots of nitrous oxide emission in fertilized and unfertilized perennial grasses on wetness-prone marginal land in New York State | journal = Soil Science Society of America Journal | volume = 81 | issue = 3| pages = 450–458 | doi = 10.2136/sssaj2016.08.0249 | bibcode = 2017SSASJ..81..450M }}</ref> |
|||
Among industrial emissions, the production of nitric acid and [[adipic acid]] are the largest sources of nitrous oxide emissions. The adipic acid emissions specifically arise from the degradation of the [[nitrolic acid]] intermediate derived from the nitration of cyclohexanone.<ref name="denman"/><ref>{{cite journal|title=Abatement of N{{ssub|2}}O emissions produced in the adipic acid industry|author1=Reimer R. A. |author2=Slaten C. S. |author3=Seapan M. |author4=Lower M. W. |author5=Tomlinson P. E. | journal = Environmental Progress| year = 1994| volume = 13| issue = 2| pages = 134–137| doi = 10.1002/ep.670130217|bibcode=1994EnvPr..13..134R }}</ref><ref>{{cite journal|title=Abatement of N{{ssub|2}}O emissions produced in the adipic acid industry|author1=Shimizu, A. |author2=Tanaka, K. |author3=Fujimori, M. | journal = Chemosphere – Global Change Science| year = 2000| volume = 2| issue = 3–4| pages = 425–434| doi = 10.1016/S1465-9972(00)00024-6|bibcode=2000ChGCS...2..425S}}</ref> |
|||
===Biological processes=== |
|||
Natural processes that generate nitrous oxide may be classified as [[nitrification]] and [[denitrification]]. Specifically, they include: |
|||
* aerobic autotrophic nitrification, the stepwise oxidation of [[ammonia]] ({{chem|NH|3}}) to [[nitrite]] ({{chem|NO|2|−}}) and to [[nitrate]] ({{chem|NO|3|−}})<!-- (Kowalchuk and Stephen, 2001)--> |
|||
* anaerobic heterotrophic denitrification, the stepwise reduction of {{chem|NO|3|−}} to {{chem|NO|2|-}}, [[nitric oxide]] (NO), {{chem|N|2|O}} and ultimately {{chem|N|2}}, where facultative anaerobe bacteria use {{chem|NO|3|−}} as an electron acceptor in the respiration of organic material in the condition of insufficient oxygen ({{chem|O|2}})<!-- (Knowles, 1982)--> |
|||
* nitrifier denitrification, which is carried out by autotrophic {{chem|NH|3}}-oxidising bacteria and the pathway whereby ammonia ({{chem|NH|3}}) is oxidised to nitrite ({{chem|NO|2|−}}), followed by the reduction of {{chem|NO|2|-}} to nitric oxide (NO), {{chem|N|2|O}} and molecular nitrogen ({{chem|N|2}})<!-- (Webster and Hopkins, 1996; Wrage et al., 2001)--> |
|||
* heterotrophic nitrification<!-- (Robertson and Kuenen, 1990)--> |
|||
* aerobic denitrification by the same heterotrophic nitrifiers<!-- (Robertson and Kuenen, 1990) --> |
|||
* fungal denitrification<!-- (Laughlin and Stevens, 2002) --> |
|||
* non-biological chemodenitrification<!-- (Chalk and Smith, 1983; Van Cleemput and Baert, 1984; Martikainen and De Boer, 1993; Daum and Schenk, 1998; Mørkved et al., 2007)--> |
|||
These processes are affected by soil chemical and physical properties such as the availability of mineral nitrogen and [[organic matter]], acidity and soil type, as well as climate-related factors such as soil temperature and water content. <!--(Mosier, 1994; Bouwman, 1996; Beauchamp, 1997; Yamulki et al. 1997; Dobbie and Smith, 2003; Smith et al. 2003; Dalal et al. 2003) --> |
|||
The emission of the gas to the atmosphere is limited greatly by its consumption inside the cells, by a process catalysed by the enzyme [[nitrous-oxide reductase|nitrous oxide reductase]].<ref>{{cite book|author1=Schneider, Lisa K. |author2=Wüst, Anja |author3=Pomowski, Anja |author4=Zhang, Lin |author5=Einsle, Oliver |chapter=No Laughing Matter: The Unmaking of the Greenhouse Gas Dinitrogen Monoxide by Nitrous Oxide Reductase |year=2014|title=The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment|pages =177–210| volume =14 |series=Metal Ions in Life Sciences |editor=Kroneck, Peter M. H. |editor2=Sosa Torres, Martha E. |publisher= Springer|doi=10.1007/978-94-017-9269-1_8|pmid=25416395|isbn=978-94-017-9268-4}}</ref> |
|||
==Environmental impact== |
|||
Global accounting of {{chem|N|2|O}} sources and sinks over the decade ending 2016 indicates that about 40% of the average 17 TgN/yr ([[Megatonne|teragram]]s, or million metric tons, of nitrogen per year) of emissions originated from human activity, and shows that emissions growth chiefly came from expanding [[agriculture]].<ref name="HTian" /><ref name=":0" /> |
|||
===Greenhouse effect=== |
|||
[[File:Major greenhouse gas trends.png|thumb|right|Trends in the atmospheric abundance of long-lived greenhouse gases]] |
|||
Nitrous oxide has significant [[global warming potential]] as a [[greenhouse gas]]. On a per-molecule basis, considered over a 100-year period, nitrous oxide has 265 times the atmospheric heat-trapping ability of [[carbon dioxide]] ({{chem|CO|2}}).<ref name="ar5" /> However, because of its low concentration (less than 1/1,000 of that of {{chem|C|O|2}}), its contribution to the [[greenhouse effect]] is less than one third that of carbon dioxide, and also less than [[methane]].<ref name="clim">US Environmental Protection Agency, "[https://www.epa.gov/climate-indicators/climate-change-indicators-atmospheric-concentrations-greenhouse-gases Climate Change Indicators: Atmospheric Concentrations of Greenhouse Gases]" Web document, accessed on 2017-02-14</ref> On the other hand, since about 40% of the {{chem|N|2|O}} entering the atmosphere is the result of human activity,<ref name="denman" /> control of nitrous oxide is part of efforts to curb greenhouse gas emissions.<ref>{{cite web |url=http://www.grida.no/publications/other/ipcc%5Ftar/?src=/climate/ipcc_tar/wg1/247.htm |title=4.1.1 Sources of Greenhouse Gases |work=IPCC TAR WG1 2001 |access-date=21 September 2012 |archive-url=https://web.archive.org/web/20121029023441/http://www.grida.no/publications/other/ipcc%5Ftar/?src=%2Fclimate%2Fipcc_tar%2Fwg1%2F247.htm |archive-date=29 October 2012 }}</ref> |
|||
Most human caused nitrous oxide released into the atmosphere is a [[Greenhouse gas emissions from agriculture|greenhouse gas emission from agriculture]], when farmers add nitrogen-based fertilizers onto the fields, and through the breakdown of animal manure. Reduction of emissions can be a hot topic in the [[politics of climate change]].<ref>{{Cite web |last=Mundschenk |first=Susanne |title=The Netherlands is showing how not to tackle climate change {{!}} The Spectator |url=https://www.spectator.co.uk/article/the-netherlands-is-showing-how-not-to-tackle-climate-change |access-date=2022-08-28 |website=www.spectator.co.uk |date=3 August 2022 |language=en}}</ref> |
|||
Nitrous oxide is also released as a by-product of burning fossil fuel, though the amount released depends on which fuel was used. It is also emitted through the manufacture of [[nitric acid]], which is used in the synthesis of nitrogen fertilizers. The production of adipic acid, a precursor to [[nylon]] and other synthetic clothing fibres, also releases nitrous oxide.<ref>{{cite web|title=Overview of Greenhouse Gases: Nitrous Oxide Emissions|url=https://19january2017snapshot.epa.gov/ghgemissions/overview-greenhouse-gases_.html|publisher=United States Environmental Protection Agency|date=October 6, 2016|access-date=July 14, 2019}}</ref> |
|||
A rise in atmospheric nitrous oxide concentrations has been implicated as a possible contributor to the extremely intense global warming during the [[Cenomanian-Turonian boundary event]].<ref>{{cite journal |last1=Naafs |first1=B. David A. |last2=Monteiro |first2=Fanny M. |last3=Pearson |first3=Ann |last4=Higgins |first4=Meytal B. |last5=Pancost |first5=Richard D. |last6=Ridgwell |first6=Andy |date=10 December 2019 |title=Fundamentally different global marine nitrogen cycling in response to severe ocean deoxygenation |journal=[[Proceedings of the National Academy of Sciences of the United States of America]] |volume=116 |issue=50 |pages=24979–24984 |doi=10.1073/pnas.1905553116 |pmid=31767742 |pmc=6911173 |bibcode=2019PNAS..11624979N |doi-access=free }}</ref> |
|||
===Ozone layer depletion=== |
|||
Nitrous oxide has also been implicated in [[ozone depletion|thinning the ozone layer]]. A 2009 study suggested that {{chem|N|2|O}} emission was the single most important ozone-depleting emission and it was expected to remain the largest throughout the 21st century.<ref name="sciozo">{{cite journal|doi=10.1126/science.1176985 |title=Nitrous Oxide (N{{ssub|2}}O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century|year=2009|last1=Ravishankara|first1=A. R.|last2=Daniel|first2=J. S.|last3=Portmann|first3=R. W. |journal=Science |volume=326 |issue=5949 |pages=123–5|pmid=19713491 |bibcode = 2009Sci...326..123R |s2cid=2100618|doi-access=free}}</ref><ref>{{cite magazine|last=Grossman |first=Lisa |date=28 August 2009 |url=https://www.newscientist.com/article/dn17698-laughing-gas-is-biggest-threat-to-ozone-layer.html |title=Laughing gas is biggest threat to ozone layer |magazine=New Scientist}}</ref> |
|||
==Legality== |
==Legality== |
||
{{See also|Recreational use of nitrous oxide#Legality}} |
|||
In the [[United States]], possession of nitrous oxide is legal under federal law and is not subject to [[Drug Enforcement Administration|DEA]] purview.<ref name="ccle">[http://www.cognitiveliberty.org/dll/N20_state_laws.htm Center for Cognitive Liberty and Ethics: State Laws Concerning Inhalation of Nitrous Oxide]</ref> It is, however, regulated by the [[Food and Drug Administration]] under the Food Drug and Cosmetics Act; prosecution is possible under its "misbranding" clauses, prohibiting the sale or distribution of nitrous oxide for the purpose of [[recreational drug use|human consumption]]. |
|||
===India=== |
|||
Transfer of nitrous oxide from bulk cylinders to smaller, more transportable E-type, 1,590-litre-capacity tanks<ref>{{cite web|url=http://www.ohiomedical.com/UserFiles/File/Medical+Gas+Cylinder+Data.pdf|title=Ohio Medical|website=www.ohiomedical.com|access-date=20 September 2017|archive-url=https://web.archive.org/web/20160417223630/http://www.ohiomedical.com/UserFiles/File/Medical%20Gas%20Cylinder%20Data.pdf|archive-date=17 April 2016}}</ref> is legal when the intended use of the gas is for medical anaesthesia. |
|||
===New Zealand=== |
|||
Many states have laws regulating the possession, sale, and distribution of nitrous oxide. Such laws usually ban distribution to minors or limit the amount of nitrous oxide that may be sold without special license.{{Citation needed|date=July 2008}} For example, in the state of California, possession for recreational use is prohibited and qualifies as a misdemeanor.<ref>[http://codes.lp.findlaw.com/cacode/PEN/3/1/10/s381b CAL. PEN. CODE § 381b : California Code – Section 381b]</ref> |
|||
The [[New Zealand Ministry of Health|Ministry of Health]] has warned that nitrous oxide is a prescription medicine, and its sale or possession without a prescription is an offense under the Medicines Act.<ref>{{cite news |last=Anderton |first=Jim |date=26 June 2005 |url=http://www.beehive.govt.nz/release/time039s-sham-sales-laughing-gas |title=Time's up for sham sales of laughing gas |publisher=Beehive.govt.nz |archive-url=https://web.archive.org/web/20150108015457/http://www.beehive.govt.nz/release/time039s-sham-sales-laughing-gas |archive-date=8 January 2015 }}</ref> This statement would seemingly prohibit all non-medicinal uses of nitrous oxide, although it is implied that only recreational use will be targeted legally. |
|||
===UK=== |
|||
