Peroxisome proliferator-activated receptor
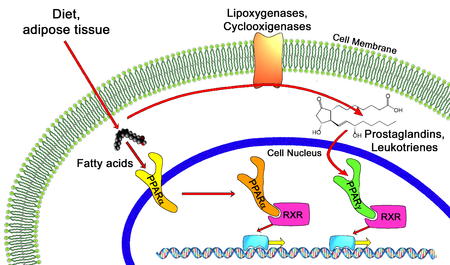
In the field of molecular biology, the peroxisome proliferator-activated receptors (PPARs) are a group of nuclear receptor proteins that function as transcription factors regulating the expression of genes.[1] PPARs play essential roles in the regulation of cellular differentiation, development, and metabolism (carbohydrate, lipid, protein),[2] and tumorigenesis[3] of higher organisms.[4][5]
Nomenclature and tissue distribution
| Peroxisome proliferator-activated receptor alpha | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | PPARA | ||||||
| Alt. symbols | PPAR | ||||||
| NCBI gene | 5465 | ||||||
| HGNC | 9232 | ||||||
| OMIM | 170998 | ||||||
| RefSeq | NM_001001928 | ||||||
| UniProt | Q07869 | ||||||
| Other data | |||||||
| Locus | Chr. 22 q12-q13.1 | ||||||
| |||||||
| Peroxisome proliferator-activated receptor gamma | |||||||
|---|---|---|---|---|---|---|---|
 | |||||||
| Identifiers | |||||||
| Symbol | PPARG | ||||||
| NCBI gene | 5468 | ||||||
| HGNC | 9236 | ||||||
| OMIM | 601487 | ||||||
| RefSeq | NM_005037 | ||||||
| UniProt | P37231 | ||||||
| Other data | |||||||
| Locus | Chr. 3 p25 | ||||||
| |||||||
| Peroxisome proliferator-activated receptor delta | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | PPARD | ||||||
| NCBI gene | 5467 | ||||||
| HGNC | 9235 | ||||||
| OMIM | 600409 | ||||||
| RefSeq | NM_006238 | ||||||
| UniProt | Q03181 | ||||||
| Other data | |||||||
| Locus | Chr. 6 p21.2 | ||||||
| |||||||
Three types of PPARs have been identified: alpha, gamma, and delta (beta):[4]
- α (alpha) - expressed in liver, kidney, heart, muscle, adipose tissue, and others[6]
- β/δ (beta/delta) - expressed in many tissues but markedly in brain, adipose tissue, and skin
- γ (gamma) - although transcribed by the same gene, this PPAR through alternative splicing is expressed in three forms:
- γ1 - expressed in virtually all tissues, including heart, muscle, colon, kidney, pancreas, and spleen
- γ2 - expressed mainly in adipose tissue (30 amino acids longer than γ1)
- γ3 - expressed in macrophages, large intestine, white adipose tissue.
History
PPARs were originally identified in Xenopus frogs as receptors that induce the proliferation of peroxisomes in cells.[7] The first PPAR (PPARα) was discovered during the search of a molecular target for a group of agents then referred to as peroxisome proliferators, as they increased peroxisomal numbers in rodent liver tissue, apart from improving insulin sensitivity.[8] These agents, pharmacologically related to the fibrates were discovered in the early 1980s. When it turned out that PPARs played a much more versatile role in biology, the agents were in turn termed PPAR ligands. The best-known PPAR ligands are the thiazolidinediones; see below for more details.
After PPARδ (delta) was identified in humans in 1992,[9] it turned out to be closely related to the PPARβ (beta) previously described during the same year in other animals (Xenopus). The name PPARδ is generally used in the US, whereas the use of the PPARβ denomination has remained in Europe where this receptor was initially discovered in Xenopus.
Physiological function
All PPARs heterodimerize with the retinoid X receptor (RXR) and bind to specific regions on the DNA of target genes. These DNA sequences are termed PPREs (peroxisome proliferator hormone response elements). The DNA consensus sequence is AGGTCANAGGTCA, with N being any nucleotide. In general, this sequence occurs in the promoter region of a gene, and, when the PPAR binds its ligand, transcription of target genes is increased or decreased, depending on the gene. The RXR also forms a heterodimer with a number of other receptors (e.g., vitamin D and thyroid hormone).
