Opioid use disorder: Difference between revisions
m Reverted edits by 72.38.187.82 (talk) to last version by AnomieBOT |
moved some way to complicated stuff out of the lead and added |
||
| Line 15: | Line 15: | ||
MeshID = D009293 | |
MeshID = D009293 | |
||
}} |
}} |
||
'''Opioid dependence''' is a medical |
'''Opioid dependence''' or '''opioid use disorder''' is a medical condition of opioid [[addiction]], and is characterized by an compulsive use of [[opioid]]s (e.g., [[morphine]], [[heroin]], [[codeine]], [[oxycodone]], [[hydrocodone]], etc.) in spite of consequences of continued use. The necessary descriptive characteristics of the medical diagnosis are preoccupation with a desire to obtain and take the drug and persistent drug-seeking behaviour. In a non-diagnostic context, opioid dependence properly refers to a dependence-withdrawal syndrome that is characterized by both [[psychological dependence]] and marked [[physical dependence]] upon opioid compounds; this dependence-withdrawal syndrome is a component of opioid [[addiction]]. |
||
<!-- Epidemiology --> |
|||
Opioid use disorders resulted in 51,000 deaths in 2013 up from 18,000 deaths in 1990.<ref name=GDB2013>{{cite journal|last1=GBD 2013 Mortality and Causes of Death|first1=Collaborators|title=Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013.|journal=Lancet|date=17 December 2014|pmid=25530442|doi=10.1016/S0140-6736(14)61682-2}}</ref> |
|||
{{TOC limit}} |
{{TOC limit}} |
||
| Line 66: | Line 69: | ||
The [[preproenkephalin]] gene, PENK, encodes for the endogenous opiates that modulate pain perception, and are implicated in reward and addiction. [[Microsatellite|(CA) repeats]] in the 3' flanking sequence of the PENK gene was associated with greater likelihood of opiate dependence in repeated studies. Variability in the MCR2 gene, encoding [[melanocortin receptor]] type 2 has been associated with both protective effects and increased susceptibility to heroin addiction. The CYP2B6 gene of the [[cytochrome P450]] family also mediates breakdown of opioids and thus may play a role in dependence and overdose.<ref>{{cite journal|last1=Khokhar|first1=Jibran Y. |author2=Ferguson, Charmaine S.; Zhu, Andy Z.X.; Tyndale, Rachel F.|title=Pharmacogenetics of Drug Dependence: Role of Gene Variations in Susceptibility and Treatment|journal=Annual Review of Pharmacology and Toxicology|date=February 2010|volume=50|issue=1|pages=39–61|doi=10.1146/annurev.pharmtox.010909.105826}}</ref> |
The [[preproenkephalin]] gene, PENK, encodes for the endogenous opiates that modulate pain perception, and are implicated in reward and addiction. [[Microsatellite|(CA) repeats]] in the 3' flanking sequence of the PENK gene was associated with greater likelihood of opiate dependence in repeated studies. Variability in the MCR2 gene, encoding [[melanocortin receptor]] type 2 has been associated with both protective effects and increased susceptibility to heroin addiction. The CYP2B6 gene of the [[cytochrome P450]] family also mediates breakdown of opioids and thus may play a role in dependence and overdose.<ref>{{cite journal|last1=Khokhar|first1=Jibran Y. |author2=Ferguson, Charmaine S.; Zhu, Andy Z.X.; Tyndale, Rachel F.|title=Pharmacogenetics of Drug Dependence: Role of Gene Variations in Susceptibility and Treatment|journal=Annual Review of Pharmacology and Toxicology|date=February 2010|volume=50|issue=1|pages=39–61|doi=10.1146/annurev.pharmtox.010909.105826}}</ref> |
||
==Mechanism== |
|||
Overexpression of the [[gene transcription factor]] "[[ΔFosB]]" in the [[nucleus accumbens]] plays a crucial role in the development of opioid addiction by directly modulating compulsive drug-seeking behaviors.<ref name="Cellular basis">{{cite journal | author = Nestler EJ | title = Cellular basis of memory for addiction | journal = Dialogues Clin Neurosci | volume = 15 | issue = 4 | pages = 431–443 |date=December 2013 | pmid = 24459410 | pmc = 3898681 | doi = | quote = DESPITE THE IMPORTANCE OF NUMEROUS PSYCHOSOCIAL FACTORS, AT ITS CORE, DRUG ADDICTION INVOLVES A BIOLOGICAL PROCESS: the ability of repeated exposure to a drug of abuse to induce changes in a vulnerable brain that drive the compulsive seeking and taking of drugs, and loss of control over drug use, that define a state of addiction. ... A large body of literature has demonstrated that such ΔFosB induction in D1-type NAc neurons increases an animal's sensitivity to drug as well as natural rewards and promotes drug self-administration, presumably through a process of positive reinforcement}}</ref><ref name="Nestler" /><ref name="Natural and drug addictions" /> [[ΔFosB]] overexpression and altered regulation of [[cAMP response element binding protein]] (CREB) in the group of [[dopaminergic projection]]s from the [[ventral tegmental area]] are also responsible for the induction of the negative emotional withdrawal state that characterizes opioid-related [[psychological dependence]];<ref name="Cellular basis" /><ref name="Natural and drug addictions" /> the associated mechanism(s) underlying the development of [[physical dependence]] are not currently understood. |
|||
==Diagnosis== |
==Diagnosis== |
||
| Line 133: | Line 139: | ||
==Epidemiology== |
==Epidemiology== |
||
Opioid use disorders resulted in 51,000 deaths in 2013 up from 18,000 deaths in 1990.<ref name=GDB2013/> |
|||
As of 2010 opioid use disorder resulted in about 43,000 deaths globally up from 8,900 in 1990.<ref name=Loz2012>{{cite journal|last=Lozano|first=R|title=Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010|journal=Lancet|date=15 December 2012|volume=380|issue=9859|pages=2095–128|pmid=23245604|doi=10.1016/S0140-6736(12)61728-0|last2=Naghavi|first2=Mohsen|last3=Foreman|first3=Kyle|last4=Lim|first4=Stephen|last5=Shibuya|first5=Kenji|last6=Aboyans|first6=Victor|last7=Abraham|first7=Jerry|last8=Adair|first8=Timothy|last9=Aggarwal|first9=Rakesh|last10=Ahn|first10=Stephanie Y|last11=Almazroa|first11=Mohammad A|last12=Alvarado|first12=Miriam|last13=Anderson|first13=H Ross|last14=Anderson|first14=Laurie M|last15=Andrews|first15=Kathryn G|last16=Atkinson|first16=Charles|last17=Baddour|first17=Larry M|last18=Barker-Collo|first18=Suzanne|last19=Bartels|first19=David H|last20=Bell|first20=Michelle L|last21=Benjamin|first21=Emelia J|last22=Bennett|first22=Derrick|last23=Bhalla|first23=Kavi|last24=Bikbov|first24=Boris|last25=Abdulhak|first25=Aref Bin|last26=Birbeck|first26=Gretchen|last27=Blyth|first27=Fiona|last28=Bolliger|first28=Ian|last29=Boufous|first29=Soufiane|last30=Bucello|first30=Chiara}}</ref> |
|||
Among adults, the rate of inpatient hospital stays in the United States related to opioid overuse increased by an average of 5% annually from 1993–2012. The percentage of inpatient stays due to opioid overuse that were admitted from the emergency department increased from 43% in 1993 to 64% in 2005, but have remained relatively constant since.<ref>{{cite web | author = Owens PL, Barrett ML, Weiss AJ, Washington RE, Kronick R | title = Hospital Inpatient Utilization Related to Opioid Overuse Among Adults, 1993–2012 | work = HCUP Statistical Brief #177 | publisher = Agency for Healthcare Research and Quality | location = Rockville, MD | date = August 2014 | url = https://www.hcup-us.ahrq.gov/reports/statbriefs/sb177-Hospitalizations-for-Opioid-Overuse.jsp}}</ref> |
Among adults, the rate of inpatient hospital stays in the United States related to opioid overuse increased by an average of 5% annually from 1993–2012. The percentage of inpatient stays due to opioid overuse that were admitted from the emergency department increased from 43% in 1993 to 64% in 2005, but have remained relatively constant since.<ref>{{cite web | author = Owens PL, Barrett ML, Weiss AJ, Washington RE, Kronick R | title = Hospital Inpatient Utilization Related to Opioid Overuse Among Adults, 1993–2012 | work = HCUP Statistical Brief #177 | publisher = Agency for Healthcare Research and Quality | location = Rockville, MD | date = August 2014 | url = https://www.hcup-us.ahrq.gov/reports/statbriefs/sb177-Hospitalizations-for-Opioid-Overuse.jsp}}</ref> |
||
Revision as of 23:02, 28 February 2015
This article needs more reliable medical references for verification or relies too heavily on primary sources. (October 2014) |  |
| Opioid use disorder | |
|---|---|
| Specialty | Psychiatry |
Opioid dependence or opioid use disorder is a medical condition of opioid addiction, and is characterized by an compulsive use of opioids (e.g., morphine, heroin, codeine, oxycodone, hydrocodone, etc.) in spite of consequences of continued use. The necessary descriptive characteristics of the medical diagnosis are preoccupation with a desire to obtain and take the drug and persistent drug-seeking behaviour. In a non-diagnostic context, opioid dependence properly refers to a dependence-withdrawal syndrome that is characterized by both psychological dependence and marked physical dependence upon opioid compounds; this dependence-withdrawal syndrome is a component of opioid addiction.