In [[New Zealand]], the [[New Zealand Ministry of Health|Ministry of Health]] has warned that nitrous oxide is a prescription medicine, and its sale or possession without a prescription is an offense under the Medicines Act.<ref>Jim Anderton [http://www.beehive.govt.nz/release/time039s-sham-sales-laughing-gas Time's up for sham sales of laughing gas], Beehive.govt.nz, 26 June 2005</ref> This statement would seemingly prohibit all non-medicinal uses of the chemical, though it is implied that only recreational use will be legally targeted. |
|||
In August 2015, the [[Lambeth London Borough Council|Council]] of the [[London Borough of Lambeth]] ([[United Kingdom|UK]]) banned the use of the drug for recreational purposes, making offenders liable to an on-the-spot fine of up to £1,000.<ref>{{cite news |url=https://www.bbc.co.uk/news/uk-33955823 |title=Lambeth Council bans laughing gas as recreational drug |work=BBC News |date=17 August 2015 |access-date=17 August 2015 }}</ref> |
|||
In September 2023, the Government announced that nitrous oxide would be made illegal by the end of the year, with possession potentially carrying up to a two-year prison sentence or an unlimited fine.<ref>{{Cite news |date=2023-09-05 |title=Nitrous oxide: Laughing gas to be illegal by end of year |language=en-GB |work=BBC News |url=https://www.bbc.com/news/uk-66718165 |access-date=2023-09-05}}</ref> |
|||
In [[India]], for general anaesthesia purposes, nitrous oxide is available as Nitrous Oxide IP. India's gas cylinder rules (1985) permit the transfer of gas from one cylinder to another for breathing purposes. This law benefits remote hospitals, which would otherwise suffer as a result of India's geographic immensity. Nitrous Oxide IP is transferred from bulk cylinders (17,000 liters capacity gas) to smaller pin-indexed valve cylinders (1,800 liters of gas), which are then connected to the yoke assembly of [[Boyle's machine]]s. Because India's Food & Drug Authority (FDA-India) rules state that transferring a drug from one container to another (refilling) is equivalent to manufacturing, anyone found doing so must possess a drug manufacturing license. |
|||
== |
===US=== |
||
Possession of nitrous oxide is legal under federal law and is not subject to [[Drug Enforcement Administration|DEA]] purview.<ref name="ccle">{{cite web |url=http://www.cognitiveliberty.org/dll/N20_state_laws.htm |title=US Nitrous Oxide Laws (alphabetically) Based on a search of online free legal databases. Conducted May 2002 |publisher=Center for Cognitive Liberty and Ethics |access-date=27 January 2008 |archive-url=https://web.archive.org/web/20080124114346/http://www.cognitiveliberty.org/dll/N20_state_laws.htm |archive-date=24 January 2008 }}</ref> It is, however, regulated by the [[Food and Drug Administration]] under the Food Drug and Cosmetics Act; prosecution is possible under its "misbranding" clauses, prohibiting the sale or distribution of nitrous oxide for the purpose of [[recreational drug use|human consumption]]. Many states have laws regulating the possession, sale and distribution of nitrous oxide. Such laws usually ban distribution to minors or limit the amount of nitrous oxide that may be sold without special license.{{Citation needed|date=July 2008}} For example, in the state of California, possession for recreational use is prohibited and qualifies as a misdemeanor.<ref>{{cite web|url=http://codes.lp.findlaw.com/cacode/PEN/3/1/10/s381b |title=CAL. PEN. CODE § 381b: California Code – Section 381b |publisher=Lp.findlaw.com}}</ref> |
|||
*[[Whipped-cream charger]] |
|||
*[[Diffusion hypoxia]] |
|||
*[[Nitrous oxide fuel blend]] |
|||
==See also== |
|||
* [[DayCent]] |
|||
* [[Fink effect]] |
|||
* [[Nitrous oxide fuel blend]] |
|||
==References== |
==References== |
||
{{Reflist |
{{Reflist}} |
||
==External links== |
== External links == |
||
{{Commons category|Nitrous oxide}} |
|||
*[http://www.osha.gov/SLTC/healthguidelines/nitrousoxide/recognition.html Occupational Safety and Health Guideline for Nitrous Oxide] |
|||
*[http://www.vega.org.uk/video/programme/111 Paul Crutzen Interview] Freeview video of Paul Crutzen Nobel Laureate for his work on decomposition of ozone talking to Harry Kroto Nobel Laureate by the Vega Science Trust. |
|||
{{div col | small=yes }} |
|||
*[http://www.npi.gov.au/database/substance-info/profiles/67.html National Pollutant Inventory – Oxide of nitrogen fact sheet] |
|||
*[ |
* [https://web.archive.org/web/20020827081011/http://osha.gov/SLTC/healthguidelines/nitrousoxide/recognition.html Occupational Safety and Health Guideline for Nitrous Oxide] |
||
* [http://www.vega.org.uk/video/programme/111 Paul Crutzen Interview] Freeview video of Paul Crutzen Nobel Laureate for his work on decomposition of ozone talking to Harry Kroto Nobel Laureate by the Vega Science Trust. |
|||
*[http://www.justsayn2o.com Nitrous Oxide FAQ] |
|||
* [https://web.archive.org/web/20040205090004/http://www.npi.gov.au/database/substance-info/profiles/67.html National Pollutant Inventory – Oxide of nitrogen fact sheet] |
|||
*[http://www.erowid.org/chemicals/nitrous/nitrous.shtml Erowid article on Nitrous Oxide] |
|||
* [https://www.cdc.gov/niosh/topics/nitrousoxide/ National Institute for Occupational Safety and Health – Nitrous Oxide] |
|||
*[http://www.sciencenews.org/view/generic/id/46776/title/Nitrous_oxide_fingered_as_monster_ozone_slayer Nitrous oxide fingered as monster ozone slayer], Science News |
|||
* [https://www.cdc.gov/niosh/npg/npgd0465.html CDC – NIOSH Pocket Guide to Chemical Hazards – Nitrous Oxide] |
|||
*[http://www.dentalfearcentral.org/help/sedation-dentistry/laughing-gas/ Dental Fear Central article on the use of nitrous oxide in dentistry] |
|||
* [http://www.justsayn2o.com/ Nitrous Oxide FAQ] |
|||
* [https://www.erowid.org/chemicals/nitrous/nitrous.shtml Erowid article on Nitrous Oxide] |
|||
* [https://www.sciencenews.org/view/generic/id/46776/title/Nitrous_oxide_fingered_as_monster_ozone_slayer Nitrous oxide fingered as monster ozone slayer] {{Webarchive|url=https://web.archive.org/web/20120929191310/http://www.sciencenews.org/view/generic/id/46776/title/Nitrous_oxide_fingered_as_monster_ozone_slayer |date=29 September 2012 }}, Science News |
|||
* [http://www.dentalfearcentral.org/help/sedation-dentistry/laughing-gas/ Dental Fear Central article on the use of nitrous oxide in dentistry] |
|||
* [http://asdb.info Altered States Database] |
|||
{{div col end}} |
|||
{{Drug use}} |
{{Drug use}} |
||
{{Hallucinogens}} |
|||
{{General anesthetics}} |
{{General anesthetics}} |
||
{{Analgesics}} |
{{Analgesics}} |
||
{{ |
{{Hallucinogens}} |
||
{{Navboxes |
|||
{{Glutamatergics}} |
|||
| title = [[Pharmacodynamics]] |
|||
{{Cholinergics}} |
|||
| titlestyle = background:#ccccff |
|||
{{Neurotransmitters}} |
|||
| list1 = |
|||
{{GABAA receptor positive modulators}} |
|||
{{Glycine receptor modulators}} |
|||
{{Ionotropic glutamate receptor modulators}} |
|||
{{Nicotinic acetylcholine receptor modulators}} |
|||
{{Serotonin receptor modulators}} |
|||
}} |
|||
{{E number infobox 930-949}} |
{{E number infobox 930-949}} |
||
{{Molecules detected in outer space}} |
|||
{{Nitrogen compounds}} |
|||
{{Oxygen compounds}}{{Portal bar | Medicine}} |
|||
{{Authority control}} |
|||
{{DEFAULTSORT:Nitrous Oxide}} |
{{DEFAULTSORT:Nitrous Oxide}} |
||
[[Category: |
[[Category:5-HT3 antagonists]] |
||
[[Category:Aerosol propellants]] |
[[Category:Aerosol propellants]] |
||
[[Category:General anesthetics]] |
|||
[[Category:Dissociative drugs]] |
[[Category:Dissociative drugs]] |
||
[[Category:E-number additives]] |
|||
[[Category:Euphoriants]] |
|||
[[Category:GABAA receptor positive allosteric modulators]] |
|||
[[Category:Gaseous signaling molecules]] |
|||
[[Category:General anesthetics]] |
|||
[[Category:Glycine receptor agonists]] |
|||
[[Category:Greenhouse gases]] |
[[Category:Greenhouse gases]] |
||
[[Category: |
[[Category:Industrial gases]] |
||
[[Category:Industrial hygiene]] |
|||
[[Category:Inhalants]] |
|||
[[Category:Nitrogen oxides]] |
|||
[[Category:Monopropellants]] |
[[Category:Monopropellants]] |
||
[[Category:Nicotinic antagonists]] |
|||
[[Category:Nitrogen cycle]] |
|||
[[Category:NMDA receptor antagonists]] |
|||
[[Category:Rocket oxidizers]] |
[[Category:Rocket oxidizers]] |
||
[[Category: |
[[Category:Trace gases]] |
||
[[Category:Industrial hygiene]] |
|||
[[Category:Vehicle modification]] |
|||
[[Category:World Health Organization essential medicines]] |
[[Category:World Health Organization essential medicines]] |
||
[[Category: |
[[Category:Neurotoxins]] |
||
[[ar:أكسيد النيتروس]] |
|||
[[bs:Dušik suboksid]] |
|||
[[bg:Диазотен оксид]] |
|||
[[ca:Òxid nitrós]] |
|||
[[cs:Oxid dusný]] |
|||
[[da:Lattergas]] |
|||
[[de:Distickstoffmonoxid]] |
|||
[[et:Dilämmastikoksiid]] |
|||
[[es:Óxido de nitrógeno (I)]] |
|||
[[eo:Ridgaso]] |
|||
[[fa:دی نیتروژن مونوکسید]] |
|||
[[fr:Protoxyde d'azote]] |
|||
[[gl:Óxido nitroso]] |
|||
[[ko:아산화 질소]] |
|||
[[hi:नाइट्रस ऑक्साइड]] |
|||
[[id:Nitrogen dioksida]] |
|||
[[it:Ossido di diazoto]] |
|||
[[he:חמצן דו-חנקני]] |
|||
[[lt:Diazoto monoksidas]] |
|||
[[hu:Dinitrogén-oxid]] |
|||
[[arz:غاز مضحك]] |
|||
[[nl:Distikstofmonoxide]] |
|||
[[ja:亜酸化窒素]] |
|||
[[no:Dinitrogenoksid]] |
|||
[[nn:Dinitrogenoksid]] |
|||
[[nds:Distickstoffmonoxid]] |
|||
[[pl:Podtlenek azotu]] |
|||
[[pt:Óxido nitroso]] |
|||
[[ro:Protoxid de azot]] |
|||
[[ru:Оксид азота(I)]] |
|||
[[simple:Nitrous oxide]] |
|||
[[sk:Oxid dusný]] |
|||
[[sl:Didušikov oksid]] |
|||
[[sr:Азотсубоксид]] |
|||
[[fi:Ilokaasu]] |
|||
[[sv:Lustgas]] |
|||
[[th:ไนตรัสออกไซด์]] |
|||
[[tr:Azot oksit]] |
|||
[[uk:Оксид азоту (I)]] |
|||
[[ur:Nitrous oxide]] |
|||
[[zh-yue:笑氣]] |
|||
[[zh:一氧化二氮]] |
|||
Latest revision as of 04:52, 11 April 2024

| |
| |
| Names | |
|---|---|
| IUPAC names | |
| Systematic IUPAC name
Oxodiazen-2-ium-1-ide | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 8137358 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.030.017 |
| E number | E942 (glazing agents, ...) |
| 2153410 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1070 (compressed) 2201 (liquid) |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| N 2O | |
| Molar mass | 44.013 g/mol |
| Appearance | colourless gas |
| Density | 1.977 g/L (gas) |
| Melting point | −90.86 °C (−131.55 °F; 182.29 K) |
| Boiling point | −88.48 °C (−127.26 °F; 184.67 K) |
| 1.5 g/L (15 °C) | |
| Solubility | soluble in alcohol, ether, sulfuric acid |
| log P | 0.35 |
| Vapor pressure | 5150 kPa (20 °C) |
| −18.9·10−6 cm3/mol | |
Refractive index (nD)
|
1.000516 (0 °C, 101.325 kPa) |
| Viscosity | 14.90 μPa·s[3] |
| Structure | |
| linear, C∞v | |
| 0.166 D | |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
219.96 J/(K·mol) |
Std enthalpy of
formation (ΔfH⦵298) |
+82.05 kJ/mol |
| Pharmacology | |
| N01AX13 (WHO) | |
| Inhalation | |
| Pharmacokinetics: | |
| 0.004% | |
| 5 minutes | |
| Respiratory | |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H270, H280, H281 | |
| P220, P244, P282, P317, P336, P370+P376, P403, P410+P403 | |
| NFPA 704 (fire diamond) | |
| Flash point | Nonflammable |
| Safety data sheet (SDS) | Ilo.org, ICSC 0067 |
| Related compounds | |
| Nitric oxide Dinitrogen trioxide Nitrogen dioxide Dinitrogen tetroxide Dinitrogen pentoxide | |
Related compounds
|
Ammonium nitrate Azide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, nitro, or nos,[4] is a chemical compound, an oxide of nitrogen with the formula N
2O. At room temperature, it is a colourless non-flammable gas, and has a slightly sweet scent and taste.[5] At elevated temperatures, nitrous oxide is a powerful oxidiser similar to molecular oxygen.