The function of PPARs is modified by the precise shape of their ligand-binding domain (see below) induced by ligand binding and by a number of coactivator and corepressor proteins, the presence of which can stimulate or inhibit receptor function, respectively.[10]
Endogenous ligands for the PPARs include free fatty acids, eicosanoids and Vitamin B3. PPARγ is activated by PGJ2 (a prostaglandin) and certain members of the 5-HETE family of arachidonic acid metabolites including 5-oxo-15(S)-HETE and 5-oxo-ETE.[11] In contrast, PPARα is activated by leukotriene B4. Certain members of the 15-hydroxyeicosatetraenoic acid family of arachidonic acid metabolites, including 15(S)-HETE, 15(R)-HETE, and 15-HpETE activate to varying degrees PPAR alpha, beta/delta, and gamma.[12] PPARγ activation by agonist RS5444 may inhibit anaplastic thyroid cancer growth.[13] See[14] for a review and critique of the roles of PPAR gamma in cancer.
Genetics
The three main forms are transcribed from different genes:
- PPARα - chromosome 22q12-13.1 (OMIM 170998)
- PPARβ/δ - chromosome 6p21.2-21.1 (OMIM 600409)
- PPARγ - chromosome 3p25 (OMIM 601487).
Hereditary disorders of all PPARs have been described, generally leading to a loss in function and concomitant lipodystrophy, insulin resistance, and/or acanthosis nigricans.[15] Of PPARγ, a gain-of-function mutation has been described and studied (Pro12Ala) which decreased the risk of insulin resistance; it is quite prevalent (allele frequency 0.03 - 0.12 in some populations).[16] In contrast, pro115gln is associated with obesity. Some other polymorphisms have high incidence in populations with elevated body mass indexes.
Structure
Like other nuclear receptors, PPARs are modular in structure and contain the following functional domains:
- (A/B) N-terminal region
- (C) DBD (DNA-binding domain)
- (D) flexible hinge region
- (E) LBD (ligand binding domain)
- (F) C-terminal region
The DBD contains two zinc finger motifs, which bind to specific sequences of DNA known as hormone response elements when the receptor is activated. The LBD has an extensive secondary structure consisting of 13 alpha helices and a beta sheet.[17] Natural and synthetic ligands bind to the LBD, either activating or repressing the receptor.
Pharmacology and PPAR modulators
PPARα and PPARγ are the molecular targets of a number of marketed drugs. For instance the hypolipidemic fibrates activate PPARα, and the anti diabetic thiazolidinediones activate PPARγ. The synthetic chemical perfluorooctanoic acid activates PPARα while the synthetic perfluorononanoic acid activates both PPARα and PPARγ. Berberine activates PPARγ, as well as other natural compounds from different chemical classes.[18][19]
See also
References
- ^ Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ, Grimaldi PA, Kadowaki T, Lazar MA, O'Rahilly S, Palmer CN, Plutzky J, Reddy JK, Spiegelman BM, Staels B, Wahli W (2006). "International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors". Pharmacol. Rev. 58 (4): 726–41. doi:10.1124/pr.58.4.5. PMID 17132851.
- ^ Dunning, Kylie R.; Anastasi, Marie R.; Zhang, Voueleng J.; Russell, Darryl L.; Robker, Rebecca L. (2014-02-05). "Regulation of Fatty Acid Oxidation in Mouse Cumulus-Oocyte Complexes during Maturation and Modulation by PPAR Agonists". PLOS ONE. 9 (2): e87327. doi:10.1371/journal.pone.0087327. ISSN 1932-6203. PMC 3914821. PMID 24505284.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Belfiore A, Genua M, Malaguarnera R (2009). "PPAR-gamma Agonists and Their Effects on IGF-I Receptor Signaling: Implications for Cancer". PPAR Res. 2009: 830501. doi:10.1155/2009/830501. PMC 2709717. PMID 19609453.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Berger J, Moller DE (2002). "The mechanisms of action of PPARs". Annu. Rev. Med. 53: 409–35. doi:10.1146/annurev.med.53.082901.104018. PMID 11818483.
- ^ Feige JN, Gelman L, Michalik L, Desvergne B, Wahli W (2006). "From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions". Prog. Lipid Res. 45 (2): 120–59. doi:10.1016/j.plipres.2005.12.002. PMID 16476485.