Opioid use disorders resulted in 51,000 deaths in 2013 up from 18,000 deaths in 1990.[1]
Symptoms of withdrawal
Symptoms of withdrawal from opiates include, but are not limited to:[2]
Early symptoms
- Agitation
- Anxiety
- Muscle aches
- Increased tearing
- Insomnia
- Runny nose
- Sweating
- Yawning
- Skin-Crawling
Late symptoms
- Abdominal cramping
- Diarrhea
- Dilated pupils
- Goose bumps
- Nausea
- Vomiting
Causes
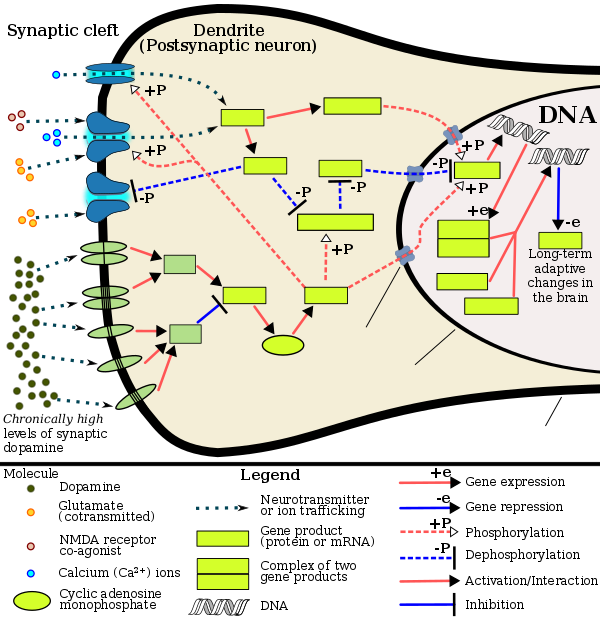
Like many other forms of behavioral addiction and drug addiction, overuse of opiates leads to increased ΔFosB expression in the nucleus accumbens.[3][4][5] Opiates affect dopamine neurotransmission in the nucleus accumbens through their disinhibition of the GABA-based negative dopaminergic feedback system in the tail of the ventral tegmental area (tVTA).[6][7][8]
It has been demonstrated that most people who are opioid-dependent have at least one other psychiatric comorbidity.[9] Since opioids used in pain therapy rarely cause any of these conditions, they are assumed to have existed prior to the development of dependence.[citation needed] Opioids are known to have strong antidepressive, anxiolytic and antipsychotic effects and thus opioid dependence often develops as a result of self medication.[citation needed] Opioids are excellent acute pain medication, but it is their ability to produce euphoria that makes them attractive to addicts.[10] Scoring systems have been derived to assess the likelihood of opiate addiction in chronic pain patients.[11]
Furthermore some studies suggest a permanent dysregulation of the endogenous opioid receptor system after chronic exposure to opioids. A recent study has shown that an increase in BDNF, brain-derived neurotrophic factor, in the ventral tegmental area (VTA) in rats can cause opiate-naive rats to begin displaying opiate-dependent behavior, including withdrawal and drug-seeking behavior.[12] It has been shown that when an opiate-naive person begins using opiates at levels inducing euphoria, this same increase in BDNF occurs.[13]
Another recent study concluded to have shown "a direct link between morphine abstinence and depressive-like symptoms" and postulates "that serotonin dysfunction represents a main mechanism contributing to mood disorders in opiate abstinence".[14]
| opioid use disorder | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | opioid abuseopioid addictionopiate abuseopiate opiateopiate use disorderopiate addiction | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | GeneCards: [1]; OMA:- orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Protein fosB, also known as FosB and G0/G1 switch regulatory protein 3 (G0S3), is a protein that in humans is encoded by the FBJ murine osteosarcoma viral oncogene homolog B (FOSB) gene.[15][16][17]
The FOS gene family consists of four members: FOS, FOSB, FOSL1, and FOSL2. These genes encode leucine zipper proteins that can dimerize with proteins of the JUN family (e.g., c-Jun, JunD), thereby forming the transcription factor complex AP-1. As such, the FOS proteins have been implicated as regulators of cell proliferation, differentiation, and transformation.[15] FosB and its truncated splice variants, ΔFosB and further truncated Δ2ΔFosB, are all involved in osteosclerosis, although Δ2ΔFosB lacks a known transactivation domain, in turn preventing it from affecting transcription through the AP-1 complex.[18]
The ΔFosB splice variant has been identified as playing a central, crucial[19][3] role in the development and maintenance of addiction.[19][4][20] ΔFosB overexpression (i.e., an abnormally and excessively high level of ΔFosB expression which produces a pronounced gene-related phenotype) triggers the development of addiction-related neuroplasticity throughout the reward system and produces a behavioral phenotype that is characteristic of an addiction.[19][20][21] ΔFosB differs from the full length FosB and further truncated Δ2ΔFosB in its capacity to produce these effects, as only accumbal ΔFosB overexpression is associated with pathological responses to drugs.[22]
DeltaFosB
DeltaFosB – more commonly written as ΔFosB – is a truncated splice variant of the FOSB gene.[23] ΔFosB has been implicated as a critical factor in the development of virtually all forms of behavioral and drug addictions.[3][4][5] In the brain's reward system, it is linked to changes in a number of other gene products, such as CREB and sirtuins.[24][25][26] In the body, ΔFosB regulates the commitment of mesenchymal precursor cells to the adipocyte or osteoblast lineage.[27]
In the nucleus accumbens, ΔFosB functions as a "sustained molecular switch" and "master control protein" in the development of an addiction.[19][28][29] In other words, once "turned on" (sufficiently overexpressed) ΔFosB triggers a series of transcription events that ultimately produce an addictive state (i.e., compulsive reward-seeking involving a particular stimulus); this state is sustained for months after cessation of drug use due to the abnormal and exceptionally long half-life of ΔFosB isoforms.[19][28][29] ΔFosB expression in D1-type nucleus accumbens medium spiny neurons directly and positively regulates drug self-administration and reward sensitization through positive reinforcement while decreasing sensitivity to aversion.[19][20] Based upon the accumulated evidence, a medical review from late 2014 argued that accumbal ΔFosB expression can be used as an addiction biomarker and that the degree of accumbal ΔFosB induction by a drug is a metric for how addictive it is relative to others.[19]
Chronic administration of anandamide, or N-arachidonylethanolamide (AEA), an endogenous cannabinoid, and additives such as sucralose, a noncaloric sweetener used in many food products of daily intake, are found to induce an overexpression of ΔFosB in the infralimbic cortex (Cx), nucleus accumbens (NAc) core, shell, and central nucleus of amygdala (Amy), that induce long-term changes in the reward system.[30]
Role in addiction
| Addiction and dependence glossary[20][31][32] | |
|---|---|
| |
Chronic addictive drug use causes alterations in gene expression in the mesocorticolimbic projection, which arise through transcriptional and epigenetic mechanisms.[3][39][40] The most important transcription factors that produce these alterations are ΔFosB, cyclic adenosine monophosphate (cAMP) response element binding protein (CREB), and nuclear factor kappa B (NF-κB).[3] ΔFosB is the most significant biomolecular mechanism in addiction because the overexpression of ΔFosB in the D1-type medium spiny neurons in the nucleus accumbens is necessary and sufficient for many of the neural adaptations and behavioral effects (e.g., expression-dependent increases in drug self-administration and reward sensitization) seen in drug addiction.[19][3][20] ΔFosB overexpression has been implicated in addictions to alcohol, cannabinoids, cocaine, methylphenidate, nicotine, opioids, phencyclidine, propofol, and substituted amphetamines, among others.[19][3][39][41][42] ΔJunD, a transcription factor, and G9a, a histone methyltransferase, both oppose the function of ΔFosB and inhibit increases in its expression.[3][20][43] Increases in nucleus accumbens ΔJunD expression (via viral vector-mediated gene transfer) or G9a expression (via pharmacological means) reduces, or with a large increase can even block, many of the neural and behavioral alterations seen in chronic drug abuse (i.e., the alterations mediated by ΔFosB).[21][3] Repression of c-Fos by ΔFosB, which consequently further induces expression of ΔFosB, forms a positive feedback loop that serves to indefinitely perpetuate the addictive state.
ΔFosB also plays an important role in regulating behavioral responses to natural rewards, such as palatable food, sex, and exercise.[3][5] Natural rewards, similar to drugs of abuse, induce gene expression of ΔFosB in the nucleus accumbens, and chronic acquisition of these rewards can result in a similar pathological addictive state through ΔFosB overexpression.[3][4][5] Consequently, ΔFosB is the key mechanism involved in addictions to natural rewards (i.e., behavioral addictions) as well;[3][4][5] in particular, ΔFosB in the nucleus accumbens is critical for the reinforcing effects of sexual reward.[5] Research on the interaction between natural and drug rewards suggests that dopaminergic psychostimulants (e.g., amphetamine) and sexual behavior act on similar biomolecular mechanisms to induce ΔFosB in the nucleus accumbens and possess bidirectional reward cross-sensitization effects[note 1] that are mediated through ΔFosB.[4][44] This phenomenon is notable since, in humans, a dopamine dysregulation syndrome, characterized by drug-induced compulsive engagement in natural rewards (specifically, sexual activity, shopping, and gambling), has also been observed in some individuals taking dopaminergic medications.[4]
ΔFosB inhibitors (drugs or treatments that oppose its action or reduce its expression) may be an effective treatment for addiction and addictive disorders.[45] Current medical reviews of research involving lab animals have identified a drug class – class I histone deacetylase inhibitors[note 2] – that indirectly inhibits the function and further increases in the expression of accumbal ΔFosB by inducing G9a expression in the nucleus accumbens after prolonged use.[21][43][46][47] These reviews and subsequent preliminary evidence which used oral administration or intraperitoneal administration of the sodium salt of butyric acid or other class I HDAC inhibitors for an extended period indicate that these drugs have efficacy in reducing addictive behavior in lab animals[note 3] that have developed addictions to ethanol, psychostimulants (i.e., amphetamine and cocaine), nicotine, and opiates;[43][47][48][49] however, as of August 2015[update], few clinical trials involving humans with addiction and any HDAC class I inhibitors have been conducted to test for treatment efficacy in humans or identify an optimal dosing regimen.[note 4]
Plasticity in cocaine addiction
ΔFosB accumulation from excessive drug use
Top: this depicts the initial effects of high dose exposure to an addictive drug on gene expression in the nucleus accumbens for various Fos family proteins (i.e., c-Fos, FosB, ΔFosB, Fra1, and Fra2).
Bottom: this illustrates the progressive increase in ΔFosB expression in the nucleus accumbens following repeated twice daily drug binges, where these phosphorylated (35–37 kilodalton) ΔFosB isoforms persist in the D1-type medium spiny neurons of the nucleus accumbens for up to 2 months.[29][37] |
ΔFosB levels have been found to increase upon the use of cocaine.[51] Each subsequent dose of cocaine continues to increase ΔFosB levels with no apparent ceiling of tolerance.[citation needed] Elevated levels of ΔFosB leads to increases in brain-derived neurotrophic factor (BDNF) levels, which in turn increases the number of dendritic branches and spines present on neurons involved with the nucleus accumbens and prefrontal cortex areas of the brain. This change can be identified rather quickly, and may be sustained weeks after the last dose of the drug.