Nitrous oxide has significant medical uses, especially in surgery and dentistry, for its anaesthetic and pain-reducing effects.[6] Its colloquial name, "laughing gas", coined by Humphry Davy, is due to the euphoric effects upon inhaling it, a property that has led to its recreational use as a dissociative anaesthetic.[6] It is on the World Health Organization's List of Essential Medicines.[7] It is also used as an oxidiser in rocket propellants, and in motor racing to increase the power output of engines.
Nitrous oxide's atmospheric concentration reached 333 parts per billion (ppb) in 2020, increasing at a rate of about 1 ppb annually.[8][9] It is a major scavenger of stratospheric ozone, with an impact comparable to that of CFCs.[10] About 40% of human-caused emissions are from agriculture.[11][12] Nitrogen is added to the soil via animal urine and dung, and synthetic fertilisers: micro-organisms then release it in nitrous oxide.[13] Being the third most important greenhouse gas, nitrous oxide substantially contributes to global warming.[14][15] Reduction of emissions is a popular topic in the politics of climate change.[16]
Nitrous oxide is used as a propellant, and has a variety of applications from rocketry to making whipped cream. It is used as a recreational drug for its potential to induce a brief "high". Most recreational users are unaware of its neurotoxic effects when abused. When used chronically, nitrous oxide has the potential to cause neurological damage through inactivation of vitamin B12.
Uses[edit]
Rocket motors[edit]
Nitrous oxide may be used as an oxidiser in a rocket motor. It has advantages over other oxidisers in that it is much less toxic, and because of its stability at room temperature, it is also easier to store and relatively safe to carry on a flight. As a secondary benefit, it may be decomposed readily to form breathing air. Its high density and low storage pressure (when maintained at low temperatures) enable it to be highly competitive with stored high-pressure gas systems.[17]
In a 1914 patent, American rocket pioneer Robert Goddard suggested nitrous oxide and gasoline as possible propellants for a liquid-fuelled rocket.[18] Nitrous oxide has been the oxidiser of choice in several hybrid rocket designs (using solid fuel with a liquid or gaseous oxidiser). The combination of nitrous oxide with hydroxyl-terminated polybutadiene fuel has been used by SpaceShipOne and others. It also is notably used in amateur and high power rocketry with various plastics as the fuel.
Nitrous oxide also may be used in a monopropellant rocket. In the presence of a heated catalyst, N
2O will decompose exothermically into nitrogen and oxygen, at a temperature of approximately 1,070 °F (577 °C).[19] Because of the large heat release, the catalytic action rapidly becomes secondary, as thermal autodecomposition becomes dominant. In a vacuum thruster, this may provide a monopropellant specific impulse (Isp) of as much as 180 s. While noticeably less than the Isp available from hydrazine thrusters (monopropellant or bipropellant with dinitrogen tetroxide), the decreased toxicity makes nitrous oxide an option worth investigating.
Nitrous oxide is said to deflagrate at approximately 600 °C (1,112 °F) at a pressure of 309 psi (21 atmospheres).[20] At 600 psi, for example, the required ignition energy is only 6 joules, whereas N
2O at 130 psi a 2,500-joule ignition energy input is insufficient.[21][22]
Internal combustion engine[edit]
In vehicle racing, nitrous oxide (often called "nitrous") allows the engine to burn more fuel by providing more oxygen during combustion. The increase in oxygen allows an increase in the injection of fuel, allowing the engine to produce more engine power. The gas is not flammable at a low pressure/temperature, but it delivers more oxygen than atmospheric air by breaking down at elevated temperatures, about 570 degrees F (~300C). Therefore, it often is mixed with another fuel that is easier to deflagrate. Nitrous oxide is a strong oxidising agent, roughly equivalent to hydrogen peroxide, and much stronger than oxygen gas.
Nitrous oxide is stored as a compressed liquid; the evaporation and expansion of liquid nitrous oxide in the intake manifold causes a large drop in intake charge temperature, resulting in a denser charge, further allowing more air/fuel mixture to enter the cylinder. Sometimes nitrous oxide is injected into (or prior to) the intake manifold, whereas other systems directly inject, right before the cylinder (direct port injection) to increase power.
The technique was used during World War II by Luftwaffe aircraft with the GM-1 system to boost the power output of aircraft engines. Originally meant to provide the Luftwaffe standard aircraft with superior high-altitude performance, technological considerations limited its use to extremely high altitudes. Accordingly, it was only used by specialised planes such as high-altitude reconnaissance aircraft, high-speed bombers and high-altitude interceptor aircraft. It sometimes could be found on Luftwaffe aircraft also fitted with another engine-boost system, MW 50, a form of water injection for aviation engines that used methanol for its boost capabilities.
One of the major problems of using nitrous oxide in a reciprocating engine is that it can produce enough power to damage or destroy the engine. Very large power increases are possible, and if the mechanical structure of the engine is not properly reinforced, the engine may be severely damaged or destroyed during this type of operation. It is important with nitrous oxide augmentation of petrol engines to maintain proper operating temperatures and fuel levels to prevent "pre-ignition",[23] or "detonation" (sometimes referred to as "knock"). Most problems that are associated with nitrous oxide do not come from mechanical failure due to the power increases. Since nitrous oxide allows a much denser charge into the cylinder, it dramatically increases cylinder pressures. The increased pressure and temperature can cause problems such as melting the pistons or valves. It also may crack or warp the piston or cylinder head and cause pre-ignition due to uneven heating.
Automotive-grade liquid nitrous oxide differs slightly from medical-grade nitrous oxide. A small amount of sulfur dioxide (SO
2) is added to prevent substance abuse.[24]
Aerosol propellant[edit]

2O whipped-cream chargers
The gas is approved for use as a food additive (E number: E942), specifically as an aerosol spray propellant. Its most common uses in this context are in aerosol whipped cream canisters and cooking sprays.
The gas is extremely soluble in fatty compounds. In aerosol whipped cream, it is dissolved in the fatty cream until it leaves the can, when it becomes gaseous and thus creates foam. Used in this way, it produces whipped cream which is four times the volume of the liquid, whereas whipping air into cream only produces twice the volume. If air were used as a propellant, oxygen would accelerate rancidification of the butterfat, but nitrous oxide inhibits such degradation. Carbon dioxide cannot be used for whipped cream because it is acidic in water, which would curdle the cream and give it a seltzer-like "sparkling" sensation.
The whipped cream produced with nitrous oxide is unstable, and will return to a more liquid state within half an hour to one hour.[25] Thus, the method is not suitable for decorating food that will not be served immediately.
In December 2016, some manufacturers reported a shortage of aerosol whipped creams in the United States due to an explosion at the Air Liquide nitrous oxide facility in Florida in late August. With a major facility offline, the disruption caused a shortage resulting in the company diverting the supply of nitrous oxide to medical customers rather than to food manufacturing. The shortage came during the Christmas and holiday season when canned whipped cream use is normally at its highest.[26]
Similarly, cooking spray, which is made from various types of oils combined with lecithin (an emulsifier), may use nitrous oxide as a propellant. Other propellants used in cooking spray include food-grade alcohol and propane.
Medicine[edit]

2O tanks used in dentistry
Nitrous oxide has been used in dentistry and surgery, as an anaesthetic and analgesic, since 1844.[27] In the early days, the gas was administered through simple inhalers consisting of a breathing bag made of rubber cloth.[28] Today, the gas is administered in hospitals by means of an automated relative analgesia machine, with an anaesthetic vaporiser and a medical ventilator, that delivers a precisely dosed and breath-actuated flow of nitrous oxide mixed with oxygen in a 2:1 ratio.
Nitrous oxide is a weak general anaesthetic, and so is generally not used alone in general anaesthesia, but used as a carrier gas (mixed with oxygen) for more powerful general anaesthetic drugs such as sevoflurane or desflurane. It has a minimum alveolar concentration of 105% and a blood/gas partition coefficient of 0.46. The use of nitrous oxide in anaesthesia can increase the risk of postoperative nausea and vomiting.[29][30][31]
Dentists use a simpler machine which only delivers an N
2O/O
2 mixture for the patient to inhale while conscious but must still be a recognised purpose designed dedicated relative analgesic flowmeter with a minimum 30% of oxygen at all times and a maximum upper limit of 70% nitrous oxide. The patient is kept conscious throughout the procedure, and retains adequate mental faculties to respond to questions and instructions from the dentist.[32]
Inhalation of nitrous oxide is used frequently to relieve pain associated with childbirth, trauma, oral surgery and acute coronary syndrome (including heart attacks). Its use during labour has been shown to be a safe and effective aid for birthing women.[33] Its use for acute coronary syndrome is of unknown benefit.[34]
In Canada and the UK, Entonox and Nitronox are used commonly by ambulance crews (including unregistered practitioners) as rapid and highly effective analgesic gas.