- ^ Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S (October 2011). "The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases". J Adv Pharm Technol Res. 2 (4): 236–40. doi:10.4103/2231-4040.90879. PMC 3255347. PMID 22247890.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W (1992). "Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors". Cell. 68 (5): 879–87. doi:10.1016/0092-8674(92)90031-7. PMID 1312391.
- ^ Issemann I, Green S (1990). "Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators". Nature. 347 (6294): 645–50. doi:10.1038/347645a0. PMID 2129546.
- ^ Schmidt A, Endo N, Rutledge SJ, Vogel R, Shinar D, Rodan GA (1992). "Identification of a new member of the steroid hormone receptor superfamily that is activated by a peroxisome proliferator and fatty acids". Mol. Endocrinol. 6 (10): 1634–41. doi:10.1210/me.6.10.1634. PMID 1333051.
- ^ Yu S, Reddy JK (2007). "Transcription coactivators for peroxisome proliferator-activated receptors". Biochim. Biophys. Acta. 1771 (8): 936–51. doi:10.1016/j.bbalip.2007.01.008. PMID 17306620.
- ^ Biochim. Biophys. Acta 1736:228-236, 2005
- ^ Mol. Pharmacol. 77-171-184, 2010
- ^ Marlow LA, Reynolds LA, Cleland AS, Cooper SJ, Gumz ML, Kurakata S, Fujiwara K, Zhang Y, Sebo T, Grant C, McIver B, Wadsworth JT, Radisky DC, Smallridge RC, Copland JA (February 2009). "Reactivation of suppressed RhoB is a critical step for the inhibition of anaplastic thyroid cancer growth". Cancer Res. 69 (4): 1536–44. doi:10.1158/0008-5472.CAN-08-3718. PMC 2644344. PMID 19208833.
- ^ Curr. Mol. Med. 7:532-540, 2007
- ^ Meirhaeghe A, Amouyel P (2004). "Impact of genetic variation of PPARgamma in humans". Mol. Genet. Metab. 83 (1–2): 93–102. doi:10.1016/j.ymgme.2004.08.014. PMID 15464424.
- ^ Buzzetti R, Petrone A, Ribaudo MC, Alemanno I, Zavarella S, Mein CA, Maiani F, Tiberti C, Baroni MG, Vecci E, Arca M, Leonetti F, Di Mario U (2004). "The common PPAR-gamma2 Pro12Ala variant is associated with greater insulin sensitivity". European Journal of Human Genetics. 12 (12): 1050–4. doi:10.1038/sj.ejhg.5201283. PMID 15367918.
- ^ Zoete V, Grosdidier A, Michielin O (2007). "Peroxisome proliferator-activated receptor structures: ligand specificity, molecular switch and interactions with regulators". Biochim. Biophys. Acta. 1771 (8): 915–25. doi:10.1016/j.bbalip.2007.01.007. PMID 17317294.
- ^ Atanasov AG, Wang JN, Gu SP, Bu J, Kramer MP, Baumgartner L, Fakhrudin N, Ladurner A, Malainer C, Vuorinen A, Noha SM, Schwaiger S, Rollinger JM, Schuster D, Stuppner H, Dirsch VM, Heiss EH (2013). "Honokiol: a non-adipogenic PPARγ agonist from nature". Biochim. Biophys. Acta. 1830 (10): 4813–9. doi:10.1016/j.bbagen.2013.06.021. PMC 3790966. PMID 23811337.
- ^ Atanasov AG, Blunder M, Fakhrudin N, Liu X, Noha SM, Malainer C, Kramer MP, Cocic A, Kunert O, Schinkovitz A, Heiss EH, Schuster D, Dirsch VM, Bauer R (2013). "Polyacetylenes from Notopterygium incisum--new selective partial agonists of peroxisome proliferator-activated receptor-gamma". PLoS ONE. 8 (4): e61755. doi:10.1371/journal.pone.0061755. PMC 3632601. PMID 23630612.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
External links
- [1] (PPAR Resource Page, Penn State University).
- [2] (Nuclear Receptor Resource).
- PPAR reference outline (Rutgers University).
- Peroxisome+Proliferator-Activated+Receptors at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Proteopedia Peroxisome_Proliferator-Activated_Receptors - the Peroxisome Proliferator-Activated Receptor Structure in Interactive 3D