Transgenic mice exhibiting inducible expression of ΔFosB primarily in the nucleus accumbens and dorsal striatum exhibit sensitized behavioural responses to cocaine.[52] They self-administer cocaine at lower doses than control,[53] but have a greater likelihood of relapse when the drug is withheld.[29][53] ΔFosB increases the expression of AMPA receptor subunit GluR2[52] and also decreases expression of dynorphin, thereby enhancing sensitivity to reward.[29]
| Target gene |
Target expression |
Neural effects | Behavioral effects |
|---|---|---|---|
| c-Fos | ↓ | Molecular switch enabling the chronic induction of ΔFosB[note 5] |
– |
| dynorphin | ↓ [note 6] |
• Downregulation of κ-opioid feedback loop | • Diminished self-extinguishing response to drug |
| NF-κB | ↑ | • Expansion of Nacc dendritic processes • NF-κB inflammatory response in the NAcc • NF-κB inflammatory response in the CP |
• Increased drug reward • Locomotor sensitization |
| GluR2 | ↑ | • Decreased sensitivity to glutamate | • Increased drug reward |
| Cdk5 | ↑ | • GluR1 synaptic protein phosphorylation • Expansion of NAcc dendritic processes |
• Decreased drug reward (net effect) |
| Form of neuroplasticity or behavioral plasticity |
Type of reinforcer | Sources | |||||
|---|---|---|---|---|---|---|---|
| Opiates | Psychostimulants | High fat or sugar food | Sexual intercourse | Physical exercise (aerobic) |
Environmental enrichment | ||
| ΔFosB expression in nucleus accumbens D1-type MSNs |
↑ | ↑ | ↑ | ↑ | ↑ | ↑ | [4] |
| Behavioral plasticity | |||||||
| Escalation of intake | Yes | Yes | Yes | [4] | |||
| Psychostimulant cross-sensitization |
Yes | Not applicable | Yes | Yes | Attenuated | Attenuated | [4] |
| Psychostimulant self-administration |
↑ | ↑ | ↓ | ↓ | ↓ | [4] | |
| Psychostimulant conditioned place preference |
↑ | ↑ | ↓ | ↑ | ↓ | ↑ | [4] |
| Reinstatement of drug-seeking behavior | ↑ | ↑ | ↓ | ↓ | [4] | ||
| Neurochemical plasticity | |||||||
| CREB phosphorylation in the nucleus accumbens |
↓ | ↓ | ↓ | ↓ | ↓ | [4] | |
| Sensitized dopamine response in the nucleus accumbens |
No | Yes | No | Yes | [4] | ||
| Altered striatal dopamine signaling | ↓DRD2, ↑DRD3 | ↑DRD1, ↓DRD2, ↑DRD3 | ↑DRD1, ↓DRD2, ↑DRD3 | ↑DRD2 | ↑DRD2 | [4] | |
| Altered striatal opioid signaling | No change or ↑μ-opioid receptors |
↑μ-opioid receptors ↑κ-opioid receptors |
↑μ-opioid receptors | ↑μ-opioid receptors | No change | No change | [4] |
| Changes in striatal opioid peptides | ↑dynorphin No change: enkephalin |
↑dynorphin | ↓enkephalin | ↑dynorphin | ↑dynorphin | [4] | |
| Mesocorticolimbic synaptic plasticity | |||||||
| Number of dendrites in the nucleus accumbens | ↓ | ↑ | ↑ | [4] | |||
| Dendritic spine density in the nucleus accumbens |
↓ | ↑ | ↑ | [4] | |||
Other functions in the brain
Viral overexpression of ΔFosB in the output neurons of the nigrostriatal dopamine pathway (i.e., the medium spiny neurons in the dorsal striatum) induces levodopa-induced dyskinesias in animal models of Parkinson's disease.[54][55] Dorsal striatal ΔFosB is overexpressed in rodents and primates with dyskinesias;[55] postmortem studies of individuals with Parkinson's disease that were treated with levodopa have also observed similar dorsal striatal ΔFosB overexpression.[55] Levetiracetam, an antiepileptic drug, has been shown to dose-dependently decrease the induction of dorsal striatal ΔFosB expression in rats when co-administered with levodopa;[55] the signal transduction involved in this effect is unknown.[55]
ΔFosB expression in the nucleus accumbens shell increases resilience to stress and is induced in this region by acute exposure to social defeat stress.[56][57][58]
Antipsychotic drugs have been shown to increase ΔFosB as well, more specifically in the prefrontal cortex. This increase has been found to be part of pathways for the negative side effects that such drugs produce.[59]
See also
Notes
- ^ In simplest terms, this means that when either amphetamine or sex is perceived as "more alluring or desirable" through reward sensitization, this effect occurs with the other as well.
- ^ Inhibitors of class I histone deacetylase (HDAC) enzymes are drugs that inhibit four specific histone-modifying enzymes: HDAC1, HDAC2, HDAC3, and HDAC8. Most of the animal research with HDAC inhibitors has been conducted with four drugs: butyrate salts (mainly sodium butyrate), trichostatin A, valproic acid, and SAHA;[46][47] butyric acid is a naturally occurring short-chain fatty acid in humans, while the latter two compounds are FDA-approved drugs with medical indications unrelated to addiction.
- ^ Specifically, prolonged administration of a class I HDAC inhibitor appears to reduce an animal's motivation to acquire and use an addictive drug without affecting an animals motivation to attain other rewards (i.e., it does not appear to cause motivational anhedonia) and reduce the amount of the drug that is self-administered when it is readily available.[43][47][48]
- ^ Among the few clinical trials that employed a class I HDAC inhibitor, one utilized valproate for methamphetamine addiction.[50]
- ^ In other words, c-Fos repression allows ΔFosB to accumulate within nucleus accumbens medium spiny neurons more rapidly because it is selectively induced in this state.[20]
- ^ ΔFosB has been implicated in causing both increases and decreases in dynorphin expression in different studies;[19][24] this table entry reflects only a decrease.
- Image legend
- ^ (Text color) Transcription factors
References
- ^ GBD 2013 Mortality and Causes of Death, Collaborators (17 December 2014). "Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013". Lancet. doi:10.1016/S0140-6736(14)61682-2. PMID 25530442.
{{cite journal}}:|first1=has generic name (help)CS1 maint: numeric names: authors list (link) - ^ "Opiate withdrawal". Medline Plus. Retrieved 1 November 2014.
- ^ a b c d e f g h i j k l Robison AJ, Nestler EJ (November 2011). "Transcriptional and epigenetic mechanisms of addiction". Nat. Rev. Neurosci. 12 (11): 623–637. doi:10.1038/nrn3111. PMC 3272277. PMID 21989194.
ΔFosB has been linked directly to several addiction-related behaviors ... Importantly, genetic or viral overexpression of ΔJunD, a dominant negative mutant of JunD which antagonizes ΔFosB- and other AP-1-mediated transcriptional activity, in the NAc or OFC blocks these key effects of drug exposure14,22–24. This indicates that ΔFosB is both necessary and sufficient for many of the changes wrought in the brain by chronic drug exposure. ΔFosB is also induced in D1-type NAc MSNs by chronic consumption of several natural rewards, including sucrose, high fat food, sex, wheel running, where it promotes that consumption14,26–30. This implicates ΔFosB in the regulation of natural rewards under normal conditions and perhaps during pathological addictive-like states.
Cite error: The named reference "Nestler" was defined multiple times with different content (see the help page). - ^ a b c d e f g h i j k l m n o p q r s t Olsen CM (December 2011). "Natural rewards, neuroplasticity, and non-drug addictions". Neuropharmacology. 61 (7): 1109–22. doi:10.1016/j.neuropharm.2011.03.010. PMC 3139704. PMID 21459101. Cite error: The named reference "Natural and drug addictions" was defined multiple times with different content (see the help page).
- ^ a b c d e f Blum K, Werner T, Carnes S, Carnes P, Bowirrat A, Giordano J, Oscar-Berman M, Gold M (2012). "Sex, drugs, and rock 'n' roll: hypothesizing common mesolimbic activation as a function of reward gene polymorphisms". J. Psychoactive Drugs. 44 (1): 38–55. doi:10.1080/02791072.2012.662112. PMC 4040958. PMID 22641964.
It has been found that deltaFosB gene in the NAc is critical for reinforcing effects of sexual reward. Pitchers and colleagues (2010) reported that sexual experience was shown to cause DeltaFosB accumulation in several limbic brain regions including the NAc, medial pre-frontal cortex, VTA, caudate, and putamen, but not the medial preoptic nucleus. Next, the induction of c-Fos, a downstream (repressed) target of DeltaFosB, was measured in sexually experienced and naive animals. The number of mating-induced c-Fos-IR cells was significantly decreased in sexually experienced animals compared to sexually naive controls. Finally, DeltaFosB levels and its activity in the NAc were manipulated using viral-mediated gene transfer to study its potential role in mediating sexual experience and experience-induced facilitation of sexual performance. Animals with DeltaFosB overexpression displayed enhanced facilitation of sexual performance with sexual experience relative to controls. In contrast, the expression of DeltaJunD, a dominant-negative binding partner of DeltaFosB, attenuated sexual experience-induced facilitation of sexual performance, and stunted long-term maintenance of facilitation compared to DeltaFosB overexpressing group. Together, these findings support a critical role for DeltaFosB expression in the NAc in the reinforcing effects of sexual behavior and sexual experience-induced facilitation of sexual performance. ... both drug addiction and sexual addiction represent pathological forms of neuroplasticity along with the emergence of aberrant behaviors involving a cascade of neurochemical changes mainly in the brain's rewarding circuitry.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "ΔFosB reward" was defined multiple times with different content (see the help page). - ^ Bourdy R, Barrot M (November 2012). "A new control center for dopaminergic systems: pulling the VTA by the tail". Trends Neurosci. 35 (11): 681–690. doi:10.1016/j.tins.2012.06.007. PMID 22824232.
In light of the crucial role of the tVTA in the opiate control of dopamine activity ...
In the context of addiction, the tVTA is a target for psychostimulant-induced plasticity [1,6,23] and is also essential for morphine action on dopamine neurons [19]. This latter finding suggests that the classical disinhibition model may need to be revisited in light of the GABAergic control that the tVTA exerts on dopamine systems. ...
The tVTA is rich in inhibitory GABA neurons expressing μ-opioid receptors and sends extensive projections toward midbrain dopamine cells. It is proposed as a major brake for dopamine systems. - ^ Barrot M, Sesack SR, Georges F, Pistis M, Hong S, Jhou TC (October 2012). "Braking dopamine systems: a new GABA master structure for mesolimbic and nigrostriatal functions". J. Neurosci. 32 (41): 14094–14101. doi:10.1523/JNEUROSCI.3370-12.2012. PMC 3513755. PMID 23055478.
The tVTA/RMTg sends dense GABA projections to VTA and substantia nigra neurons. ...
Indeed, tVTA/RMTg cells express high levels of mu-opioid receptors (Jhou et al., 2009a, 2012; Jalabert et al., 2011), and in vivo, ex vivo and optogenetic electrophysiological approaches demonstrated that morphine excites dopamine neurons by targeting receptors localized to tVTA/RMTg cell bodies as well as its terminals within the VTA (Jalabert et al., 2011; Lecca et al., 2011; Matsui and Williams, 2011; Lecca et al., 2012).{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Morphine addiction – Homo sapiens (human)". KEGG. Kanehisa Laboratories. 18 June 2013. Retrieved 11 September 2014.
- ^ Chen, Kevin W.; Banducci, Annie N.; Guller, Leila; MacAtee, Richard J.; Lavelle, Anna; Daughters, Stacey B.; Lejuez, C.W. (2011). "An examination of psychiatric comorbidities as a function of gender and substance type within an inpatient substance use treatment program". Drug and Alcohol Dependence. 118 (2–3): 92–9. doi:10.1016/j.drugalcdep.2011.03.003. PMC 3188332. PMID 21514751.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 23062049, please use {{cite journal}} with
|pmid= 23062049instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 16336480, please use {{cite journal}} with
|pmid= 16336480instead. - ^ Vargas-Perez, H.; Ting-A-Kee, R.; Walton, C. H.; Hansen, D. M.; Razavi, R.; Clarke, L.; Bufalino, M. R.; Allison, D. W.; Steffensen, S. C. (2009). "Ventral Tegmental Area BDNF Induces an Opiate-Dependent-Like Reward State in Naive Rats". Science. 324 (5935): 1732–34. doi:10.1126/science.1168501. PMC 2913611. PMID 19478142.