Fifty per cent nitrous oxide can be considered for use by trained non-professional first aid responders in prehospital settings, given the relative ease and safety of administering 50% nitrous oxide as an analgesic. The rapid reversibility of its effect would also prevent it from precluding diagnosis.[35]
Recreational use[edit]



Recreational inhalation of nitrous oxide, with the purpose of causing euphoria and/or slight hallucinations, began as a phenomenon for the British upper class in 1799, known as "laughing gas parties".[36]
Starting in the 19th century, the widespread availability of the gas for medical and culinary purposes allowed for recreational use to expand greatly globally. In the UK as of 2014, nitrous oxide was estimated to be used by almost half a million young people at nightspots, festivals and parties.[37]
Widespread recreational use of the drug throughout the UK was featured in the 2017 Vice documentary Inside The Laughing Gas Black Market, in which journalist Matt Shea met with dealers of the drug who stole it from hospitals.[38]
A significant issue cited in London's press is the effect of nitrous oxide canister littering, which is highly visible and causes significant complaints from communities.[39]
Prior to 8 November 2023, nitrous oxide was subject to the Psychoactive Substances Act 2016 in the UK. It was already illegal to produce, supply, import or export nitrous oxide for recreational use. However, the UK government updated the law on 8 November 2023 to include possession of nitrous oxide by classifying it as a Class C drug under the Misuse of Drugs Act 1971.[40]
While casual use of nitrous oxide is understood by most recreational users to be a route to a "safe high", many are unaware that excessive consumption has the potential to cause neurological harm which, if left untreated, can result in permanent neurological damage.[41] In Australia, recreation use became a public health concern following a rise in reported cases of neurotoxicity and a rise in emergency room admissions, and in (the state of) South Australia legislation was passed in 2020 to restrict canister sales.[42]
Safety[edit]
Nitrous oxide is a significant occupational hazard for surgeons, dentists and nurses. Because nitrous oxide is minimally metabolised in humans (with a rate of 0.004%), it retains its potency when exhaled into the room by the patient, and can pose an intoxicating and prolonged exposure hazard to the clinic staff if the room is poorly ventilated. Where nitrous oxide is administered, a continuous-flow fresh-air ventilation system or N
2O scavenger system is used to prevent a waste-gas buildup.[citation needed]
The National Institute for Occupational Safety and Health recommends that workers' exposure to nitrous oxide should be controlled during the administration of anaesthetic gas in medical, dental and veterinary operators.[43] It set a recommended exposure limit (REL) of 25 ppm (46 mg/m3) to escaped anaesthetic.[44]
Mental and manual impairment[edit]
Exposure to nitrous oxide causes short-term decreases in mental performance, audiovisual ability and manual dexterity.[45] These effects coupled with the induced spatial and temporal disorientation could result in physical harm to the user from environmental hazards.[46]
Neurotoxicity and neuroprotection[edit]
Nitrous oxide is neurotoxic and there is evidence that medium or long-term habitual consumption of significant quantities can cause neurological harm with the potential for permanent damage if left untreated.[42][41]
Like other NMDA receptor antagonists, it has been suggested that N
2O produces neurotoxicity in the form of Olney's lesions in rodents upon prolonged (several hour) exposure.[47][48][49][50]
It has been argued that, because N
2O is rapidly expelled from the body under normal circumstances, it is less likely to be neurotoxic than other NMDAR antagonists.[51] Indeed, in rodents, short-term exposure results in only mild injury that is rapidly reversible, and neuronal death occurs only after constant and sustained exposure.[47] Nitrous oxide also may cause neurotoxicity after extended exposure because of hypoxia. This is especially true of non-medical formulations such as whipped-cream chargers (also known as "whippets" or "nangs"),[52] which never contain oxygen, since oxygen makes cream rancid.[53]
In heavy (≥400 g or ≥200 L of N2O gas in one session) or frequent (regular, i.e., daily or weekly) users reported to poison control centers, signs of peripheral neuropathy have been noted: the presence of ataxia (gait abnormalities) or paresthesia (perception of abnormal sensations, e.g. tingling, numbness, prickling, mostly in the extremities). These are considered an early sign of neurological damage and indicates chronic toxicity.[54]
Nitrous oxide at 75% by volume reduces ischemia-induced neuronal death induced by occlusion of the middle cerebral artery in rodents, and decreases NMDA-induced Ca2+ influx in neuronal cell cultures, a critical event involved in excitotoxicity.[51]
DNA damage[edit]
Occupational exposure to ambient nitrous oxide has been associated with DNA damage, due to interruptions in DNA synthesis.[55] This correlation is dose-dependent[56][57] and does not appear to extend to casual recreational use; however, further research is needed to confirm the duration and quantity of exposure needed to cause damage.
Oxygen deprivation[edit]
If pure nitrous oxide is inhaled without oxygen, oxygen deprivation can occur, resulting in low blood pressure, fainting, and even heart attacks. This can occur if the user inhales large quantities continuously, as with a strap-on mask connected to a gas canister. It can also happen if the user engages in excessive breath-holding or uses any other inhalation system that cuts off a supply of fresh air.[58]
Vitamin B12 deficiency[edit]
Long-term exposure to nitrous oxide may cause vitamin B12 deficiency. This can cause serious neurotoxicity if the user has preexisting vitamin B12 deficiency.[59] It inactivates the cobalamin form of vitamin B12 by oxidation. Symptoms of vitamin B12 deficiency, including sensory neuropathy, myelopathy and encephalopathy, may occur within days or weeks of exposure to nitrous oxide anaesthesia in people with subclinical vitamin B12 deficiency.
Symptoms are treated with high doses of vitamin B12, but recovery can be slow and incomplete.[60]
People with normal vitamin B12 levels have stores to make the effects of nitrous oxide insignificant, unless exposure is repeated and prolonged (nitrous oxide abuse). Vitamin B12 levels should be checked in people with risk factors for vitamin B12 deficiency prior to using nitrous oxide anaesthesia.[61]
Prenatal development[edit]
Several experimental studies in rats indicate that chronic exposure of pregnant females to nitrous oxide may have adverse effects on the developing fetus.[62][63][64]
Chemical/physical risks[edit]
At room temperature (20 °C [68 °F]) the saturated vapour pressure is 50.525 bar, rising up to 72.45 bar at 36.4 °C (97.5 °F)—the critical temperature. The pressure curve is thus unusually sensitive to temperature.[65]
As with many strong oxidisers, contamination of parts with fuels have been implicated in rocketry accidents, where small quantities of nitrous/fuel mixtures explode due to "water hammer"-like effects (sometimes called "dieseling"—heating due to adiabatic compression of gases can reach decomposition temperatures).[66] Some common building materials such as stainless steel and aluminium can act as fuels with strong oxidisers such as nitrous oxide, as can contaminants that may ignite due to adiabatic compression.[67]
There also have been incidents where nitrous oxide decomposition in plumbing has led to the explosion of large tanks.[20]
Mechanism of action[edit]
The pharmacological mechanism of action of N
2O in medicine is not fully known. However, it has been shown to directly modulate a broad range of ligand-gated ion channels, and this likely plays a major role in many of its effects. It moderately blocks NMDAR and β2-subunit-containing nACh channels, weakly inhibits AMPA, kainate, GABAC and 5-HT3 receptors, and slightly potentiates GABAA and glycine receptors.[68][69] It also has been shown to activate two-pore-domain K+
channels.[70] While N
2O affects quite a few ion channels, its anaesthetic, hallucinogenic and euphoriant effects are likely caused predominantly, or fully, via inhibition of NMDA receptor-mediated currents.[68][71] In addition to its effects on ion channels, N
2O may act to imitate nitric oxide (NO) in the central nervous system, and this may be related to its analgesic and anxiolytic properties.[71] Nitrous oxide is 30 to 40 times more soluble than nitrogen.
The effects of inhaling sub-anaesthetic doses of nitrous oxide have been known to vary, based on several factors, including settings and individual differences;[72][73] however, from his discussion, Jay (2008)[46] suggests that it has been reliably known to induce the following states and sensations:
- Intoxication
- Euphoria/dysphoria
- Spatial disorientation
- Temporal disorientation
- Reduced pain sensitivity
A minority of users also will present with uncontrolled vocalisations and muscular spasms. These effects generally disappear minutes after removal of the nitrous oxide source.[46]
Anxiolytic effect[edit]
In behavioural tests of anxiety, a low dose of N
2O is an effective anxiolytic, and this anti-anxiety effect is associated with enhanced activity of GABAA receptors, as it is partially reversed by benzodiazepine receptor antagonists. Mirroring this, animals that have developed tolerance to the anxiolytic effects of benzodiazepines are partially tolerant to N
2O.[74] Indeed, in humans given 30% N
2O, benzodiazepine receptor antagonists reduced the subjective reports of feeling "high", but did not alter psychomotor performance, in human clinical studies.[75][76]
Analgesic effect[edit]
The analgesic effects of N
2O are linked to the interaction between the endogenous opioid system and the descending noradrenergic system. When animals are given morphine chronically, they develop tolerance to its pain-killing effects, and this also renders the animals tolerant to the analgesic effects of N
2O.[77] Administration of antibodies that bind and block the activity of some endogenous opioids (not β-endorphin) also block the antinociceptive effects of N
2O.[78] Drugs that inhibit the breakdown of endogenous opioids also potentiate the antinociceptive effects of N
2O.[78] Several experiments have shown that opioid receptor antagonists applied directly to the brain block the antinociceptive effects of N
2O, but these drugs have no effect when injected into the spinal cord.
Apart from an indirect action, nitrous oxide, like morphine [79] also interacts directly with the endogenous opioid system by binding at opioid receptor binding sites.[80][81]
Conversely, α2-adrenoceptor antagonists block the pain-reducing effects of N
2O when given directly to the spinal cord, but not when applied directly to the brain.[82] Indeed, α2B-adrenoceptor knockout mice or animals depleted in norepinephrine are nearly completely resistant to the antinociceptive effects of N
2O.[83] Apparently N
2O-induced release of endogenous opioids causes disinhibition of brainstem noradrenergic neurons, which release norepinephrine into the spinal cord and inhibit pain signalling.[84] Exactly how N
2O causes the release of endogenous opioid peptides remains uncertain.
Properties and reactions[edit]
Nitrous oxide is a colourless gas with a faint, sweet odour.
Nitrous oxide supports combustion by releasing the dipolar bonded oxygen radical, and can thus relight a glowing splint.
N
2O is inert at room temperature and has few reactions. At elevated temperatures, its reactivity increases. For example, nitrous oxide reacts with NaNH
2 at 187 °C (369 °F) to give NaN
3:
- 2 NaNH2 + N2O → NaN3 + NaOH + NH3
The above reaction is the route adopted by the commercial chemical industry to produce azide salts, which are used as detonators.[85]
History[edit]
The gas was first synthesised in 1772 by English natural philosopher and chemist Joseph Priestley who called it dephlogisticated nitrous air (see phlogiston theory)[86] or inflammable nitrous air.[87] Priestley published his discovery in the book Experiments and Observations on Different Kinds of Air (1775), where he described how to produce the preparation of "nitrous air diminished", by heating iron filings dampened with nitric acid.[88]
Early use[edit]

The first important use of nitrous oxide was made possible by Thomas Beddoes and James Watt, who worked together to publish the book Considerations on the Medical Use and on the Production of Factitious Airs (1794). This book was important for two reasons. First, James Watt had invented a novel machine to produce "factitious airs" (including nitrous oxide) and a novel "breathing apparatus" to inhale the gas. Second, the book also presented the new medical theories by Thomas Beddoes, that tuberculosis and other lung diseases could be treated by inhalation of "Factitious Airs".[27]

The machine to produce "Factitious Airs" had three parts: a furnace to burn the needed material, a vessel with water where the produced gas passed through in a spiral pipe (for impurities to be "washed off"), and finally the gas cylinder with a gasometer where the gas produced, "air", could be tapped into portable air bags (made of airtight oily silk). The breathing apparatus consisted of one of the portable air bags connected with a tube to a mouthpiece. With this new equipment being engineered and produced by 1794, the way was paved for clinical trials,[clarification needed] which began in 1798 when Thomas Beddoes established the "Pneumatic Institution for Relieving Diseases by Medical Airs" in Hotwells (Bristol). In the basement of the building, a large-scale machine was producing the gases under the supervision of a young Humphry Davy, who was encouraged to experiment with new gases for patients to inhale.[27] The first important work of Davy was examination of the nitrous oxide, and the publication of his results in the book: Researches, Chemical and Philosophical (1800). In that publication, Davy notes the analgesic effect of nitrous oxide at page 465 and its potential to be used for surgical operations at page 556.[89] Davy coined the name "laughing gas" for nitrous oxide.[90]
Despite Davy's discovery that inhalation of nitrous oxide could relieve a conscious person from pain, another 44 years elapsed before doctors attempted to use it for anaesthesia. The use of nitrous oxide as a recreational drug at "laughing gas parties", primarily arranged for the British upper class, became an immediate success beginning in 1799. While the effects of the gas generally make the user appear stuporous, dreamy and sedated, some people also "get the giggles" in a state of euphoria, and frequently erupt in laughter.[91]
One of the earliest commercial producers in the U.S. was George Poe, cousin of the poet Edgar Allan Poe, who also was the first to liquefy the gas.[92]
Anaesthetic use[edit]
The first time nitrous oxide was used as an anaesthetic drug in the treatment of a patient was when dentist Horace Wells, with assistance by Gardner Quincy Colton and John Mankey Riggs, demonstrated insensitivity to pain from a dental extraction on 11 December 1844.[93] In the following weeks, Wells treated the first 12 to 15 patients with nitrous oxide in Hartford, Connecticut, and, according to his own record, only failed in two cases.[94] In spite of these convincing results having been reported by Wells to the medical society in Boston in December 1844, this new method was not immediately adopted by other dentists. The reason for this was most likely that Wells, in January 1845 at his first public demonstration to the medical faculty in Boston, had been partly unsuccessful, leaving his colleagues doubtful regarding its efficacy and safety.[95] The method did not come into general use until 1863, when Gardner Quincy Colton successfully started to use it in all his "Colton Dental Association" clinics, that he had just established in New Haven and New York City.[27] Over the following three years, Colton and his associates successfully administered nitrous oxide to more than 25,000 patients.[28] Today, nitrous oxide is used in dentistry as an anxiolytic, as an adjunct to local anaesthetic.
Nitrous oxide was not found to be a strong enough anaesthetic for use in major surgery in hospital settings, however. Instead, diethyl ether, being a stronger and more potent anaesthetic, was demonstrated and accepted for use in October 1846, along with chloroform in 1847.[27] When Joseph Thomas Clover invented the "gas-ether inhaler" in 1876, however, it became a common practice at hospitals to initiate all anaesthetic treatments with a mild flow of nitrous oxide, and then gradually increase the anaesthesia with the stronger ether or chloroform. Clover's gas-ether inhaler was designed to supply the patient with nitrous oxide and ether at the same time, with the exact mixture being controlled by the operator of the device. It remained in use by many hospitals until the 1930s.[28] Although hospitals today use a more advanced anaesthetic machine, these machines still use the same principle launched with Clover's gas-ether inhaler, to initiate the anaesthesia with nitrous oxide, before the administration of a more powerful anaesthetic.
As a patent medicine[edit]
Colton's popularisation of nitrous oxide led to its adoption by a number of less than reputable quacksalvers, who touted it as a cure for consumption, scrofula, catarrh and other diseases of the blood, throat and lungs. Nitrous oxide treatment was administered and licensed as a patent medicine by the likes of C. L. Blood and Jerome Harris in Boston and Charles E. Barney of Chicago.[96][97]
Production[edit]
Reviewing various methods of producing nitrous oxide is published.[98]
Industrial methods[edit]
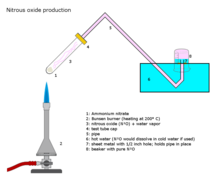
Nitrous oxide is prepared on an industrial scale by carefully heating ammonium nitrate[98] at about 250 °C, which decomposes into nitrous oxide and water vapour.[99]
- NH4NO3 → 2 H2O + N2O
The addition of various phosphate salts favours formation of a purer gas at slightly lower temperatures. This reaction may be difficult to control, resulting in detonation.[100]
Laboratory methods[edit]
The decomposition of ammonium nitrate is also a common laboratory method for preparing the gas. Equivalently, it can be obtained by heating a mixture of sodium nitrate and ammonium sulfate:[101]
- 2 NaNO3 + (NH4)2SO4 → Na2SO4 + 2 N2O + 4 H2O
Another method involves the reaction of urea, nitric acid and sulfuric acid:[102]
- 2 (NH2)2CO + 2 HNO3 + H2SO4 → 2 N2O + 2 CO2 + (NH4)2SO4 + 2 H2O
Direct oxidation of ammonia with a manganese dioxide-bismuth oxide catalyst has been reported:[103] cf. Ostwald process.
- 2 NH3 + 2 O2 → N2O + 3 H2O
Hydroxylammonium chloride reacts with sodium nitrite to give nitrous oxide. If the nitrite is added to the hydroxylamine solution, the only remaining by-product is salt water. If the hydroxylamine solution is added to the nitrite solution (nitrite is in excess), however, then toxic higher oxides of nitrogen also are formed:
- NH3OHCl + NaNO2 → N2O + NaCl + 2 H2O
Treating HNO
3 with SnCl
2 and HCl also has been demonstrated:
- 2 HNO3 + 8 HCl + 4 SnCl2 → 5 H2O + 4 SnCl4 + N2O
Hyponitrous acid decomposes to N2O and water with a half-life of 16 days at 25 °C at pH 1–3.[104]
- H2N2O2 → H2O + N2O
Atmospheric occurrence[edit]
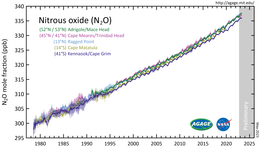



Nitrous oxide is a minor component of Earth's atmosphere and is an active part of the planetary nitrogen cycle. Based on analysis of air samples gathered from sites around the world, its concentration surpassed 330 ppb in 2017.[8] The growth rate of about 1 ppb per year has also accelerated during recent decades.[9] Nitrous oxide's atmospheric abundance has grown more than 20% from a base level of about 270 ppb in year 1750.[106]
Important atmospheric properties of N
2O are summarized in the following table:
| Property | Value |
|---|---|
| Ozone depletion potential (ODP) | 0.017[107] (CCl3F = 1) |
| Global warming potential (GWP: 100-year) | 265[108] (CO2 = 1) |
| Atmospheric lifetime | 116 ± 9 years[109] |
In 2022 the IPCC reported that: "The human perturbation of the natural nitrogen cycle through the use of synthetic fertilizers and manure, as well as nitrogen deposition resulting from land-based agriculture and fossil fuel burning has been the largest driver of the increase in atmospheric N2O of 31.0 ± 0.5 ppb (10%) between 1980 and 2019."[109]
Emissions by source[edit]
17.0 (12.2 to 23.5) million tonnes total annual average nitrogen in N
2O was emitted in 2007–2016.[109] About 40% of N
2O emissions are from humans and the rest are part of the natural nitrogen cycle.[110] The N
2O emitted each year by humans has a greenhouse effect equivalent to about 3 billion tonnes of carbon dioxide: for comparison humans emitted 37 billion tonnes of actual carbon dioxide in 2019, and methane equivalent to 9 billion tonnes of carbon dioxide.[111]
Most of the N
2O emitted into the atmosphere, from natural and anthropogenic sources, is produced by microorganisms such as denitrifying bacteria and fungi in soils and oceans.[112] Soils under natural vegetation are an important source of nitrous oxide, accounting for 60% of all naturally produced emissions. Other natural sources include the oceans (35%) and atmospheric chemical reactions (5%).[113] Wetlands can also be emitters of nitrous oxide.[114][115] Emissions from thawing permafrost may be significant, but as of 2022 this is not certain.[109]
The main components of anthropogenic emissions are fertilised agricultural soils and livestock manure (42%), runoff and leaching of fertilisers (25%), biomass burning (10%), fossil fuel combustion and industrial processes (10%), biological degradation of other nitrogen-containing atmospheric emissions (9%) and human sewage (5%).[116][117][118][119][120] Agriculture enhances nitrous oxide production through soil cultivation, the use of nitrogen fertilisers and animal waste handling.[121] These activities stimulate naturally occurring bacteria to produce more nitrous oxide. Nitrous oxide emissions from soil can be challenging to measure as they vary markedly over time and space,[122] and the majority of a year's emissions may occur when conditions are favorable during "hot moments"[123][124] and/or at favorable locations known as "hotspots".[125]
Among industrial emissions, the production of nitric acid and adipic acid are the largest sources of nitrous oxide emissions. The adipic acid emissions specifically arise from the degradation of the nitrolic acid intermediate derived from the nitration of cyclohexanone.[116][126][127]
Biological processes[edit]
Natural processes that generate nitrous oxide may be classified as nitrification and denitrification. Specifically, they include:
- aerobic autotrophic nitrification, the stepwise oxidation of ammonia (NH
3) to nitrite (NO−
2) and to nitrate (NO−
3) - anaerobic heterotrophic denitrification, the stepwise reduction of NO−
3 to NO−
2, nitric oxide (NO), N
2O and ultimately N
2, where facultative anaerobe bacteria use NO−
3 as an electron acceptor in the respiration of organic material in the condition of insufficient oxygen (O
2) - nitrifier denitrification, which is carried out by autotrophic NH
3-oxidising bacteria and the pathway whereby ammonia (NH
3) is oxidised to nitrite (NO−
2), followed by the reduction of NO−
2 to nitric oxide (NO), N
2O and molecular nitrogen (N
2) - heterotrophic nitrification
- aerobic denitrification by the same heterotrophic nitrifiers
- fungal denitrification
- non-biological chemodenitrification
These processes are affected by soil chemical and physical properties such as the availability of mineral nitrogen and organic matter, acidity and soil type, as well as climate-related factors such as soil temperature and water content.
The emission of the gas to the atmosphere is limited greatly by its consumption inside the cells, by a process catalysed by the enzyme nitrous oxide reductase.[128]
Environmental impact[edit]
Global accounting of N
2O sources and sinks over the decade ending 2016 indicates that about 40% of the average 17 TgN/yr (teragrams, or million metric tons, of nitrogen per year) of emissions originated from human activity, and shows that emissions growth chiefly came from expanding agriculture.[11][12]
Greenhouse effect[edit]

Nitrous oxide has significant global warming potential as a greenhouse gas. On a per-molecule basis, considered over a 100-year period, nitrous oxide has 265 times the atmospheric heat-trapping ability of carbon dioxide (CO
2).[108] However, because of its low concentration (less than 1/1,000 of that of CO
2), its contribution to the greenhouse effect is less than one third that of carbon dioxide, and also less than methane.[129] On the other hand, since about 40% of the N
2O entering the atmosphere is the result of human activity,[116] control of nitrous oxide is part of efforts to curb greenhouse gas emissions.[130]
Most human caused nitrous oxide released into the atmosphere is a greenhouse gas emission from agriculture, when farmers add nitrogen-based fertilizers onto the fields, and through the breakdown of animal manure. Reduction of emissions can be a hot topic in the politics of climate change.[131]
Nitrous oxide is also released as a by-product of burning fossil fuel, though the amount released depends on which fuel was used. It is also emitted through the manufacture of nitric acid, which is used in the synthesis of nitrogen fertilizers. The production of adipic acid, a precursor to nylon and other synthetic clothing fibres, also releases nitrous oxide.[132]
A rise in atmospheric nitrous oxide concentrations has been implicated as a possible contributor to the extremely intense global warming during the Cenomanian-Turonian boundary event.[133]
Ozone layer depletion[edit]
Nitrous oxide has also been implicated in thinning the ozone layer. A 2009 study suggested that N
2O emission was the single most important ozone-depleting emission and it was expected to remain the largest throughout the 21st century.[10][134]
Legality[edit]
India[edit]
Transfer of nitrous oxide from bulk cylinders to smaller, more transportable E-type, 1,590-litre-capacity tanks[135] is legal when the intended use of the gas is for medical anaesthesia.