- ^ Laviolette, Steven R.; Van Der Kooy, Derek (2001). "GABAA receptors in the ventral tegmental area control bidirectional reward signalling between dopaminergic and non-dopaminergic neural motivational systems". European Journal of Neuroscience. 13 (5): 1009–15. doi:10.1046/j.1460-9568.2001.01458.x. PMID 11264674.
- ^ Goeldner, Celia; Lutz, Pierre-Eric; Darcq, Emmanuel; Halter, Thomas; Clesse, Daniel; Ouagazzal, Abdel-Mouttalib; Kieffer, Brigitte L. (2011). "Impaired Emotional-Like Behavior and Serotonergic Function During Protracted Abstinence from Chronic Morphine". Biological Psychiatry. 69 (3): 236–44. doi:10.1016/j.biopsych.2010.08.021. PMC 3014999. PMID 20947067.
- ^ a b "Entrez Gene: FOSB FBJ murine osteosarcoma viral oncogene homolog B".
- ^ Siderovski DP, Blum S, Forsdyke RE, Forsdyke DR (October 1990). "A set of human putative lymphocyte G0/G1 switch genes includes genes homologous to rodent cytokine and zinc finger protein-encoding genes". DNA and Cell Biology. 9 (8): 579–87. doi:10.1089/dna.1990.9.579. PMID 1702972.
- ^ Martin-Gallardo A, McCombie WR, Gocayne JD, FitzGerald MG, Wallace S, Lee BM, Lamerdin J, Trapp S, Kelley JM, Liu LI (April 1992). "Automated DNA sequencing and analysis of 106 kilobases from human chromosome 19q13.3". Nature Genetics. 1 (1): 34–9. doi:10.1038/ng0492-34. PMID 1301997. S2CID 1986255.
- ^ Sabatakos G, Rowe GC, Kveiborg M, Wu M, Neff L, Chiusaroli R, Philbrick WM, Baron R (May 2008). "Doubly truncated FosB isoform (Delta2DeltaFosB) induces osteosclerosis in transgenic mice and modulates expression and phosphorylation of Smads in osteoblasts independent of intrinsic AP-1 activity". Journal of Bone and Mineral Research. 23 (5): 584–95. doi:10.1359/jbmr.080110. PMC 2674536. PMID 18433296.
- ^ a b c d e f g h i j k Ruffle JK (November 2014). "Molecular neurobiology of addiction: what's all the (Δ)FosB about?". The American Journal of Drug and Alcohol Abuse. 40 (6): 428–37. doi:10.3109/00952990.2014.933840. PMID 25083822. S2CID 19157711.
ΔFosB as a therapeutic biomarker
The strong correlation between chronic drug exposure and ΔFosB provides novel opportunities for targeted therapies in addiction (118), and suggests methods to analyze their efficacy (119). Over the past two decades, research has progressed from identifying ΔFosB induction to investigating its subsequent action (38). It is likely that ΔFosB research will now progress into a new era – the use of ΔFosB as a biomarker. If ΔFosB detection is indicative of chronic drug exposure (and is at least partly responsible for dependence of the substance), then its monitoring for therapeutic efficacy in interventional studies is a suitable biomarker (Figure 2). Examples of therapeutic avenues are discussed herein. ...
Conclusions
ΔFosB is an essential transcription factor implicated in the molecular and behavioral pathways of addiction following repeated drug exposure. The formation of ΔFosB in multiple brain regions, and the molecular pathway leading to the formation of AP-1 complexes is well understood. The establishment of a functional purpose for ΔFosB has allowed further determination as to some of the key aspects of its molecular cascades, involving effectors such as GluR2 (87,88), Cdk5 (93) and NFkB (100). Moreover, many of these molecular changes identified are now directly linked to the structural, physiological and behavioral changes observed following chronic drug exposure (60,95,97,102). New frontiers of research investigating the molecular roles of ΔFosB have been opened by epigenetic studies, and recent advances have illustrated the role of ΔFosB acting on DNA and histones, truly as a molecular switch (34). As a consequence of our improved understanding of ΔFosB in addiction, it is possible to evaluate the addictive potential of current medications (119), as well as use it as a biomarker for assessing the efficacy of therapeutic interventions (121,122,124). Some of these proposed interventions have limitations (125) or are in their infancy (75). However, it is hoped that some of these preliminary findings may lead to innovative treatments, which are much needed in addiction. - ^ a b c d e f g Nestler EJ (December 2013). "Cellular basis of memory for addiction". Dialogues in Clinical Neuroscience. 15 (4): 431–443. PMC 3898681. PMID 24459410.
Despite the importance of numerous psychosocial factors, at its core, drug addiction involves a biological process: the ability of repeated exposure to a drug of abuse to induce changes in a vulnerable brain that drive the compulsive seeking and taking of drugs, and loss of control over drug use, that define a state of addiction. ... A large body of literature has demonstrated that such ΔFosB induction in D1-type [nucleus accumbens] neurons increases an animal's sensitivity to drug as well as natural rewards and promotes drug self-administration, presumably through a process of positive reinforcement ... Another ΔFosB target is cFos: as ΔFosB accumulates with repeated drug exposure it represses c-Fos and contributes to the molecular switch whereby ΔFosB is selectively induced in the chronic drug-treated state.41 ... Moreover, there is increasing evidence that, despite a range of genetic risks for addiction across the population, exposure to sufficiently high doses of a drug for long periods of time can transform someone who has relatively lower genetic loading into an addict.
- ^ a b c Biliński P, Wojtyła A, Kapka-Skrzypczak L, Chwedorowicz R, Cyranka M, Studziński T (2012). "Epigenetic regulation in drug addiction". Annals of Agricultural and Environmental Medicine. 19 (3): 491–6. PMID 23020045.
For these reasons, ΔFosB is considered a primary and causative transcription factor in creating new neural connections in the reward centre, prefrontal cortex, and other regions of the limbic system. This is reflected in the increased, stable and long-lasting level of sensitivity to cocaine and other drugs, and tendency to relapse even after long periods of abstinence. These newly constructed networks function very efficiently via new pathways as soon as drugs of abuse are further taken ... In this way, the induction of CDK5 gene expression occurs together with suppression of the G9A gene coding for dimethyltransferase acting on the histone H3. A feedback mechanism can be observed in the regulation of these 2 crucial factors that determine the adaptive epigenetic response to cocaine. This depends on ΔFosB inhibiting G9a gene expression, i.e. H3K9me2 synthesis which in turn inhibits transcription factors for ΔFosB. For this reason, the observed hyper-expression of G9a, which ensures high levels of the dimethylated form of histone H3, eliminates the neuronal structural and plasticity effects caused by cocaine by means of this feedback which blocks ΔFosB transcription
- ^ Ohnishi YN, Ohnishi YH, Vialou V, Mouzon E, LaPlant Q, Nishi A, Nestler EJ (January 2015). "Functional role of the N-terminal domain of ΔFosB in response to stress and drugs of abuse". Neuroscience. 284: 165–70. doi:10.1016/j.neuroscience.2014.10.002. PMC 4268105. PMID 25313003.
- ^ Nakabeppu Y, Nathans D (February 1991). "A naturally occurring truncated form of FosB that inhibits Fos/Jun transcriptional activity". Cell. 64 (4): 751–9. doi:10.1016/0092-8674(91)90504-R. PMID 1900040. S2CID 23904956.
- ^ a b c Nestler EJ (October 2008). "Review. Transcriptional mechanisms of addiction: role of DeltaFosB". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 363 (1507): 3245–55. doi:10.1098/rstb.2008.0067. PMC 2607320. PMID 18640924.
Recent evidence has shown that ΔFosB also represses the c-fos gene that helps create the molecular switch—from the induction of several short-lived Fos family proteins after acute drug exposure to the predominant accumulation of ΔFosB after chronic drug exposure—cited earlier (Renthal et al. in press). The mechanism responsible for ΔFosB repression of c-fos expression is complex and is covered below. ...
Examples of validated targets for ΔFosB in nucleus accumbens ... GluR2 ... dynorphin ... Cdk5 ... NFκB ... c-Fos
Table 3 - ^ Renthal W, Nestler EJ (August 2008). "Epigenetic mechanisms in drug addiction". Trends in Molecular Medicine. 14 (8): 341–50. doi:10.1016/j.molmed.2008.06.004. PMC 2753378. PMID 18635399.
- ^ Renthal W, Kumar A, Xiao G, Wilkinson M, Covington HE, Maze I, Sikder D, Robison AJ, LaPlant Q, Dietz DM, Russo SJ, Vialou V, Chakravarty S, Kodadek TJ, Stack A, Kabbaj M, Nestler EJ (May 2009). "Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins". Neuron. 62 (3): 335–48. doi:10.1016/j.neuron.2009.03.026. PMC 2779727. PMID 19447090.
- ^ Sabatakos G, Sims NA, Chen J, Aoki K, Kelz MB, Amling M, Bouali Y, Mukhopadhyay K, Ford K, Nestler EJ, Baron R (September 2000). "Overexpression of DeltaFosB transcription factor(s) increases bone formation and inhibits adipogenesis". Nature Medicine. 6 (9): 985–90. doi:10.1038/79683. PMID 10973317. S2CID 20302360.
- ^ a b c d e Robison AJ, Nestler EJ (November 2011). "Transcriptional and epigenetic mechanisms of addiction". Nature Reviews Neuroscience. 12 (11): 623–637. doi:10.1038/nrn3111. PMC 3272277. PMID 21989194.
ΔFosB serves as one of the master control proteins governing this structural plasticity. ... ΔFosB also represses G9a expression, leading to reduced repressive histone methylation at the cdk5 gene. The net result is gene activation and increased CDK5 expression. ... In contrast, ΔFosB binds to the c-fos gene and recruits several co-repressors, including HDAC1 (histone deacetylase 1) and SIRT 1 (sirtuin 1). ... The net result is c-fos gene repression.
Figure 4: Epigenetic basis of drug regulation of gene expression - ^ a b c d e Nestler EJ, Barrot M, Self DW (September 2001). "DeltaFosB: a sustained molecular switch for addiction". Proceedings of the National Academy of Sciences of the United States of America. 98 (20): 11042–6. Bibcode:2001PNAS...9811042N. doi:10.1073/pnas.191352698. PMC 58680. PMID 11572966.
- ^ Salaya-Velazquez NF, López-Muciño LA, Mejía-Chávez S, Sánchez-Aparicio P, Domínguez-Guadarrama AA, Venebra-Muñoz A (February 2020). "Anandamide and sucralose change ΔFosB expression in the reward system". NeuroReport. 31 (3): 240–244. doi:10.1097/WNR.0000000000001400. PMID 31923023. S2CID 210149592.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and Addictive Disorders". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 364–375. ISBN 978-0-07-148127-4.
- ^ Volkow ND, Koob GF, McLellan AT (January 2016). "Neurobiologic Advances from the Brain Disease Model of Addiction". New England Journal of Medicine. 374 (4): 363–371. doi:10.1056/NEJMra1511480. PMC 6135257. PMID 26816013.
Substance-use disorder: A diagnostic term in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) referring to recurrent use of alcohol or other drugs that causes clinically and functionally significant impairment, such as health problems, disability, and failure to meet major responsibilities at work, school, or home. Depending on the level of severity, this disorder is classified as mild, moderate, or severe.
Addiction: A term used to indicate the most severe, chronic stage of substance-use disorder, in which there is a substantial loss of self-control, as indicated by compulsive drug taking despite the desire to stop taking the drug. In the DSM-5, the term addiction is synonymous with the classification of severe substance-use disorder. - ^ a b c Renthal W, Nestler EJ (September 2009). "Chromatin regulation in drug addiction and depression". Dialogues in Clinical Neuroscience. 11 (3): 257–268. doi:10.31887/DCNS.2009.11.3/wrenthal. PMC 2834246. PMID 19877494.
[Psychostimulants] increase cAMP levels in striatum, which activates protein kinase A (PKA) and leads to phosphorylation of its targets. This includes the cAMP response element binding protein (CREB), the phosphorylation of which induces its association with the histone acetyltransferase, CREB binding protein (CBP) to acetylate histones and facilitate gene activation. This is known to occur on many genes including fosB and c-fos in response to psychostimulant exposure. ΔFosB is also upregulated by chronic psychostimulant treatments, and is known to activate certain genes (eg, cdk5) and repress others (eg, c-fos) where it recruits HDAC1 as a corepressor. ... Chronic exposure to psychostimulants increases glutamatergic [signaling] from the prefrontal cortex to the NAc. Glutamatergic signaling elevates Ca2+ levels in NAc postsynaptic elements where it activates CaMK (calcium/calmodulin protein kinases) signaling, which, in addition to phosphorylating CREB, also phosphorylates HDAC5.
Figure 2: Psychostimulant-induced signaling events - ^ Broussard JI (January 2012). "Co-transmission of dopamine and glutamate". The Journal of General Physiology. 139 (1): 93–96. doi:10.1085/jgp.201110659. PMC 3250102. PMID 22200950.
Coincident and convergent input often induces plasticity on a postsynaptic neuron. The NAc integrates processed information about the environment from basolateral amygdala, hippocampus, and prefrontal cortex (PFC), as well as projections from midbrain dopamine neurons. Previous studies have demonstrated how dopamine modulates this integrative process. For example, high frequency stimulation potentiates hippocampal inputs to the NAc while simultaneously depressing PFC synapses (Goto and Grace, 2005). The converse was also shown to be true; stimulation at PFC potentiates PFC–NAc synapses but depresses hippocampal–NAc synapses. In light of the new functional evidence of midbrain dopamine/glutamate co-transmission (references above), new experiments of NAc function will have to test whether midbrain glutamatergic inputs bias or filter either limbic or cortical inputs to guide goal-directed behavior.
- ^ Kanehisa Laboratories (10 October 2014). "Amphetamine – Homo sapiens (human)". KEGG Pathway. Retrieved 31 October 2014.
Most addictive drugs increase extracellular concentrations of dopamine (DA) in nucleus accumbens (NAc) and medial prefrontal cortex (mPFC), projection areas of mesocorticolimbic DA neurons and key components of the "brain reward circuit". Amphetamine achieves this elevation in extracellular levels of DA by promoting efflux from synaptic terminals. ... Chronic exposure to amphetamine induces a unique transcription factor delta FosB, which plays an essential role in long-term adaptive changes in the brain.
- ^ Cadet JL, Brannock C, Jayanthi S, Krasnova IN (2015). "Transcriptional and epigenetic substrates of methamphetamine addiction and withdrawal: evidence from a long-access self-administration model in the rat". Molecular Neurobiology. 51 (2): 696–717 (Figure 1). doi:10.1007/s12035-014-8776-8. PMC 4359351. PMID 24939695.
- ^ a b c d Nestler EJ (December 2012). "Transcriptional mechanisms of drug addiction". Clinical Psychopharmacology and Neuroscience. 10 (3): 136–143. doi:10.9758/cpn.2012.10.3.136. PMC 3569166. PMID 23430970.
The 35-37 kD ΔFosB isoforms accumulate with chronic drug exposure due to their extraordinarily long half-lives. ... As a result of its stability, the ΔFosB protein persists in neurons for at least several weeks after cessation of drug exposure. ... ΔFosB overexpression in nucleus accumbens induces NFκB ... In contrast, the ability of ΔFosB to repress the c-Fos gene occurs in concert with the recruitment of a histone deacetylase and presumably several other repressive proteins such as a repressive histone methyltransferase
- ^ Nestler EJ (October 2008). "Transcriptional mechanisms of addiction: Role of ΔFosB". Philosophical Transactions of the Royal Society B: Biological Sciences. 363 (1507): 3245–3255. doi:10.1098/rstb.2008.0067. PMC 2607320. PMID 18640924.
Recent evidence has shown that ΔFosB also represses the c-fos gene that helps create the molecular switch—from the induction of several short-lived Fos family proteins after acute drug exposure to the predominant accumulation of ΔFosB after chronic drug exposure
- ^ a b Hyman SE, Malenka RC, Nestler EJ (2006). "Neural mechanisms of addiction: the role of reward-related learning and memory". Annual Review of Neuroscience. 29: 565–98. doi:10.1146/annurev.neuro.29.051605.113009. PMID 16776597.
- ^ Steiner H, Van Waes V (January 2013). "Addiction-related gene regulation: risks of exposure to cognitive enhancers vs. other psychostimulants". Progress in Neurobiology. 100: 60–80. doi:10.1016/j.pneurobio.2012.10.001. PMC 3525776. PMID 23085425.
- ^ Kanehisa Laboratories (29 October 2014). "Alcoholism – Homo sapiens (human)". KEGG Pathway. Retrieved 31 October 2014.
- ^ Kim Y, Teylan MA, Baron M, Sands A, Nairn AC, Greengard P (February 2009). "Methylphenidate-induced dendritic spine formation and DeltaFosB expression in nucleus accumbens". Proceedings of the National Academy of Sciences of the United States of America. 106 (8): 2915–20. Bibcode:2009PNAS..106.2915K. doi:10.1073/pnas.0813179106. PMC 2650365. PMID 19202072.
- ^ a b c d Nestler EJ (January 2014). "Epigenetic mechanisms of drug addiction". Neuropharmacology. 76 Pt B: 259–268. doi:10.1016/j.neuropharm.2013.04.004. PMC 3766384. PMID 23643695.
Short-term increases in histone acetylation generally promote behavioral responses to the drugs, while sustained increases oppose cocaine's effects, based on the actions of systemic or intra-NAc administration of HDAC inhibitors. ... Genetic or pharmacological blockade of G9a in the NAc potentiates behavioral responses to cocaine and opiates, whereas increasing G9a function exerts the opposite effect (Maze et al., 2010; Sun et al., 2012a). Such drug-induced downregulation of G9a and H3K9me2 also sensitizes animals to the deleterious effects of subsequent chronic stress (Covington et al., 2011). Downregulation of G9a increases the dendritic arborization of NAc neurons, and is associated with increased expression of numerous proteins implicated in synaptic function, which directly connects altered G9a/H3K9me2 in the synaptic plasticity associated with addiction (Maze et al., 2010).
G9a appears to be a critical control point for epigenetic regulation in NAc, as we know it functions in two negative feedback loops. It opposes the induction of ΔFosB, a long-lasting transcription factor important for drug addiction (Robison and Nestler, 2011), while ΔFosB in turn suppresses G9a expression (Maze et al., 2010; Sun et al., 2012a). ... Also, G9a is induced in NAc upon prolonged HDAC inhibition, which explains the paradoxical attenuation of cocaine's behavioral effects seen under these conditions, as noted above (Kennedy et al., 2013). GABAA receptor subunit genes are among those that are controlled by this feedback loop. Thus, chronic cocaine, or prolonged HDAC inhibition, induces several GABAA receptor subunits in NAc, which is associated with increased frequency of inhibitory postsynaptic currents (IPSCs). In striking contrast, combined exposure to cocaine and HDAC inhibition, which triggers the induction of G9a and increased global levels of H3K9me2, leads to blockade of GABAA receptor and IPSC regulation. - ^ Pitchers KK, Vialou V, Nestler EJ, Laviolette SR, Lehman MN, Coolen LM (February 2013). "Natural and drug rewards act on common neural plasticity mechanisms with ΔFosB as a key mediator". The Journal of Neuroscience. 33 (8): 3434–42. doi:10.1523/JNEUROSCI.4881-12.2013. PMC 3865508. PMID 23426671.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and addictive disorders". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 384–385. ISBN 9780071481274.
- ^ a b McCowan TJ, Dhasarathy A, Carvelli L (February 2015). "The Epigenetic Mechanisms of Amphetamine". J. Addict. Prev. 2015 (Suppl 1). PMC 4955852. PMID 27453897.
Epigenetic modifications caused by addictive drugs play an important role in neuronal plasticity and in drug-induced behavioral responses. Although few studies have investigated the effects of AMPH on gene regulation (Table 1), current data suggest that AMPH acts at multiple levels to alter histone/DNA interaction and to recruit transcription factors which ultimately cause repression of some genes and activation of other genes. Importantly, some studies have also correlated the epigenetic regulation induced by AMPH with the behavioral outcomes caused by this drug, suggesting therefore that epigenetics remodeling underlies the behavioral changes induced by AMPH. If this proves to be true, the use of specific drugs that inhibit histone acetylation, methylation or DNA methylation might be an important therapeutic alternative to prevent and/or reverse AMPH addiction and mitigate the side effects generate by AMPH when used to treat ADHD.
- ^ a b c d Walker DM, Cates HM, Heller EA, Nestler EJ (February 2015). "Regulation of chromatin states by drugs of abuse". Curr. Opin. Neurobiol. 30: 112–121. doi:10.1016/j.conb.2014.11.002. PMC 4293340. PMID 25486626.