New Zealand[edit]
The Ministry of Health has warned that nitrous oxide is a prescription medicine, and its sale or possession without a prescription is an offense under the Medicines Act.[136] This statement would seemingly prohibit all non-medicinal uses of nitrous oxide, although it is implied that only recreational use will be targeted legally.
UK[edit]
In August 2015, the Council of the London Borough of Lambeth (UK) banned the use of the drug for recreational purposes, making offenders liable to an on-the-spot fine of up to £1,000.[137]
In September 2023, the Government announced that nitrous oxide would be made illegal by the end of the year, with possession potentially carrying up to a two-year prison sentence or an unlimited fine.[138]
US[edit]
Possession of nitrous oxide is legal under federal law and is not subject to DEA purview.[139] It is, however, regulated by the Food and Drug Administration under the Food Drug and Cosmetics Act; prosecution is possible under its "misbranding" clauses, prohibiting the sale or distribution of nitrous oxide for the purpose of human consumption. Many states have laws regulating the possession, sale and distribution of nitrous oxide. Such laws usually ban distribution to minors or limit the amount of nitrous oxide that may be sold without special license.[citation needed] For example, in the state of California, possession for recreational use is prohibited and qualifies as a misdemeanor.[140]
See also[edit]
References[edit]
- ^ "[Nitrous oxide]". Degruyter.com. Retrieved 24 July 2022.
- ^ IUPAC nomenclature of inorganic chemistry 2005. PDF, p. 317.
- ^ Takahashi M, Shibasaki-Kitakawa N, Yokoyama C, Takahashi S (1996). "Viscosity of Gaseous Nitrous Oxide from 298.15 K to 398.15 K at Pressures up to 25 MPa". Journal of Chemical & Engineering Data. 41 (6): 1495–1498. doi:10.1021/je960060d. ISSN 0021-9568.
- ^ Tarendash AS (2001). Let's review: chemistry, the physical setting (3rd ed.). Barron's Educational Series. p. 44. ISBN 978-0-7641-1664-3.
- ^ PubChem. "Nitrous oxide". pubchem.ncbi.nlm.nih.gov. Retrieved 29 March 2022.
- ^ a b Quax ML, Van Der Steenhoven TJ, Bronkhorst MW, Emmink BL (July 2020). "Frostbite injury: An unknown risk when using nitrous oxide as a party drug". Acta Chirurgica Belgica. 120 (1–4). Taylor & Francis on behalf of the Royal Belgian Society for Surgery: 140–143. doi:10.1080/00015458.2020.1782160. ISSN 0001-5458. PMID 32543291. S2CID 219702849.
- ^ Organization WH (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771.
- ^ a b "Nitrous Oxide (N2O) Mole Fraction" (PDF). Massachusetts Institute of Technology. Archived (PDF) from the original on 9 October 2022. Retrieved 15 February 2021.
- ^ a b "Trends in Atmospheric Nitrous Oxide". National Oceanic and Atmospheric Administration / Earth System Research Laboratories. Retrieved 15 February 2021.
- ^ a b Ravishankara AR, Daniel JS, Portmann RW (2009). "Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century". Science. 326 (5949): 123–5. Bibcode:2009Sci...326..123R. doi:10.1126/science.1176985. PMID 19713491. S2CID 2100618.
- ^ a b Tian H, Xu R, Canadell JG, Thompson RL, Winiwarter W, Suntharalingam P, et al. (October 2020). "A comprehensive quantification of global nitrous oxide sources and sinks". Nature. 586 (7828): 248–256. Bibcode:2020Natur.586..248T. doi:10.1038/s41586-020-2780-0. hdl:1871.1/c74d4b68-ecf4-4c6d-890d-a1d0aaef01c9. ISSN 1476-4687. PMID 33028999. S2CID 222217027. Archived from the original on 3 December 2020. Retrieved 9 November 2020.
{{cite journal}}: CS1 maint: bot: original URL status unknown (link) - ^ a b Thompson, R. L., Lassaletta, L., Patra, P. K. (2019). "Acceleration of global N2O emissions seen from two decades of atmospheric inversion". Nat. Clim. Change. 9 (12): 993–998. Bibcode:2019NatCC...9..993T. doi:10.1038/s41558-019-0613-7. hdl:11250/2646484. S2CID 208302708.
- ^ "Reduce nitrous oxide emissions". Ag Matters. 13 December 2021. Retrieved 1 April 2024.
- ^ "Chapter 8". AR5 Climate Change 2013: The Physical Science Basis. pp. 677–678.
- ^ "Nitrous oxide emissions pose an increasing climate threat, study finds". phys.org. Retrieved 9 November 2020.
- ^ Mundschenk S (3 August 2022). "The Netherlands is showing how not to tackle climate change | The Spectator". www.spectator.co.uk. Retrieved 28 August 2022.
- ^ Berger, Bruno (5 October 2007). "Is nitrous oxide safe?" (PDF). Swiss Propulsion Laboratory. pp. 1–2. Archived (PDF) from the original on 9 October 2022.
...Self pressurizing (Vapor pressure at 20°C is ~50.1 bar...Nontoxic, low reactivity -> rel. safe handling (General safe ???)...Additional energy from decomposition (as a monopropellant: ISP of 170 s)...Specific impulse doesn't change much with O/F...[page 2] N2O is a monopropellant (as H2O2 or Hydrazine...)
- ^ Goddard, R. H. (1914) "Rocket apparatus" U.S. patent 1,103,503
- ^ Nitrous Oxide Safety. Space Propulsion Group (2012)
- ^ a b Munke, Konrad (2 July 2001) Nitrous Oxide Trailer Rupture, Report at CGA Seminar "Safety and Reliability of Industrial Gases, Equipment and Facilities", 15–17 October 2001, St. Louis, Missouri
- ^ "Scaled Composites Safety Guidelines for N
2O" (PDF). Scaled Composites. 17 June 2009. Archived from the original (PDF) on 12 July 2011. Retrieved 29 December 2013.For example, N2O flowing at 130 psi in an epoxy composite pipe would not react even with a 2500 J ignition energy input. At 600 psi, however, the required ignition energy was only 6 J.
- ^ FR-5904. Pratt & Whitney Aircraft.
- ^ Cline, Allen W. (January 2000) "Engine Basics: Detonation and Pre-Ignition". CONTACT! Magazine
- ^ "Holley performance products, FAQ for Nitrous Oxide Systems". Holley. Retrieved 18 December 2013.
- ^ "Explora Science | Nitrous use as a propellant and in cooking". Archived from the original on 27 February 2019. Retrieved 19 February 2019.
- ^ Dewey C (21 December 2016). "The real reason grocery stores are running out of whipped cream this Christmas". The Washington Post. Retrieved 22 December 2016.
- ^ a b c d e Sneader W (2005). "Systematic Medicine". Drug Discovery –A History. John Wiley and Sons. pp. 74–87. ISBN 978-0-471-89980-8.
- ^ a b c Miller AH (1941). "Technical Development of Gas Anesthesia". Anesthesiology. 2 (4): 398–409. doi:10.1097/00000542-194107000-00004. S2CID 71117361.
- ^ Divatia JV, Vaidya JS, Badwe RA, Hawaldar RW (1996). "Omission of Nitrous Oxide during Anesthesia Reduces the Incidence of Postoperative Nausea and Vomiting". Anesthesiology. 85 (5): 1055–1062. doi:10.1097/00000542-199611000-00014. PMID 8916823. S2CID 41549796.
- ^ Hartung J (1996). "Twenty-Four of Twenty-Seven Studies Show a Greater Incidence of Emesis Associated with Nitrous Oxide than with Alternative Anesthetics". Anesthesia & Analgesia. 83 (1): 114–116. doi:10.1213/00000539-199607000-00020.
- ^ Tramèr M, Moore A, McQuay H (February 1996). "Omitting nitrous oxide in general anaesthesia: meta-analysis of intraoperative awareness and postoperative emesis in randomized controlled trials". British Journal of Anaesthesia. 76 (2): 186–193. doi:10.1093/bja/76.2.186. PMID 8777095.
- ^ Council on Clinical Affairs (2013). "Guideline on use of nitrous oxide for pediatric dental patients" (PDF). Reference Manual V37. 6: 206–210. Archived (PDF) from the original on 9 October 2022.
- ^ Copeland C. "Nitrous Oxide Analgesia for Childbirth". Pregnancy.org. Archived from the original on 25 May 2011.
- ^ O'Connor RE, Brady W, Brooks SC, Diercks D, Egan J, Ghaemmaghami C, et al. (2010). "Part 10: acute coronary syndromes: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care". Circulation. 122 (18 Suppl 3): S787–817. doi:10.1161/CIRCULATIONAHA.110.971028. PMID 20956226.
- ^ Faddy SC, Garlick SR (1 December 2005). "A systematic review of the safety of analgesia with 50% nitrous oxide: can lay responders use analgesic gases in the prehospital setting?". Emergency Medicine Journal. 22 (12): 901–908. doi:10.1136/emj.2004.020891. PMC 1726638. PMID 16299211.
- ^ Davy H (1800). Researches, chemical and philosophical: chiefly concerning nitrous oxide, or diphlogisticated nitrous air, and its respiration. Francis A. Countway Library of Medicine. London : printed for J. Johnson, St. Paul's Church-Yard, by Biggs and Cottle, Bristol.
- ^ "Warning over laughing gas misuse". The Guardian. London. Press Association. 9 August 2014. Retrieved 9 August 2014.
- ^ VICE (7 February 2017), Inside The Laughing Gas Black Market, archived from the original on 29 October 2021, retrieved 29 March 2019
- ^ "Recycling used laughing gas canisters for cash could help create a cleaner Britain". Metro. 10 July 2018. Retrieved 15 July 2019.
- ^ "Nitrous oxide ban: guidance". GOV.UK. Retrieved 6 December 2023.
- ^ a b "Nitrous oxide: Laughing gas users risk spine damage, say doctors". Retrieved 26 March 2023.
- ^ a b Evans EB, Evans MR (November 2021). "Nangs, balloons and crackers: Recreational nitrous oxide neurotoxicity". Aust J Gen Pract (Review). 50 (11): 834–838. doi:10.31128/AJGP-10-20-5668. PMID 34713284. S2CID 240153502.
- ^ CDC.gov NIOSH Alert: Controlling Exposures to Nitrous Oxide During Anesthetic Administration. Cincinnati, OH: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 94-100
- ^ "NIOSH Pocket Guide to Chemical Hazards – Nitrous oxide". CDC. Retrieved 21 November 2015.
- ^ Criteria for a recommended standard: occupational exposure to waste anesthetic gases and vapors. Cincinnati, OH: U.S. Department of Health, Education, and Welfare, Public Health Service, Center for Disease Control, National Institute for Occupational Safety and Health, DHEW (NIOSH) Publication No. 77B140.