Studies investigating general HDAC inhibition on behavioral outcomes have produced varying results but it seems that the effects are specific to the timing of exposure (either before, during or after exposure to drugs of abuse) as well as the length of exposure
- ^ a b Primary references involving sodium butyrate:
• Kennedy PJ, Feng J, Robison AJ, Maze I, Badimon A, Mouzon E, Chaudhury D, Damez-Werno DM, Haggarty SJ, Han MH, Bassel-Duby R, Olson EN, Nestler EJ (April 2013). "Class I HDAC inhibition blocks cocaine-induced plasticity by targeted changes in histone methylation". Nat. Neurosci. 16 (4): 434–440. doi:10.1038/nn.3354. PMC 3609040. PMID 23475113.While acute HDAC inhibition enhances the behavioral effects of cocaine or amphetamine1,3,4,13,14, studies suggest that more chronic regimens block psychostimulant-induced plasticity3,5,11,12. ... The effects of pharmacological inhibition of HDACs on psychostimulant-induced plasticity appear to depend on the timecourse of HDAC inhibition. Studies employing co-administration procedures in which inhibitors are given acutely, just prior to psychostimulant administration, report heightened behavioral responses to the drug1,3,4,13,14. In contrast, experimental paradigms like the one employed here, in which HDAC inhibitors are administered more chronically, for several days prior to psychostimulant exposure, show inhibited expression3 or decreased acquisition of behavioral adaptations to drug5,11,12. The clustering of seemingly discrepant results based on experimental methodologies is interesting in light of our present findings. Both HDAC inhibitors and psychostimulants increase global levels of histone acetylation in NAc. Thus, when co-administered acutely, these drugs may have synergistic effects, leading to heightened transcriptional activation of psychostimulant-regulated target genes. In contrast, when a psychostimulant is given in the context of prolonged, HDAC inhibitor-induced hyperacetylation, homeostatic processes may direct AcH3 binding to the promoters of genes (e.g., G9a) responsible for inducing chromatin condensation and gene repression (e.g., via H3K9me2) in order to dampen already heightened transcriptional activation. Our present findings thus demonstrate clear cross talk among histone PTMs and suggest that decreased behavioral sensitivity to psychostimulants following prolonged HDAC inhibition might be mediated through decreased activity of HDAC1 at H3K9 KMT promoters and subsequent increases in H3K9me2 and gene repression.
• Simon-O'Brien E, Alaux-Cantin S, Warnault V, Buttolo R, Naassila M, Vilpoux C (July 2015). "The histone deacetylase inhibitor sodium butyrate decreases excessive ethanol intake in dependent animals". Addict Biol. 20 (4): 676–689. doi:10.1111/adb.12161. PMID 25041570. S2CID 28667144.Altogether, our results clearly demonstrated the efficacy of NaB in preventing excessive ethanol intake and relapse and support the hypothesis that HDACi may have a potential use in alcohol addiction treatment.
• Castino MR, Cornish JL, Clemens KJ (April 2015). "Inhibition of histone deacetylases facilitates extinction and attenuates reinstatement of nicotine self-administration in rats". PLOS ONE. 10 (4): e0124796. Bibcode:2015PLoSO..1024796C. doi:10.1371/journal.pone.0124796. PMC 4399837. PMID 25880762.treatment with NaB significantly attenuated nicotine and nicotine + cue reinstatement when administered immediately ... These results provide the first demonstration that HDAC inhibition facilitates the extinction of responding for an intravenously self-administered drug of abuse and further highlight the potential of HDAC inhibitors in the treatment of drug addiction.
- ^ Kyzar EJ, Pandey SC (August 2015). "Molecular mechanisms of synaptic remodeling in alcoholism". Neurosci. Lett. 601: 11–9. doi:10.1016/j.neulet.2015.01.051. PMC 4506731. PMID 25623036.
Increased HDAC2 expression decreases the expression of genes important for the maintenance of dendritic spine density such as BDNF, Arc, and NPY, leading to increased anxiety and alcohol-seeking behavior. Decreasing HDAC2 reverses both the molecular and behavioral consequences of alcohol addiction, thus implicating this enzyme as a potential treatment target (Fig. 3). HDAC2 is also crucial for the induction and maintenance of structural synaptic plasticity in other neurological domains such as memory formation [115]. Taken together, these findings underscore the potential usefulness of HDAC inhibition in treating alcohol use disorders ... Given the ability of HDAC inhibitors to potently modulate the synaptic plasticity of learning and memory [118], these drugs hold potential as treatment for substance abuse-related disorders. ... Our lab and others have published extensively on the ability of HDAC inhibitors to reverse the gene expression deficits caused by multiple models of alcoholism and alcohol abuse, the results of which were discussed above [25,112,113]. This data supports further examination of histone modifying agents as potential therapeutic drugs in the treatment of alcohol addiction ... Future studies should continue to elucidate the specific epigenetic mechanisms underlying compulsive alcohol use and alcoholism, as this is likely to provide new molecular targets for clinical intervention.
- ^ Kheirabadi GR, Ghavami M, Maracy MR, Salehi M, Sharbafchi MR (2016). "Effect of add-on valproate on craving in methamphetamine depended patients: A randomized trial". Advanced Biomedical Research. 5: 149. doi:10.4103/2277-9175.187404. PMC 5025910. PMID 27656618.
- ^ Hope BT (May 1998). "Cocaine and the AP-1 transcription factor complex". Annals of the New York Academy of Sciences. 844 (1): 1–6. Bibcode:1998NYASA.844....1H. doi:10.1111/j.1749-6632.1998.tb08216.x. PMID 9668659. S2CID 11683570.
- ^ a b Kelz MB, Chen J, Carlezon WA, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, Tkatch T, Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Picciotto MR, Nestler EJ (September 1999). "Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine". Nature. 401 (6750): 272–6. Bibcode:1999Natur.401..272K. doi:10.1038/45790. PMID 10499584. S2CID 4390717.
- ^ a b Colby CR, Whisler K, Steffen C, Nestler EJ, Self DW (March 2003). "Striatal cell type-specific overexpression of DeltaFosB enhances incentive for cocaine". The Journal of Neuroscience. 23 (6): 2488–93. doi:10.1523/JNEUROSCI.23-06-02488.2003. PMC 6742034. PMID 12657709.
- ^ Cao X, Yasuda T, Uthayathas S, Watts RL, Mouradian MM, Mochizuki H, Papa SM (May 2010). "Striatal overexpression of DeltaFosB reproduces chronic levodopa-induced involuntary movements". The Journal of Neuroscience. 30 (21): 7335–43. doi:10.1523/JNEUROSCI.0252-10.2010. PMC 2888489. PMID 20505100.
- ^ a b c d e Du H, Nie S, Chen G, Ma K, Xu Y, Zhang Z, Papa SM, Cao X (2015). "Levetiracetam Ameliorates L-DOPA-Induced Dyskinesia in Hemiparkinsonian Rats Inducing Critical Molecular Changes in the Striatum". Parkinson's Disease. 2015: 253878. doi:10.1155/2015/253878. PMC 4322303. PMID 25692070.
Furthermore, the transgenic overexpression of ΔFosB reproduces AIMs in hemiparkinsonian rats without chronic exposure to L-DOPA [13]. ... FosB/ΔFosB immunoreactive neurons increased in the dorsolateral part of the striatum on the lesion side with the used antibody that recognizes all members of the FosB family. All doses of levetiracetam decreased the number of FosB/ΔFosB positive cells (from 88.7 ± 1.7/section in the control group to 65.7 ± 0.87, 42.3 ± 1.88, and 25.7 ± 1.2/section in the 15, 30, and 60 mg groups, resp.; Figure 2). These results indicate dose-dependent effects of levetiracetam on FosB/ΔFosB expression. ... In addition, transcription factors expressed with chronic events such as ΔFosB (a truncated splice variant of FosB) are overexpressed in the striatum of rodents and primates with dyskinesias [9, 10]. ... Furthermore, ΔFosB overexpression has been observed in postmortem striatal studies of Parkinsonian patients chronically treated with L-DOPA [26]. ... Of note, the most prominent effect of levetiracetam was the reduction of ΔFosB expression, which cannot be explained by any of its known actions on vesicular protein or ion channels. Therefore, the exact mechanism(s) underlying the antiepileptic effects of levetiracetam remains uncertain.
- ^ "ROLE OF ΔFOSB IN THE NUCLEUS ACCUMBENS". Mount Sinai School of Medicine. NESTLER LAB: LABORATORY OF MOLECULAR PSYCHIATRY. Archived from the original on 28 June 2017. Retrieved 6 September 2014.
- ^ Furuyashiki T, Deguchi Y (August 2012). "[Roles of altered striatal function in major depression]". Brain and Nerve = Shinkei Kenkyū No Shinpo (in Japanese). 64 (8): 919–26. PMID 22868883.
- ^ Nestler EJ (April 2015). "∆FosB: a transcriptional regulator of stress and antidepressant responses". European Journal of Pharmacology. 753: 66–72. doi:10.1016/j.ejphar.2014.10.034. PMC 4380559. PMID 25446562.
In more recent years, prolonged induction of ∆FosB has also been observed within NAc in response to chronic administration of certain forms of stress. Increasing evidence indicates that this induction represents a positive, homeostatic adaptation to chronic stress, since overexpression of ∆FosB in this brain region promotes resilience to stress, whereas blockade of its activity promotes stress susceptibility. Chronic administration of several antidepressant medications also induces ∆FosB in the NAc, and this induction is required for the therapeutic-like actions of these drugs in mouse models. Validation of these rodent findings is the demonstration that depressed humans, examined at autopsy, display reduced levels of ∆FosB within the NAc. As a transcription factor, ΔFosB produces this behavioral phenotype by regulating the expression of specific target genes, which are under current investigation. These studies of ΔFosB are providing new insight into the molecular basis of depression and antidepressant action, which is defining a host of new targets for possible therapeutic development.
- ^ Dietz DM, Kennedy PJ, Sun H, Maze I, Gancarz AM, Vialou V, Koo JW, Mouzon E, Ghose S, Tamminga CA, Nestler EJ (February 2014). "ΔFosB induction in prefrontal cortex by antipsychotic drugs is associated with negative behavioral outcomes". Neuropsychopharmacology. 39 (3): 538–44. doi:10.1038/npp.2013.255. PMC 3895248. PMID 24067299.
Further reading
- Schuermann M, Jooss K, Müller R (April 1991). "fosB is a transforming gene encoding a transcriptional activator". Oncogene. 6 (4): 567–76. PMID 1903195.
- Brown JR, Ye H, Bronson RT, Dikkes P, Greenberg ME (July 1996). "A defect in nurturing in mice lacking the immediate early gene fosB". Cell. 86 (2): 297–309. doi:10.1016/S0092-8674(00)80101-4. PMID 8706134. S2CID 17266171.
- Heximer SP, Cristillo AD, Russell L, Forsdyke DR (December 1996). "Sequence analysis and expression in cultured lymphocytes of the human FOSB gene (G0S3)". DNA and Cell Biology. 15 (12): 1025–38. doi:10.1089/dna.1996.15.1025. PMID 8985116.
- Liberati NT, Datto MB, Frederick JP, Shen X, Wong C, Rougier-Chapman EM, Wang XF (April 1999). "Smads bind directly to the Jun family of AP-1 transcription factors". Proceedings of the National Academy of Sciences of the United States of America. 96 (9): 4844–9. Bibcode:1999PNAS...96.4844L. doi:10.1073/pnas.96.9.4844. PMC 21779. PMID 10220381.
- Yamamura Y, Hua X, Bergelson S, Lodish HF (November 2000). "Critical role of Smads and AP-1 complex in transforming growth factor-beta -dependent apoptosis". The Journal of Biological Chemistry. 275 (46): 36295–302. doi:10.1074/jbc.M006023200. PMID 10942775.
- Bergman MR, Cheng S, Honbo N, Piacentini L, Karliner JS, Lovett DH (February 2003). "A functional activating protein 1 (AP-1) site regulates matrix metalloproteinase 2 (MMP-2) transcription by cardiac cells through interactions with JunB-Fra1 and JunB-FosB heterodimers". Biochemical Journal. 369 (Pt 3): 485–96. doi:10.1042/BJ20020707. PMC 1223099. PMID 12371906.
- Milde-Langosch K, Kappes H, Riethdorf S, Löning T, Bamberger AM (February 2003). "FosB is highly expressed in normal mammary epithelia, but down-regulated in poorly differentiated breast carcinomas". Breast Cancer Research and Treatment. 77 (3): 265–75. doi:10.1023/A:1021887100216. PMID 12602926. S2CID 987857.
- Baumann S, Hess J, Eichhorst ST, Krueger A, Angel P, Krammer PH, Kirchhoff S (March 2003). "An unexpected role for FosB in activation-induced cell death of T cells". Oncogene. 22 (9): 1333–9. doi:10.1038/sj.onc.1206126. PMID 12618758. S2CID 10696422.
- Holmes DI, Zachary I (January 2004). "Placental growth factor induces FosB and c-Fos gene expression via Flt-1 receptors". FEBS Letters. 557 (1–3): 93–8. doi:10.1016/S0014-5793(03)01452-2. PMID 14741347. S2CID 6596900.
- Konsman JP, Blomqvist A (May 2005). "Forebrain patterns of c-Fos and FosB induction during cancer-associated anorexia-cachexia in rat". The European Journal of Neuroscience. 21 (10): 2752–66. doi:10.1111/j.1460-9568.2005.04102.x. PMID 15926923. S2CID 40045788.
External links
- ROLE OF ΔFOSB IN THE NUCLEUS ACCUMBENS Archived 28 June 2017 at the Wayback Machine
- KEGG Pathway – human alcohol addiction
- KEGG Pathway – human amphetamine addiction
- KEGG Pathway – human cocaine addiction
- FOSB+protein,+human at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
Opioid receptor pharmacogenomics
A genetic basis for the efficacy of opioids in the treatment of pain has been demonstrated for a number of specific variations; however, the evidence for clinical differences in opioid effects is ambiguous. The pharmacogenomics of the opioid receptors and their endogenous ligands has been the subject of intensive activity in association studies. These studies test broadly for a number of phenotypes, including opioid dependence, cocaine dependence, alcohol dependence, methamphetamine dependence/psychosis, response to naltrexone treatment, personality traits, and others. Major and minor variants have been reported for every receptor and ligand coding gene in both coding sequences, as well as regulatory regions. Newer approaches shift away from analysis of specific genes and regions, and are based on an unbiased screen of genes across the entire genome, which have no apparent relationship to the phenotype in question. These GWAS studies yield a number of implicated genes, although many of them code for seemingly unrelated proteins in processes such as cell adhesion, transcriptional regulation, cell structure determination, and RNA, DNA, and protein handling/modifying.[1]
Currently there are no specific pharmacogenomic dosing recommendations for opioids due to a lack of clear evidence connecting genotype to drug effect, toxicity, or likelihood of dependence.
118A>G variant
While over 100 variants have been identified for the opioid mu-receptor, the most studied mu-receptor variant is the non-synonymous 118A>G variant, which results in functional changes to the receptor, including lower binding site availability, reduced mRNA levels, altered signal transduction, and increased affinity for beta-endorphin. In theory, all of these functional changes would reduce the impact of exogenous opioids, requiring a higher dose to achieve the same therapeutic effect. This points to a potential for a greater addictive capacity in these individuals who require dosages to achieve pain control. However, evidence linking the 118A>G variant to opioid dependence is mixed, with associations shown in a number of study groups, but negative results in other groups. One explanation for the mixed results is the possibility of other variants which are in linkage disequilibrium with the 118A>G variant and thus contribute to different haplotype patterns that more specifically associate with opioid dependence.[2]
Non-opioid receptor genes
The preproenkephalin gene, PENK, encodes for the endogenous opiates that modulate pain perception, and are implicated in reward and addiction. (CA) repeats in the 3' flanking sequence of the PENK gene was associated with greater likelihood of opiate dependence in repeated studies. Variability in the MCR2 gene, encoding melanocortin receptor type 2 has been associated with both protective effects and increased susceptibility to heroin addiction. The CYP2B6 gene of the cytochrome P450 family also mediates breakdown of opioids and thus may play a role in dependence and overdose.[3]
Mechanism
Overexpression of the gene transcription factor "ΔFosB" in the nucleus accumbens plays a crucial role in the development of opioid addiction by directly modulating compulsive drug-seeking behaviors.[4][5][6] ΔFosB overexpression and altered regulation of cAMP response element binding protein (CREB) in the group of dopaminergic projections from the ventral tegmental area are also responsible for the induction of the negative emotional withdrawal state that characterizes opioid-related psychological dependence;[4][6] the associated mechanism(s) underlying the development of physical dependence are not currently understood.
Diagnosis
The core concept of the WHO's definition of a "drug dependence" is exactly the same as a drug addiction: compulsive drug use. The WHO and DSM-IV-TR clinical guidelines for a definite diagnosis of "dependence" require that three or more of the following six characteristic features be experienced or exhibited:
- A strong desire or sense of compulsion to take the drug;
- Difficulties in controlling drug-taking behaviour in terms of its onset, termination, or levels of use;
- A physiological withdrawal state when drug use is stopped or reduced, as evidenced by: the characteristic withdrawal syndrome for the substance; or use of the same (or a closely related) substance with the intention of relieving or avoiding withdrawal symptoms;
- Evidence of tolerance, such that increased doses of the drug are required in order to achieve effects originally produced by lower doses;
- Progressive neglect of alternative pleasures or interests because of drug use, increased amount of time necessary to obtain or take the drug or to recover from its effects;
- Persisting with drug use despite clear evidence of overtly harmful consequences, such as harm to the liver, depressive mood states or impairment of cognitive functioning.
According to position papers on the treatment of opioid dependence published by the United Nations Office on Drugs and Crime and the World Health Organization, care providers should not mistake opioid dependence for a weakness of character or will.[7][8] Accordingly, detoxification alone does not constitute adequate treatment.
Treatment
Opioid dependence is a complex health condition that often requires long-term treatment and care. The treatment of opioid dependence is important to reduce its health and social consequences and to improve the well-being and social functioning of people affected. The main objectives of treating and rehabilitating persons with opioid dependence are to reduce dependence on illicit drugs; to reduce the morbidity and mortality caused by the use of illicit opioids, or associated with their use, such as infectious diseases; to improve physical and psychological health; to reduce criminal behaviour; to facilitate reintegration into the workforce and education system and to improve social functioning.
As no single treatment is effective for all individuals with opioid dependence, diverse treatment options are needed, including psychosocial approaches and pharmacological treatment.[9]
Relapse following detoxification alone is extremely common, and therefore detoxification rarely constitutes an adequate treatment of substance dependence on its own. However, it is a first step for many forms of longer-term abstinence-based treatment. Both detoxification with subsequent abstinence-oriented treatment and substitution maintenance treatment are essential components of an effective treatment system for people with opioid dependence.[10]
Current trends in the US reveal a significant increase of prescription opioid abuse compared to illicit opiates such as heroin.[11] This development has also implications for the prevention, treatment and therapy of opioid dependence.[12]
Methadone
MMT (Methadone Maintenance Treatment), a form of opioid replacement therapy, reduces and/or eliminates the use of illicit opiates, the criminality associated with opiate use, and allows patients to improve their health and social productivity.[13][14] In addition, enrollment in methadone maintenance has the potential to reduce the transmission of infectious diseases associated with opiate injection, such as hepatitis and HIV.[13] The principal effects of methadone maintenance are to relieve narcotic craving, suppress the abstinence syndrome, and block the euphoric effects associated with opiates. Methadone maintenance has been found to be medically safe and non-sedating.[13] It is also indicated for pregnant women addicted to opiates.[13] Methadone maintenance treatment is given to addicted individuals who feel unable to go the whole way and get clean. For those individuals who wish to completely move away from drugs, a methadone reduction program is indicated, where the individual is prescribed an amount of methadone which is titrated up until withdrawal symptoms subside, followed by a period of stability, the dose will then be gradually reduced until the individual is either free of the need for methadone or is at a level which allows a switch to a different opiate with an easier withdrawal profile, such as Suboxone.[15] Methadone toxicity has been shown to be associated with specific phenotypes of CYP2B6.[16]
Buprenorphine

Studies have shown buprenorphine to be a safer alternative over methadone in opiate replacement therapy, primarily due to its lower instance of overdose related deaths during the course of treatment.[17] Buprenorphine sublingual preparations are often used in the management of opioid dependence (that is, dependence on heroin, oxycodone, hydrocodone, morphine, oxymorphone, fentanyl or other opioids). The Suboxone and Subutex preparations were approved for this indication by the United States Food and Drug Administration in October 2002.[18]
Naltrexone
Naltrexone was approved by the FDA in 1984 for the treatment of opioid dependence. It is available both as an oral medication and as a monthly injectable (approved in 2010). Some authors question whether oral Naltrexone is as effective in the treatment of opioid dependence as methadone and buprenorphine mainly due to non-compliance.[19]
Diamorphine
In Switzerland, Germany, the Netherlands, and the United Kingdom, longterm injecting drug users who do not benefit from methadone and other medication options are being treated with pure injectable diamorphine that is administered under the supervision of medical staff. For this group of patients, diamorphine treatment has proven superior in improving their social and health situation.[20] Studies show that even after years of homelessness and delinquency and despite severe comorbidities, about half of the patients find employment within the first year of treatment.[21]
LAAM
LAAM was previously used to treat opioid dependence. In 2003 the drug's manufacturer discontinued production. There are no available generic versions. LAAM produced long lasting effects, which allowed the person receiving treatment to visit a clinic only three times per week, as opposed to daily as with methadone.[22]
Experimental treatments
Each of these treatments is experimental, and some remain quite far from having been proven to be effective.