- ^ a b c Jay M (1 September 2008). "Nitrous oxide: recreational use, regulation and harm reduction". Drugs and Alcohol Today. 8 (3): 22–25. doi:10.1108/17459265200800022.
- ^ a b Jevtovic-Todorovic V, Beals J, Benshoff N, Olney JW (2003). "Prolonged exposure to inhalational anesthetic nitrous oxide kills neurons in adult rat brain". Neuroscience. 122 (3): 609–16. doi:10.1016/j.neuroscience.2003.07.012. PMID 14622904. S2CID 9407096.
- ^ Nakao S, Nagata A, Masuzawa M, Miyamoto E, Yamada M, Nishizawa N, et al. (2003). "NMDA receptor antagonist neurotoxicity and psychotomimetic activity". Masui. The Japanese Journal of Anesthesiology (in Japanese). 52 (6): 594–602. PMID 12854473.
- ^ Jevtovic-Todorovic V, Benshoff N, Olney JW (2000). "Ketamine potentiates cerebrocortical damage induced by the common anaesthetic agent nitrous oxide in adult rats". British Journal of Pharmacology. 130 (7): 1692–8. doi:10.1038/sj.bjp.0703479. PMC 1572233. PMID 10928976.
- ^ Jevtovic-Todorovic V, Carter LB, Carter (2005). "The anesthetics nitrous oxide and ketamine are more neurotoxic to old than to young rat brain". Neurobiology of Aging. 26 (6): 947–56. doi:10.1016/j.neurobiolaging.2004.07.009. PMID 15718054. S2CID 25095727.
- ^ a b Abraini JH, David HN, Lemaire M (2005). "Potentially neuroprotective and therapeutic properties of nitrous oxide and xenon". Annals of the New York Academy of Sciences. 1053 (1): 289–300. Bibcode:2005NYASA1053..289A. doi:10.1111/j.1749-6632.2005.tb00036.x. PMID 16179534. S2CID 34160112.
- ^ De Vasconcellos K, Sneyd JR (2013). "Nitrous oxide: Are we still in equipoise? A qualitative review of current controversies". British Journal of Anaesthesia. 111 (6): 877–85. doi:10.1093/bja/aet215. PMID 23801743.
- ^ Middleton B (2012). Physics in anaesthesia. Banbury, Oxfordshire, UK: Scion Pub. Ltd. ISBN 978-1-904842-98-9.
- ^ van Riel A (2022). "Alarming increase in poisonings from recreational nitrous oxide use after a change in EU-legislation, inquiries to the Dutch Poisons Information Center". International Journal of Drug Policy. 100: 103519. doi:10.1016/j.drugpo.2021.103519. PMID 34753046.
- ^ Randhawa G, Bodenham A (1 March 2016). "The increasing recreational use of nitrous oxide: history revisited". British Journal of Anaesthesia. pp. 321–324. doi:10.1093/bja/aev297. PMID 26323292.
- ^ Wrońska-Nofer T, Nofer JR, Jajte J, Dziubałtowska E, Szymczak W, Krajewski W, et al. (1 March 2012). "Oxidative DNA damage and oxidative stress in subjects occupationally exposed to nitrous oxide (N2O)". Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 731 (1): 58–63. doi:10.1016/j.mrfmmm.2011.10.010. PMID 22085808.
- ^ Wrońska-Nofer T, Palus J, Krajewski W, Jajte J, Kucharska M, Stetkiewicz J, et al. (18 June 2009). "DNA damage induced by nitrous oxide: Study in medical personnel of operating rooms". Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 666 (1–2): 39–43. doi:10.1016/j.mrfmmm.2009.03.012. PMID 19439331.
- ^ Dangers of Nitrous Oxide. Just Say N2O
- ^ Flippo TS, Holder WD Jr (1993). "Neurologic Degeneration Associated with Nitrous Oxide Anesthesia in Patients with Vitamin B12 Deficiency". Archives of Surgery. 128 (12): 1391–5. doi:10.1001/archsurg.1993.01420240099018. PMID 8250714.
- ^ Giannini A (1999). Drug Abuse. Los Angeles: Health Information Press. ISBN 978-1-885987-11-2.
- ^ Conrad M (4 October 2006). "Pernicious Anemia". Medscape. Retrieved 2 June 2008.
- ^ Vieira E, Cleaton-Jones P, Austin J, Moyes D, Shaw R (1980). "Effects of low concentrations of nitrous oxide on rat fetuses". Anesthesia and Analgesia. 59 (3): 175–7. doi:10.1213/00000539-198003000-00002. PMID 7189346. S2CID 41966990.
- ^ Vieira E (1979). "Effect of the chronic administration of nitrous oxide 0.5% to gravid rats". British Journal of Anaesthesia. 51 (4): 283–7. doi:10.1093/bja/51.4.283. PMID 465253.
- ^ Vieira E, Cleaton-Jones P, Moyes D (1983). "Effects of low intermittent concentrations of nitrous oxide on the developing rat fetus". British Journal of Anaesthesia. 55 (1): 67–9. doi:10.1093/bja/55.1.67. PMID 6821624.
- ^ Nitrous oxide Archived 30 March 2016 at the Wayback Machine. Air Liquide Gas Encyclopedia.
- ^ "Vaseline triggered explosion of hybrid rocket". Ukrocketman.com.
- ^ "Safetygram 20: Nitrous Oxide" (PDF). Airproducts.com. Archived from the original (PDF) on 1 September 2006.
- ^ a b Yamakura T, Harris RA (2000). "Effects of gaseous anaesthetics nitrous oxide and xenon on ligand-gated ion channels. Comparison with isoflurane and ethanol". Anesthesiology. 93 (4): 1095–101. doi:10.1097/00000542-200010000-00034. PMID 11020766. S2CID 4684919.
- ^ Mennerick S, Jevtovic-Todorovic V, Todorovic SM, Shen W, Olney JW, Zorumski CF (1998). "Effect of nitrous oxide on excitatory and inhibitory synaptic transmission in hippocampal cultures". Journal of Neuroscience. 18 (23): 9716–26. doi:10.1523/JNEUROSCI.18-23-09716.1998. PMC 6793274. PMID 9822732.
- ^ Gruss M, Bushell TJ, Bright DP, Lieb WR, Mathie A, Franks NP (2004). "Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane". Molecular Pharmacology. 65 (2): 443–52. doi:10.1124/mol.65.2.443. PMID 14742687. S2CID 7762447.
- ^ a b Emmanouil DE, Quock RM (2007). "Advances in Understanding the Actions of Nitrous Oxide". Anesthesia Progress. 54 (1): 9–18. doi:10.2344/0003-3006(2007)54[9:AIUTAO]2.0.CO;2. PMC 1821130. PMID 17352529.
- ^ Atkinson RM, Green JD, Chenoweth DE, Atkinson JH (1 October 1979). "Subjective Effects of Nitrous Oxide: Cognitive, Emotional, Perceptual and Transcendental Experiences". Journal of Psychedelic Drugs. 11 (4): 317–330. doi:10.1080/02791072.1979.10471415. PMID 522172.
- ^ Walker DJ, Zacny JP (1 September 2001). "Within- and between-subject variability in the reinforcing and subjective effects of nitrous oxide in healthy volunteers". Drug and Alcohol Dependence. 64 (1): 85–96. doi:10.1016/s0376-8716(00)00234-9. PMID 11470344.
- ^ Emmanouil DE, Johnson CH, Quock RM (1994). "Nitrous oxide anxiolytic effect in mice in the elevated plus maze: mediation by benzodiazepine receptors". Psychopharmacology. 115 (1–2): 167–72. doi:10.1007/BF02244768. PMID 7862891. S2CID 21652496.
- ^ Zacny JP, Yajnik S, Coalson D, Lichtor JL, Apfelbaum JL, Rupani G, et al. (1995). "Flumazenil may attenuate some subjective effects of nitrous oxide in humans: a preliminary report". Pharmacology Biochemistry and Behavior. 51 (4): 815–9. doi:10.1016/0091-3057(95)00039-Y. PMID 7675863. S2CID 39068081.
- ^ Gillman MA (2022). "What is better for psychiatry: Titrated or fixed concentrations of nitrous oxide?". Front. Psychiatry. 13 (773190): 460–3. doi:10.3389/fpsyt.2022.773190. PMC 9441863. PMID 36072452.
- ^ Berkowitz BA, Finck AD, Hynes MD, Ngai SH (1979). "Tolerance to nitrous oxide analgesia in rats and mice". Anesthesiology. 51 (4): 309–12. doi:10.1097/00000542-197910000-00006. PMID 484891. S2CID 26281498.
- ^ a b Branda EM, Ramza JT, Cahill FJ, Tseng LF, Quock RM (2000). "Role of brain dynorphin in nitrous oxide antinociception in mice". Pharmacology Biochemistry and Behavior. 65 (2): 217–21. doi:10.1016/S0091-3057(99)00202-6. PMID 10672972. S2CID 1978597.
- ^ Gillman M.A. [1986a]. Minireview: Analgesic [sub anaesthetic] nitrous oxide interacts with the endogenous opioid system : A review of the evidence. Life Sciences 39: l209-l22l
- ^ (Daras, C., Cantrill, R. C., Gillman, M. A. [1983]. 3[H]-Naloxone displacement: evidence for nitrous oxide as an opioid agonist. European Journal of Pharmacology 89: 177-8.
- ^ Ori, C., Ford-Rice, F., London, E. D. [1989]. Effects of nitrous oxide and halothane on mu and kappa opioid receptors in guinea-pig brain. Anesthesiology 70: 541-544.)
- ^ Guo TZ, Davies MF, Kingery WS, Patterson AJ, Limbird LE, Maze M (1999). "Nitrous oxide produces antinociceptive response via alpha2B and/or alpha2C adrenoceptor subtypes in mice". Anesthesiology. 90 (2): 470–6. doi:10.1097/00000542-199902000-00022. PMID 9952154.
- ^ Sawamura S, Kingery WS, Davies MF, Agashe GS, Clark JD, Koblika BK, et al. (2000). "Antinociceptive action of nitrous oxide is mediated by stimulation of noradrenergic neurons in the brainstem and activation of [alpha]2B adrenoceptors". J. Neurosci. 20 (24): 9242–51. doi:10.1523/JNEUROSCI.20-24-09242.2000. PMC 6773006. PMID 11125002.
- ^ Maze M, Fujinaga M (2000). "Recent advances in understanding the actions and toxicity of nitrous oxide". Anaesthesia. 55 (4): 311–4. doi:10.1046/j.1365-2044.2000.01463.x. PMID 10781114. S2CID 39823627.
- ^ Housecroft, Catherine E., Sharpe, Alan G. (2008). "Chapter 15: The group 15 elements". Inorganic Chemistry (3rd ed.). Pearson. p. 464. ISBN 978-0-13-175553-6.
- ^ Keys T (1941). "The Development of Anesthesia". Anesthesiology. 2 (5): 552–574. Bibcode:1982AmSci..70..522D. doi:10.1097/00000542-194109000-00008. S2CID 73062366.
- ^ McEvoy JG (6 March 2015). "Gases, God and the balance of nature: a commentary on Priestley (1772) 'Observations on different kinds of air'". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 373 (2039): 20140229. Bibcode:2015RSPTA.37340229M. doi:10.1098/rsta.2014.0229. PMC 4360083. PMID 25750146.
- ^ Priestley J (1776). "Experiments and Observations on Different Kinds of Air". Erowid.
- ^ Davy H (1800). Researches, chemical and philosophical –chiefly concerning nitrous oxide or dephlogisticated nitrous air, and its respiration. Printed for J. Johnson.