- Clonidine[23][24]
- Dextromethorphan[25]
- Ibogaine[26]
- Ketamine[25]
- Kratom
- Psilocybin
- Heantos
- Medical cannabis[27][28]
- Mitragynine[29]
- Tramadol[30][31]
12-step support groups
While medical treatment may help with the initial symptoms of opioid withdrawal, once an opiate addict overcomes the first stages of withdrawal, a method for long-term preventative care is attendance at 12-step groups such as Alcoholics Anonymous or Narcotics Anonymous. Attendance and participation in a 12 step program is an effective way to obtain and maintain sobriety.[32] Among primarily inner city minorities who had a "long severe history of (primarily) crack and/or heroin use", 51.7% of the individuals with continuous 12-step attendance had over 3 years of sustained abstinence, in contrast to 13.5% among those who had less than continuous 12-step attendance.[33][34]
Epidemiology
Opioid use disorders resulted in 51,000 deaths in 2013 up from 18,000 deaths in 1990.[35]
Among adults, the rate of inpatient hospital stays in the United States related to opioid overuse increased by an average of 5% annually from 1993–2012. The percentage of inpatient stays due to opioid overuse that were admitted from the emergency department increased from 43% in 1993 to 64% in 2005, but have remained relatively constant since.[36]
See also
- Benzodiazepine withdrawal syndrome
- Doctor shopping
- Opioid receptor
- Physical dependence
- Post Acute Withdrawal Syndrome
- Prescription drug abuse
- Walid–Robinson Opioid-Dependence Questionnaire
References
- ^ Hall, F. Scott; Drgonova, Jana; Jain, Siddharth; Uhl, George R. (December 2013). "Implications of genome wide association studies for addiction: Are our a priori assumptions all wrong?". Pharmacology & Therapeutics. 140 (3): 267–279. doi:10.1016/j.pharmthera.2013.07.006.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bruehl, Stephen; Apkarian, A. Vania; Ballantyne, Jane C.; Berger, Ann; Borsook, David; Chen, Wen G.; Farrar, John T.; Haythornthwaite, Jennifer A.; Horn, Susan D.; Iadarola, Michael J.; Inturrisi, Charles E.; Lao, Lixing; Mackey, Sean; Mao, Jianren; Sawczuk, Andrea; Uhl, George R.; Witter, James; Woolf, Clifford J.; Zubieta, Jon-Kar; Lin, Yu (February 2013). "Personalized Medicine and Opioid Analgesic Prescribing for Chronic Pain: Opportunities and Challenges". The Journal of Pain. 14 (2): 103–113. doi:10.1016/j.jpain.2012.10.016. PMC 3564046. PMID 23374939.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Khokhar, Jibran Y.; Ferguson, Charmaine S.; Zhu, Andy Z.X.; Tyndale, Rachel F. (February 2010). "Pharmacogenetics of Drug Dependence: Role of Gene Variations in Susceptibility and Treatment". Annual Review of Pharmacology and Toxicology. 50 (1): 39–61. doi:10.1146/annurev.pharmtox.010909.105826.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Nestler EJ (December 2013). "Cellular basis of memory for addiction". Dialogues Clin Neurosci. 15 (4): 431–443. PMC 3898681. PMID 24459410.
DESPITE THE IMPORTANCE OF NUMEROUS PSYCHOSOCIAL FACTORS, AT ITS CORE, DRUG ADDICTION INVOLVES A BIOLOGICAL PROCESS: the ability of repeated exposure to a drug of abuse to induce changes in a vulnerable brain that drive the compulsive seeking and taking of drugs, and loss of control over drug use, that define a state of addiction. ... A large body of literature has demonstrated that such ΔFosB induction in D1-type NAc neurons increases an animal's sensitivity to drug as well as natural rewards and promotes drug self-administration, presumably through a process of positive reinforcement
- ^ Cite error: The named reference
Nestlerwas invoked but never defined (see the help page). - ^ a b Cite error: The named reference
Natural and drug addictionswas invoked but never defined (see the help page). - ^ Substitution maintenance therapy in the management of opioid dependence and HIV/AIDS prevention. World Health Organization. 2004. ISBN 92-4-159115-3.
- ^ http://whqlibdoc.who.int/unaids/2004/9241591153_eng.pdf
- ^ Nicholls L, Bragaw L, Ruetsch C. (February 2010). "Opioid Dependence Treatment and guidelines". J Manag Care Pharm. 16 (1 Suppl B): S14–21. PMID 20146550.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ – Guidelines for the psychosocially assisted pharmacological treatment of opioid dependence – World Health Organization
- ^ Matthew Daubresse, Patrick P. Gleason, Yi Peng, Nilay D. Shah, Stephen T. Ritter, G. Caleb Alexander (2013). "Impact of a drug utilization review program on high-risk use of prescription controlled substances". Pharmacoepidemiology and Drug Safety. doi:10.1002/pds.3487.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Amy Maxmen (June 2012), "Tackling the US pain epidemic". Nature News doi:10.1038/nature.2012.10766
- ^ a b c d Joseph, H; Stancliff, S; Langrod, J (2000). "Methadone maintenance treatment (MMT): A review of historical and clinical issues". The Mount Sinai journal of medicine, New York. 67 (5–6): 347–64. PMID 11064485.
- ^ Connock, M; Juarez-Garcia, A; Jowett, S; Frew, E; Liu, Z; Taylor, RJ; Fry-Smith, A; Day, E; Lintzeris, N (2007). "Methadone and buprenorphine for the management of opioid dependence: A systematic review and economic evaluation". Health technology assessment. 11 (9): 1–171, iii–iv. doi:10.3310/hta11090. PMID 17313907.
- ^ http-www.rcgp.org.uk-PDF-drug_meth%20guidance.pdf
- ^ Bunten, H; Liang, W J; Pounder, D J; Seneviratne, C; Osselton, D (28 July 2010). "OPRM1 and CYP2B6 Gene Variants as Risk Factors in Methadone-Related Deaths". Clinical Pharmacology & Therapeutics. 88 (3): 383–389. doi:10.1038/clpt.2010.127. PMID 20668445.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bell, J.R; Butler, B., Lawrence, A., Batey, R. & Salmelainen (2009). "Comparing overdose mortality associated with methadone and buprenorphine treatment". Drug and Alcohol Dependence. 104 (1–2): 73–7. doi:10.1016/j.drugalcdep.2009.03.020. PMID 19443138.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Subutex and Suboxone Approved to Treat Opiate Dependence". FDA. 8 October 2002. Retrieved 1 November 2014.
- ^ Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database of Systematic Reviews 2011, Issue 4. PMID 21491383
- ^ Haasen, C.; Verthein, U.; Degkwitz, P.; Berger, J.; Krausz, M.; Naber, D. (2007). "Heroin-assisted treatment for opioid dependence: Randomised controlled trial". The British Journal of Psychiatry. 191: 55–62. doi:10.1192/bjp.bp.106.026112. PMID 17602126.
- ^ http://relaunch.bundestag.de/bundestag/ausschuesse/a14/anhoerungen/113/stllg/ZIS.pdf[dead link]
- ^ James W. Kalat, Biological Psychology. Cengage Learning. Page 81.
- ^ Brewer, C; H Rezae, C Bailey (1988). "Opioid withdrawal and naltrexone induction in 48–72 hours with minimal drop-out, using a modification of the naltrexone-clonidine technique". The British Journal of Psychiatry. 153 (3): 340–343. doi:10.1192/bjp.153.3.340. PMID 3250670.
- ^ Ling, W; L Amass, S Shoptaw, JJ Annon, M Hillhouse, D Babcock, G Brigham, J Harrer, M Reid, J Muir, B Buchan, D Orr, G Woody, J Krejci, D Ziedonis (2005). "A multi-center randomized trial of buprenorphine–naloxone versus clonidine for opioid, detoxification: findings from the National Institute on Drug Abuse Clinical Trials Network". Addiction. 100 (8): 1090–1100. doi:10.1111/j.1360-0443.2005.01154.x. PMC 1480367. PMID 16042639.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Herman, BH; F Vocci, P Bridge (1995). "The effects of NMDA receptor antagonists and nitric oxide synthase inhibitors on opioid tolerance and withdrawal: Medication development issues for opiate addiction" (PDF). Neuropsychopharmacology. 13 (4): 269–293. doi:10.1016/0893-133X(95)00140-9. PMID 8747752.
- ^ Alper, KR; HS Lotsof, GMN Frenken, DJ Luciano, J Bastiaans (1999). "Treatment of Acute Opioid Withdrawal with Ibogaine". The American Journal of Addictions. 8 (3): 234–243. doi:10.1080/105504999305848. PMID 10506904.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Morel LJ, Giros B, Daugé V (2009). "Adolescent Exposure to Chronic Delta-9-Tetrahydrocannabinol Blocks Opiate Dependence in Maternally Deprived Rats". Neuropsychopharmacology. 34 (11): 2469–76. doi:10.1038/npp.2009.70. PMID 19553915.
{{cite journal}}: Unknown parameter|laydate=ignored (help); Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Raby, Wilfrid Noel; Carpenter, Kenneth M.; Rothenberg, Jami; Brooks, Adam C.; Jiang, Huiping; Sullivan, Maria; Bisaga, Adam; Comer, Sandra; Nunes, Edward V. (2009). "Intermittent Marijuana Use Is Associated with Improved Retention in Naltrexone Treatment for Opiate-Dependence". The American Journal on Addictions. 18 (4): 301–8. doi:10.1080/10550490902927785. PMC 2753886. PMID 19444734.
- ^ Boyer, Edward W.; Kavita M. Babu, Jessica E. Adkins, Christopher R. McCurdy, John H. Halpern (28 June 2008). "Self-treatment of opioid withdrawal using kratom (Mitragynia speciosa korth)". Addiction. 103 (6): 1048–1050. doi:10.1111/j.1360-0443.2008.02209.x. PMC 3670991. PMID 18482427.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sobey, PW; Parran Tv, TV; Grey, SF; Adelman, CL; Yu, J (2003). "The use of tramadol for acute heroin withdrawal: a comparison to clonidine". Journal of addictive diseases. 22 (4): 13–25. doi:10.1300/J069v22n04_03. PMID 14723475.
- ^ Threlkeld, M; Parran, TV; Adelman, CA; Grey, SF; Yu, J; Yu, Jaehak (2006). "Tramadol versus buprenorphine for the management of acute heroin withdrawal: a retrospective matched cohort controlled study". The American journal on addictions. 15 (2): 186–91. doi:10.1080/10550490500528712. PMID 16595358.
- ^ Brigham, Gregory (2003). "12-Step Participation as a Pathway to Recovery: The Maryhaven Experience and Implications for Treatment and Research". Science & Practice Perspectives. 2 (1): 43–51. doi:10.1151/spp032143. PMC 2851040. PMID 18552722.
- ^ Alexandre B. Laudet, Ph.D – PowerPoint PPT Presentation – 12-step attendance and involvement over 3 years on odds of sustained abstinence
- ^ Manning, V; Best D., Faulkner N., Titherington E., Morinan A., Keaney F., Gossop M., Strang J. (November 2012). "Does active referral by a doctor or 12-Step peer improve 12-Step meeting attendance? Results from a pilot randomised control trial". Drug Alcohol Depend; Research study. 126 (1–2). NCBI Pubmed.gov: 131–7. doi:10.1016/j.drugalcdep.2012.05.004. PMID 22677458.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cite error: The named reference
GDB2013was invoked but never defined (see the help page). - ^ Owens PL, Barrett ML, Weiss AJ, Washington RE, Kronick R (August 2014). "Hospital Inpatient Utilization Related to Opioid Overuse Among Adults, 1993–2012". HCUP Statistical Brief #177. Rockville, MD: Agency for Healthcare Research and Quality.
{{cite web}}: CS1 maint: multiple names: authors list (link)