- ^ Hardman JG (2017). Oxford Textbook of Anaesthesia. Oxford University Press. p. 529. ISBN 978-0-19-964204-5.
- ^ Brecher EM (1972). "Consumers Union Report on Licit and Illicit Drugs, Part VI – Inhalants and Solvents and Glue-Sniffing". Consumer Reports Magazine. Retrieved 18 December 2013.
- ^ "George Poe is Dead". Washington Post. 3 February 1914. Archived from the original on 1 March 2013. Retrieved 29 December 2007.
- ^ Erving HW (1933). "The Discoverer of Anæsthesia: Dr. Horace Wells of Hartford". The Yale Journal of Biology and Medicine. 5 (5): 421–430. PMC 2606479. PMID 21433572.
- ^ Wells H (1847). A history of the discovery, of the application of nitrous oxide gas, ether, and other vapours, to surgical operations. J. Gaylord Wells.
- ^ Desai SP, Desai MS, Pandav CS (2007). "The discovery of modern anaesthesia-contributions of Davy, Clarke, Long, Wells and Morton". Indian J Anaesth. 51 (6): 472–8.
- ^ "Alleged Forgery". The Inter Ocean. 28 September 1877. p. 8. Retrieved 26 October 2015.
- ^ "A Man of Ominous Name". The Inter Ocean. 19 February 1890. Retrieved 26 October 2015.
- ^ a b Parmon VN, Panov GI, Uriarte A, Noskov AS (2005). "Nitrous oxide in oxidation chemistry and catalysis application and production". Catalysis Today. 100 (2005): 115–131. doi:10.1016/j.cattod.2004.12.012.
- ^ Holleman AF, Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 978-0-12-352651-9.
- ^ "Nitrous oxide plant". Sanghi Organization. Archived from the original on 27 November 2013. Retrieved 18 December 2013.
- ^ "Nitrogen Family" Archived 21 October 2014 at the Wayback Machine. chemistry.tutorvista.com
- ^ "Preparation of Nitrous Oxide from Urea, Nitric Acid and Sulfuric Acid".
- ^ Suwa T, Matsushima A, Suziki Y, Namina Y (1961). "Manufacture of Nitrous Oxide by the Catalytic Oxidation of Ammonia". The Journal of the Society of Chemical Industry, Japan. 64 (11): 1879–1888. doi:10.1246/nikkashi1898.64.11_1879.
- ^ Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5
- ^ "N
2O Budget". Global Carbon Project. Retrieved 9 November 2020. - ^ "Chapter 6". TAR Climate Change 2001: The Scientific Basis. p. 358.
- ^ Ravishankara AR, Daniel JS, Portmann RW (27 August 2009), "Supporting Online Material for - Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century" (PDF), Science, 326 (5949): 123–125, Bibcode:2009Sci...326..123R, doi:10.1126/science.1176985, PMID 19713491, S2CID 2100618, archived (PDF) from the original on 9 October 2022
- ^ a b "Chapter 8". AR5 Climate Change 2013: The Physical Science Basis. p. 731.
- ^ a b c d Chapter 5: Global Carbon and other Biogeochemical Cycles and Feedbacks. www.ipcc.ch (Report). Retrieved 6 May 2023.
- ^ US EPA O (23 December 2015). "Overview of Greenhouse Gases". www.epa.gov. Retrieved 4 May 2023.
- ^ "| Greenhouse Gas (GHG) Emissions | Climate Watch". www.climatewatchdata.org. Retrieved 4 May 2023.
- ^ Sloss LL (1992). Nitrogen Oxides Control Technology Fact Book. William Andrew. p. 6. ISBN 978-0-8155-1294-3.
- ^ U.S. Environmental Protection Agency (2010), "Methane and Nitrous Oxide Emissions from Natural Sources". Report EPA 430-R-10-001.
- ^ Bange HW (2006). "Nitrous oxide and methane in European coastal waters". Estuarine, Coastal and Shelf Science. 70 (3): 361–374. Bibcode:2006ECSS...70..361B. doi:10.1016/j.ecss.2006.05.042.
- ^ Thompson AJ, Giannopoulos G, Pretty J, Baggs EM, Richardson DJ (2012). "Biological sources and sinks of nitrous oxide and strategies to mitigate emissions". Philosophical Transactions of the Royal Society B. 367 (1593): 1157–1168. doi:10.1098/rstb.2011.0415. PMC 3306631. PMID 22451101.
- ^ a b c K. L. Denman, G. Brasseur, et al. (2007), "Couplings Between Changes in the Climate System and Biogeochemistry". In Fourth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press.
- ^ Steinfeld, H., Gerber, P., Wassenaar, T., Castel, V., Rosales, M., de Haan, C. (2006). Livestock's long shadow: Environmental issues and options. Fao.org. Retrieved 2 February 2008.
- ^ "Overview of Greenhouse Gases: Nitrous Oxide". U.S. Environmental Protection Agency. 23 December 2015. Archived from the original on 12 August 2016. Retrieved 31 March 2016.
- ^ "Nitrous Oxide: Sources and Emissions". U.S. Environmental Protection Agency. 2006. Archived from the original on 16 January 2008. Retrieved 2 February 2008.
- ^ IPCC. 2013. Climate change: the physical basis (WG I, full report). p. 512.
- ^ Thompson RL, Lassaletta L, Patra PK, Wilson C, Wells KC, Gressent A, et al. (18 November 2019). "Acceleration of global N 2 O emissions seen from two decades of atmospheric inversion". Nature Climate Change. 9 (12): 993–998. Bibcode:2019NatCC...9..993T. doi:10.1038/s41558-019-0613-7. hdl:11250/2646484. ISSN 1758-6798. S2CID 208302708.
- ^ Molodovskaya M, Warland J, Richards BK, Öberg G, Steenhuis TS (2011). "Nitrous Oxide from Heterogeneous Agricultural Landscapes: Source Contribution Analysis by Eddy Covariance and Chambers". Soil Science Society of America Journal. 75 (5): 1829. Bibcode:2011SSASJ..75.1829M. doi:10.2136/SSSAJ2010.0415.
- ^ Molodovskaya M, Singurindy O, Richards BK, Warland JS, Johnson M, Öberg G, et al. (2012). "Temporal variability of nitrous oxide from fertilized croplands: hot moment analysis". Soil Science Society of America Journal. 76 (5): 1728–1740. Bibcode:2012SSASJ..76.1728M. doi:10.2136/sssaj2012.0039. S2CID 54795634.
- ^ Singurindy O, Molodovskaya M, Richards BK, Steenhuis TS (July 2009). "Nitrous oxide emission at low temperatures from manure-amended soils under corn (Zea mays L.)". Agriculture, Ecosystems & Environment. 132 (1–2): 74–81. Bibcode:2009AgEE..132...74S. doi:10.1016/j.agee.2009.03.001.
- ^ Mason C, Stoof C, Richards B, Das S, Goodale C, Steenhuis T (2017). "Hotspots of nitrous oxide emission in fertilized and unfertilized perennial grasses on wetness-prone marginal land in New York State". Soil Science Society of America Journal. 81 (3): 450–458. Bibcode:2017SSASJ..81..450M. doi:10.2136/sssaj2016.08.0249.
- ^ Reimer R. A., Slaten C. S., Seapan M., Lower M. W., Tomlinson P. E. (1994). "Abatement of N2O emissions produced in the adipic acid industry". Environmental Progress. 13 (2): 134–137. Bibcode:1994EnvPr..13..134R. doi:10.1002/ep.670130217.
- ^ Shimizu, A., Tanaka, K., Fujimori, M. (2000). "Abatement of N2O emissions produced in the adipic acid industry". Chemosphere – Global Change Science. 2 (3–4): 425–434. Bibcode:2000ChGCS...2..425S. doi:10.1016/S1465-9972(00)00024-6.
- ^ Schneider, Lisa K., Wüst, Anja, Pomowski, Anja, Zhang, Lin, Einsle, Oliver (2014). "No Laughing Matter: The Unmaking of the Greenhouse Gas Dinitrogen Monoxide by Nitrous Oxide Reductase". In Kroneck, Peter M. H., Sosa Torres, Martha E. (eds.). The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment. Metal Ions in Life Sciences. Vol. 14. Springer. pp. 177–210. doi:10.1007/978-94-017-9269-1_8. ISBN 978-94-017-9268-4. PMID 25416395.
- ^ US Environmental Protection Agency, "Climate Change Indicators: Atmospheric Concentrations of Greenhouse Gases" Web document, accessed on 2017-02-14
- ^ "4.1.1 Sources of Greenhouse Gases". IPCC TAR WG1 2001. Archived from the original on 29 October 2012. Retrieved 21 September 2012.
- ^ Mundschenk S (3 August 2022). "The Netherlands is showing how not to tackle climate change | The Spectator". www.spectator.co.uk. Retrieved 28 August 2022.
- ^ "Overview of Greenhouse Gases: Nitrous Oxide Emissions". United States Environmental Protection Agency. 6 October 2016. Retrieved 14 July 2019.
- ^ Naafs BD, Monteiro FM, Pearson A, Higgins MB, Pancost RD, Ridgwell A (10 December 2019). "Fundamentally different global marine nitrogen cycling in response to severe ocean deoxygenation". Proceedings of the National Academy of Sciences of the United States of America. 116 (50): 24979–24984. Bibcode:2019PNAS..11624979N. doi:10.1073/pnas.1905553116. PMC 6911173. PMID 31767742.
- ^ Grossman L (28 August 2009). "Laughing gas is biggest threat to ozone layer". New Scientist.
- ^ "Ohio Medical" (PDF). www.ohiomedical.com. Archived from the original (PDF) on 17 April 2016. Retrieved 20 September 2017.
- ^ Anderton J (26 June 2005). "Time's up for sham sales of laughing gas". Beehive.govt.nz. Archived from the original on 8 January 2015.
- ^ "Lambeth Council bans laughing gas as recreational drug". BBC News. 17 August 2015. Retrieved 17 August 2015.
- ^ "Nitrous oxide: Laughing gas to be illegal by end of year". BBC News. 5 September 2023. Retrieved 5 September 2023.
- ^ "US Nitrous Oxide Laws (alphabetically) Based on a search of online free legal databases. Conducted May 2002". Center for Cognitive Liberty and Ethics. Archived from the original on 24 January 2008. Retrieved 27 January 2008.
- ^ "CAL. PEN. CODE § 381b: California Code – Section 381b". Lp.findlaw.com.
External links[edit]
- Occupational Safety and Health Guideline for Nitrous Oxide
- Paul Crutzen Interview Freeview video of Paul Crutzen Nobel Laureate for his work on decomposition of ozone talking to Harry Kroto Nobel Laureate by the Vega Science Trust.
- National Pollutant Inventory – Oxide of nitrogen fact sheet
- National Institute for Occupational Safety and Health – Nitrous Oxide
- CDC – NIOSH Pocket Guide to Chemical Hazards – Nitrous Oxide
- Nitrous Oxide FAQ
- Erowid article on Nitrous Oxide
- Nitrous oxide fingered as monster ozone slayer Archived 29 September 2012 at the Wayback Machine, Science News
- Dental Fear Central article on the use of nitrous oxide in dentistry
- Altered States Database
- 5-HT3 antagonists
- Aerosol propellants
- Dissociative drugs
- E-number additives
- Euphoriants
- GABAA receptor positive allosteric modulators
- Gaseous signaling molecules
- General anesthetics
- Glycine receptor agonists
- Greenhouse gases
- Industrial gases
- Industrial hygiene
- Inhalants
- Nitrogen oxides
- Monopropellants
- Nicotinic antagonists
- Nitrogen cycle
- NMDA receptor antagonists
- Rocket oxidizers
- Trace gases
- World Health Organization essential medicines
- Neurotoxins


